Iridoids and Amino Acid Derivatives from the Paraguayan Crude Drug Adenocalymma marginatum (ysypó hû)
Abstract
1. Introduction
2. Results and Discussion
2.1. CCC Isolation of the Main Compounds from Ysypó Hû
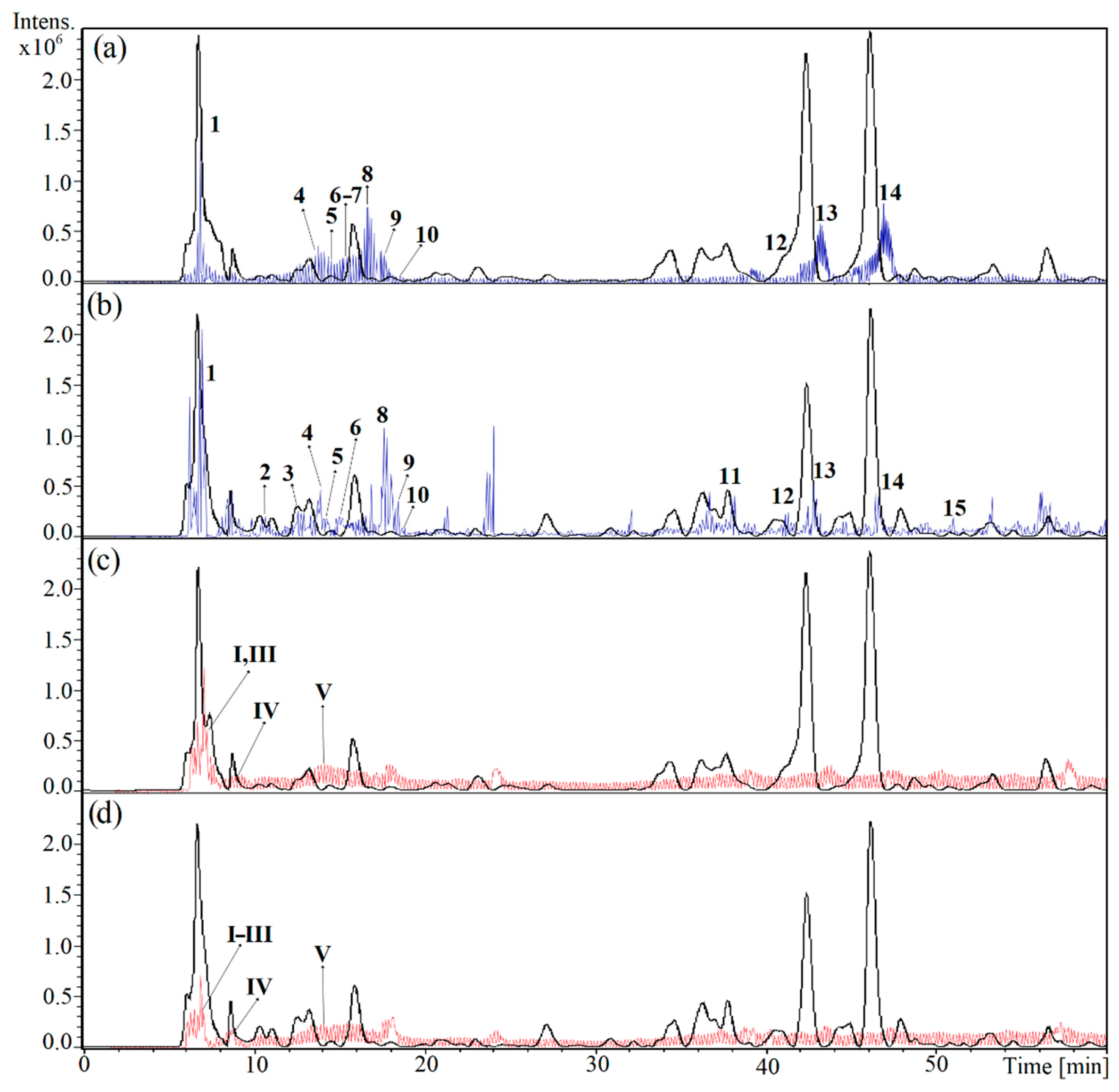
2.2. Chemical Profiling of the Crude Drug
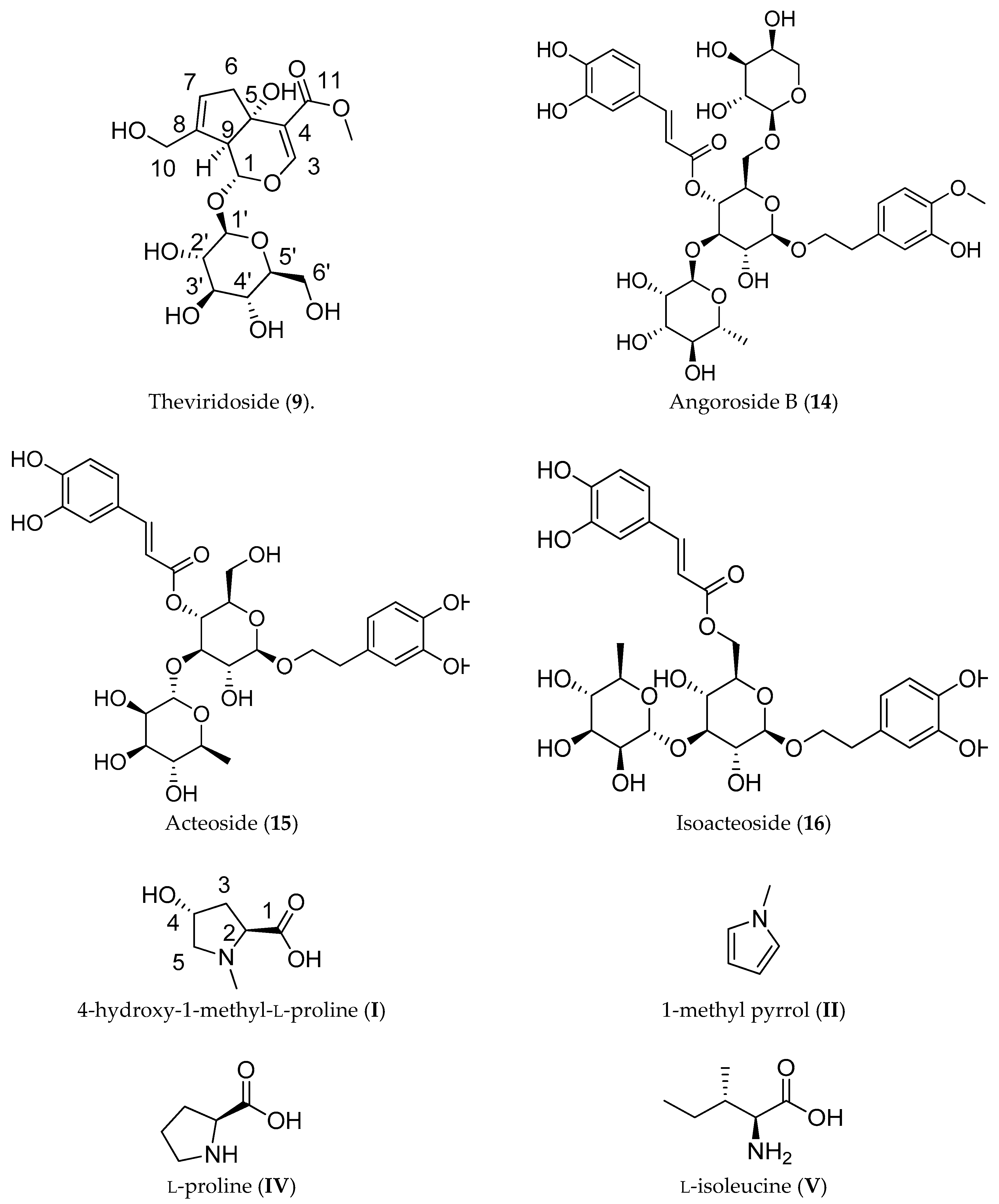
2.2.1. Iridoid Glycosides
2.2.2. Phenylpropanoid Glycosides
2.2.3. Amino Acids and Derivatives
2.3. Effect of the Extracts and Main Constituents on Phosphodiesterase-5 (PDE-5) Activity
3. Materials and Methods
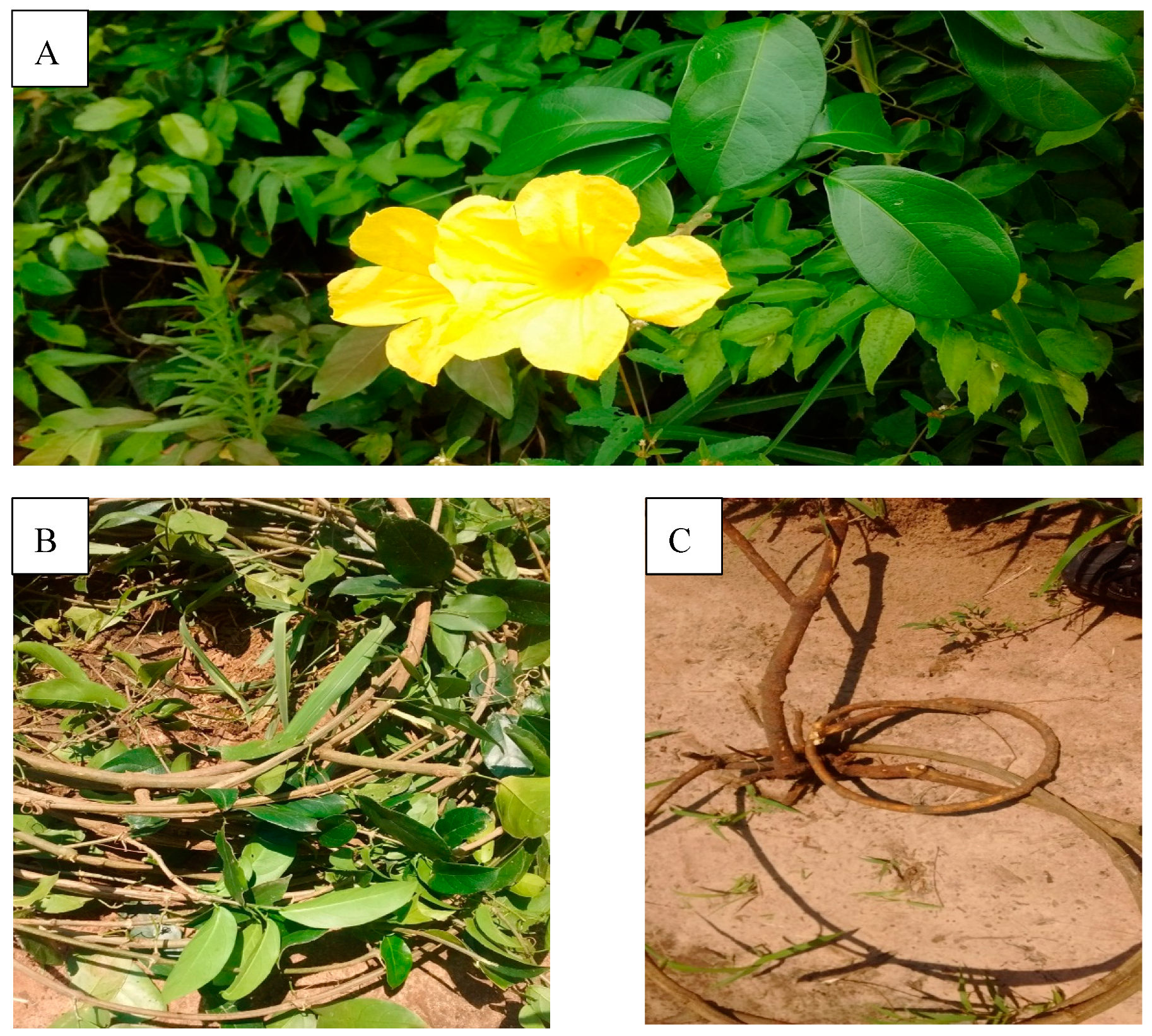
3.1. Plant Material
3.2. Isolation of the Main Compounds
3.3. Counter-Current Chromatography (CCC) Isolation of Main Compounds
3.4. HPLC-DAD-MS/MSn Analysis
3.5. Inhibition of Phosphodiesterase (PDE-5)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, F. Drogas y medicamentos de los indios Guaraní (Mbyá, Pai y Chiripá) en las regiones orientales de la selva del Paraguay. Parodiana 1997, 10, 197–209. [Google Scholar]
- Kujawska, M. Yerba Mate (Ilex paraguariensis) beverage: Nutraceutical ingredient or conveyor for the intake of medicinal plants? Evidence from Paraguayan folk medicine. EBCAM 2018, 2018, 6849317. [Google Scholar]
- Soejarto, D.D. Ethnobotany of Stevia and Stevia rebaudiana. In The Genus Stevia; Kinghorn, D., Ed.; eBook; CRC Press: London, UK, 2001; pp. 1–224. [Google Scholar]
- Montenegro, P.; Quintana, R. Materia Medica Misionera: Herbolario Guaraní sigo XVII; Buena Vista Editores: Córdoba, Argentina, 2009. [Google Scholar]
- Arenas, P.; Moreno, A.R. Plants used as means of abortion, contraception, sterilization and fecundation by Paraguayan indigenous people. Econ. Bot. 1977, 31, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Udulutsch, R.; Assis, M.A.; Dias, P. Taxonomic update of Adenocalymma (Bignoniaceae): Emendations, new synonyms, typification, and status change. Turk. J. Bot. 2013, 37, 630–643. [Google Scholar]
- Basualdo, I.; Soria, N.; Ortiz, M.; Degen, R. Uso medicinal de plantas comercializadas en los mercados de Asunción y Gran Asunción, Paraguay. Rev. Soc. Cient. Paraguay 2003, 14, 5–22. [Google Scholar]
- Basualdo, I.; Soria, N.; Ortiz, M.; Degen, R. Plantas comercializadas en los mercados de Asunción y Gran Asunción (Parte I). Rojasiana 2004, 6, 95–114. [Google Scholar]
- West, E.; Krychman, M. Natural aphrodisiacs—A review of selected sexual enhancers. Sex. Med. Rev. 2015, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Wozniak, K.; Georgiev, M.I.; Orhan, I.E. Adulteration of herbal sexual enhancers and slimmers: The wish for better sexual well-being and perfect body can be risky. Food Chem. Toxicol. 2017, 108, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Gilard, V.; Balayssac, S.; Tinaugus, A.; Martins, N.; Martino, R.; Malet-Martino, M. Detection, identification and quantification by 1H NMR of adulterants in 150 herbal dietary supplements marketed for improving sexual performance. J. Pharm. Biomed. Anal. 2015, 102, 476–493. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Burgos-Edwards, A.; Theoduloz, C.; Jiménez-Aspee, F.; Vargas- Arana, G. Male sexual enhancers from the Peruvian Amazon. J. Ethnopharmacol. 2019, 229, 167–179. [Google Scholar] [CrossRef]
- Von Poser, G.L.; Schripsema, J.; Henriques, A.T.; Jensen, S.R. The distribution of iridoids in Bignoniaceae. Biochem. Syst. Ecol. 2000, 28, 351–366. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Chen, R.-F.; Yamauchi, T.; Ohashi, H. Iridoids from the roots of Thevetia peruviana. Chem. Pharm. Bull. 1995, 43, 499–500. [Google Scholar] [CrossRef][Green Version]
- Abe, F.; Yamauchi, T.; Yahara, S.; Nohara, R. Minor iridoids from Thevetia peruviana. Phytochemistry 1995, 38, 793–794. [Google Scholar] [CrossRef]
- Jones, G.P.; Naidu, B.P.; Waisel, Y.; Solomon, A.; Paleg, L.G. Occurrence and stress response of N-methylproline compounds in Tamarix species. Phytochemistry 2006, 67, 156–160. [Google Scholar] [CrossRef]
- Haraguchi, M.; Gorniak, S.L.; Ikeda, K.; Minami, Y.; Kato, A.; Watson, A.A.; Nash, R.J.; Molyneaux, R.J.; Asano, N. Alkaloidal componentes in the poisonous plant, Ipomoea carnea (Convolvulaceae). J. Agric. Food Chem. 2003, 51, 4995–5000. [Google Scholar] [CrossRef]
- Ren, L.; Xue, X.; Zhang, F.; Wang, Y.; Liu, Y.; Li, C.; Liang, X. Studies of iridoid glycosides using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Sp. 2007, 21, 3039–3050. [Google Scholar] [CrossRef]
- Noor, A.T.; Perveen, S.; Begum, A.; Fatima, I.; Malik, A.; Tareen, R.B. Aitchisonides A and B, new iridoid glucosides from Aitchisonia rosea. J. Asian Nat. Prod. Res. 2009, 11, 985–989. [Google Scholar] [CrossRef]
- Ono, M.; Ishimatsu, N.; Masuoka, C.; Yoshimitsu, H.; Tsuchihashi, R.; Okawa, M.; Kinjo, J.; Ikeda, T.; Nohara, T. Three new monoterpenoids from the fruit of Genipa americana. Chem. Pharm. Bull. 2007, 55, 632–634. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Kerhoas, L.; Ducrot, P.H. Fragmentation study of iridoid glucosides through positive and negative electrospray ionization collision-induced dissociation and tandem mass spectrometry. Rapid Commun. Mass Sp. 2007, 21, 1165–1175. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull. 2007, 55, 159–222. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.-K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. Sulfur-containing bis iridoid glucosides and iridoid glucosides from Saprosma scortechinii. J. Nat. Prod. 2002, 65, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.-K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. Iridoids and anthraquinones from the Malaysian medicinal plant Saprosma scortchenii (Rubiaceae). Chem. Pharm. Bull. 2002, 50, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xu, D.; Luo, J.; Kong, L. Main iridoid glycosides and HPLC/DAD-Q-TOF-MS/MS profile of glycosides from the antioxidant extract of Eucommia ulmoides Oliver seeds. Ind. Crops Prod. 2016, 79, 160–169. [Google Scholar] [CrossRef]
- Calis, I.; Kirmizibekmez, H.; Sticher, O. Iridoid glycosides from Globularia trichosantha. J. Nat. Prod. 2001, 64, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Imakura, Y.; Kobayashi, S.; Yamahara, Y.; Kihara, M.; Tagawa, M.; Murai, F. Studies on constituents of Bignoniaceae plants. IV. Isolation and structure of a new iridoid glucoside, campsiside from Campsis chinensis. Chem. Pharm. Bull. 1985, 33, 2220–2227. [Google Scholar] [CrossRef][Green Version]
- Martin, F.; Hay, A.-E.; Corno, L.; Gupta, M.P.; Hostettmann, K. Iridoid glycosides from the stems of Pithecoctenium crucigerum (Bignoniaceae). Phytochemistry 2007, 68, 1307–1311. [Google Scholar] [CrossRef]
- Buckingham, J. (Ed.) Dictionary of Natural Products on DVD; CRC Press, Taylor & Francis Group: Abingdon, UK, 2019. [Google Scholar]
- Chen, Y.-H.; Qi, J.; Hua, J.; Yu, B.-Y. Structural characterization and identification of major constituents in Radix Scrophulariae by HPLC coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Nat. Med. 2014, 12, 47–54. [Google Scholar]
- Li, L.; Tsao, R.; Liu, Z.; Liu, S.; Yang, R.; Young, J.C.; Zhu, H.; Deng, Z.; Xie, M.; Fu, Z. Isolation and purification of acteoside and isoacteoside from Plantago psyllium L. by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1063, 161–169. [Google Scholar] [CrossRef]
- Pettit, G.R.; Numata, A.; Takemura, T.; Ode, R.H.; Narula, A.S.; Schmidt, J.M.; Cragg, G.M.; Pase, C.P. Antineoplastic agents, 107. Isolation of acteoside and isoacteoside from Castilleja linariaefolia. J. Nat. Prod. 1990, 53, 456–458. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T. Comments on piperidone derivatives from Dalbergia sympathetica. Magn. Reson. Chem. 2006, 44, 571–572. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, P.E.; Magalhaes, T.R.; Nicolau, L.A.; Leal, L.K.; de Aquino, N.C.; Dos Santos, S.M.; Neves, K.R.; Silveira, E.R.; Viana, G.S. The anti-inflammatory effects of N-methyl-(2S,4R)-trans-4-hydroxy-L-proline from Sideroxylon obtusifolium are related to its inhibition of TNF-alpha and inflammatory enzymes. Phytomedicine 2017, 24, 14–23. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, P.E.A.; de Souza, T.F.G.; Santos, F.A.; Viana, A.F.S.C.; Louchard, B.O.; Leal, L.K.A.M.; Rocha, T.M.; Evangelista, J.S.A.M.; de Aquino, N.C.; de Alencar, N.M.N.; et al. The wound healing property of N-methyl-(2S,4R)-trans-4-hydroxy-L-proline from Sideroxylon obtusifolium is related to its anti-inflammatory and antioxidant actions. J. Evid. Based Integr. Med. 2019, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.K.; Chan, W.; Ang, I.L.; Wei, R.; Lam, M.M.T.; Lei, K.M.K.; Poon, T.C.W. Revisiting fragmentation reactions of protonated α-amino acids by high-resolution electrospray ionization tandem mass spectrometry with collision-induced dissociation. Sci. Rep. 2019, 9, 6453. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.A.; Zampini, I.C.; Nuñez, M.B.; Isla, M.I.; Castro, M.P.; Gonzalez, A.M. In vitro antimicrobial activity of 20 selected climber species from the Bignoniaceae family. Nat. Prod. Res. 2013, 27, 2144–2148. [Google Scholar] [CrossRef]
- Granados-Echegoyen, C.; Pérez-Pacheco, R.; Alexander-Aguilera, A.; Lagunez-Rivera, L.; Alonso-Hernández, N.; Chairez-Martinez, E.d.J. Effects of aqueous and ethanol extract of dried leaves of Pseudocalymma alliaceum (Bignonaceae) on haematological and biochemical parameters of Wistar rats. Asian Pac. J. Reproduc. 2015, 4, 129–134. [Google Scholar] [CrossRef]
- Mendes, F.R. Tonic, fortifier and aphrodisiac: Adaptogens in the Brazilian folk medicine. Rev. Bras. Farmacog. 2011, 21, 754–763. [Google Scholar] [CrossRef]
- Corbin, J.D.; Francis, S.H. Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction. J. Androl. 2003, 24, S38–S41. [Google Scholar] [CrossRef]
- Drewes, S.E.; George, J.; Khan, F. Recent findings on natural products with erectile-dysfunction activity. Phytochemistry 2003, 62, 1019–1025. [Google Scholar] [CrossRef]
- Kaufman, D.M. Neurologic aspects of sexual function. In Clinical Neurology for Psychiatrist; Kaufman, D.M., Ed.; Saunders: Philadelphia, PA, USA, 2007; pp. 355–370. [Google Scholar]
- Chen, Y.; Wu, Y.; Gan, X.; Liu, K.; Lv, X.; Shen, H.; Dai, G.; Xu, H. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J. Ethnopharmacol. 2016, 194, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Bandieri, E.; Arletti, R. Lepidium meyenii Walp. improves sexual behavior in male rats independently from its action on spontaneous locomotor activity. J. Ethnopharmacol. 2001, 75, 225–229. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Pacente, S.; Plaza, A.; Sala, E.; Arletti, R.; Pizza, C. Hexanic maca extract improves rat sexual performance more effectively than methanolic and chloroformic Maca extracts. Andrologia 2002, 34, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Zenico, T.; Cicero, A.F.G.; Valmorri, L.; Mercuriali, M.; Bercovich, E. Subjective effects of Lepidium meyenii (Maca) extract on well-being and sexual performance in patients with mild erectile dysfunction: A randomized, double blind clinical trial. Andrologia 2009, 41, 95–99. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of theviridoside are available from the authors. |
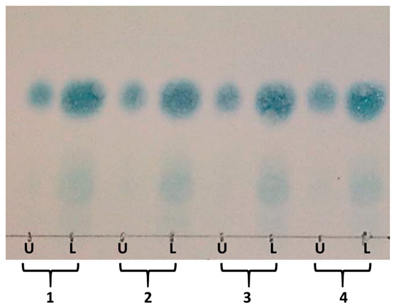 | ||
|---|---|---|
| System Number | Proportions of TBME:n-BuOH:ACN:H2O +0.1% TFA | KD Main Compound |
| 1 | 1:3:1:5 * | 0.21 |
| 2 | 1:4:1:5 | 0.33 |
| 3 | 1:5:1:5 | 0.41 |
| 4 | 2:2:2.5:5 | 0.40 |
| 1H | 13C | 1H | 13C | HMBC | |
|---|---|---|---|---|---|
| MeOH-d4 | MeOH-d4 | CDCl3 | CDCl3 | CDCl3 | |
| 1 | 5.55 d (5.6) | 95.67 d | 5.32 d (4.4) | 95.26 d | |
| 3 | 7.52 s | 152.71 d | 7.31 s | 151.36 d | 166.63, 113.78, 95.26, 74.91 |
| 4 | - | 113.14 s | - | 113.78 s | |
| 5 | - | 75.12 s | - | 74.91 s | 5.32 |
| 6 | 2.85 br s | 45.54 t | 2.75 d (19.6) 2.80 d (19.6) | 45.54 t | 129.81, 134.68, 74.91 |
| 7 | 5.72 s | 125.44 d | 5.68 br s | 129.81 d | |
| 8 | - | 140.59 s | - | 134.68 s | |
| 9 | 3.06 d (6.0) | 55.37 d | 3.08 d (2.4) | 54.94 d | |
| 10 | 4.25 d (14.4) 4.14 d (14.4) | 59.63 t | 4.58 d (13.6) 4.51 d (13.6) | 61.44 t | |
| 11 | - | 166.96 s | - | 166.63 s | |
| OMe | 3.75 s | 50.41 q | 3.67 s | 51.41 q | |
| 1’ | 4.65 d (8.0) | 98.66 d | 4.73 d (8) | 96.51 d | |
| 2’ | 3.25 dd (8.8, 8.0) | 73.18 d | 4.91 t (8.8) | 70.73 d | |
| 3’ | 3.33 m | 77.03 d | 5.16 t (9.6) | 72.03 d | |
| 4’ | 3.42 m | 76.18 d | 5.00 t (9.6) | 68.15 d | |
| 5’ | 3.32 m | 70.15 d | 3.67 m | 72.10 d | |
| 6’ | 3.83-3.85 m, 3.60-3.65 m | 62.87 t | 4.17 dd (12.4, 4) 4.07 dd (12.4, 1) | 61.44 t | |
| Ac | |||||
| COO | - | - | - | 170.51 s (2C), 170.05 s, 169.76 s, 169.27 s | |
| CH3 | - | - | 2.00 s, 1.98 s, 1.93 s, 1.91 s, 1.89 s | 20.74 q, 20.62 q, 20.51 q (2C), 20.39 q |
| H | C | HMBC | Configuration | |
|---|---|---|---|---|
| 1 | - | 170.38 s | 4.36, 3.08, 2.27 | |
| 2 | 4.36 dd (11.2, 7.6) | 69.03 d | 3.88, 3.16, 3.08, 2.27 | S |
| 3 | 2.50 ddt (14, 7.2, 1.6), 2.27 ddd (14, 11.2, 4.8) | 38.48 t | ||
| 4 | 4.55 m | 68.84 d | R | |
| 5 | 3.88 dd (12.4, 4.4), 3.16 dt (12.4, 2) | 63.29 t | 3.08 | |
| N-CH3 | 3.08 s | 42.79 q | 3.88, 4.36; 63.29, 68.84 |
| Peak | Rt (min) | [M + H]+/[M − H]− m/z | MS/MS (%) | Tentative Identification | Roots | Stems |
|---|---|---|---|---|---|---|
| [M + H]+ | ||||||
| I | 6.0 | 145.9 | 99.50 (100) | 4-Hydroxy-1-methyl-l-proline * | X | X |
| II | 6.0 | 99.6 | 81.5(100) | 1-methyl-2,3-dihydro-3-hydroxypyrrol | - | X |
| III | 6.1 | 81.5 | 1-methylpyrrol | X | X | |
| IV | 6.4 | 115.7 | 70.0(100), 28.1 (23), 43.1(20) | l-proline | X | X |
| V | 15.3 | 131.9 | 86.1 (100), 69.1(45), 56.9(4) | l-isoleucine * | X | X |
| [M − H]− | ||||||
| 1 | 5.5 | 340.9 | 178.4(100) | Caffeoyl hexoside | X | X |
| 2 | 9.0 | 727.2 | 564.8(100), 341(14), 240.5(3) | Theviridoside dihexoside 1 | - | X |
| 3 | 11.2 | 763.3 | 727.0 (100), 484.9 (29), 383.1 (43), 340.7 (29), 240.6 (11) | Chlortheviridoside dihexoside derivative | - | X |
| 4 | 12.3 | 601.4 | 564.9 (100), 385.1 (73), 301 (27), 240.6 (4), 222.6 (15) | Chlortheviridoside hexoside derivative | X | X |
| 5 | 12.6 | 726.9 | 502.5(42), 385.1 (73), 240.5(3) | Theviridoside dihexoside 2 | X | X |
| 6 | 13.5 | 601.2 | 564.9 (100), 385.1 (13), 240.7 (5) | Chlortheviridoside hexoside derivative | X | X |
| 7 | 13.6 | 659.1 | 402.3(93), 254.9(78), 240.6(100) | Methyl-theviridoside aglycon theviridoside | X | - |
| 8 | 15.7 | 403.4 | 240.67(100), 222.60(21) | Theviridoside* | X | X |
| 9 | 15.9-16.2 | 600.9 | 565.0 (100), 240.8 (13) | Chlortheviridoside hexoside derivative | X | X |
| 10 | 16.1-16.3 | 565.5 | 240.65(100) | Theviridoside hexoside | X | X |
| 11 | 39.4 | 625.6 | 461.2(100), 315.3(3) | Dihydro acteoside | - | X |
| 12 | 41.3 | 769.6 | 607.3(100), 461.2(2) | Angoroside B | X | X |
| 13 | 41.7–42.1 | 623.6 | 477.1(4), 461.2(100), 315.2(2) | Acteoside | X | X |
| 14 | 43.8–45.5 | 623.5 | 461.1(100), 315.2(2), 178.6(3) | Isoacteoside | X | X |
| 15 | 51.3 | 515.7 | 352.9(100), 172.6(7) | Dicaffeoylquinic acid | - | X |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmeda-Hirschmann, G.; Burgos-Edwards, A.; Jiménez-Aspee, F.; Mieres-Castro, D.; Theoduloz, C.; Pormetter, L.; Fogel, R.; Céspedes, C.; Soria, N.; Valdez, S. Iridoids and Amino Acid Derivatives from the Paraguayan Crude Drug Adenocalymma marginatum (ysypó hû). Molecules 2020, 25, 180. https://doi.org/10.3390/molecules25010180
Schmeda-Hirschmann G, Burgos-Edwards A, Jiménez-Aspee F, Mieres-Castro D, Theoduloz C, Pormetter L, Fogel R, Céspedes C, Soria N, Valdez S. Iridoids and Amino Acid Derivatives from the Paraguayan Crude Drug Adenocalymma marginatum (ysypó hû). Molecules. 2020; 25(1):180. https://doi.org/10.3390/molecules25010180
Chicago/Turabian StyleSchmeda-Hirschmann, Guillermo, Alberto Burgos-Edwards, Felipe Jiménez-Aspee, Daniel Mieres-Castro, Cristina Theoduloz, Lisa Pormetter, Ramon Fogel, Claudia Céspedes, Nelida Soria, and Sintya Valdez. 2020. "Iridoids and Amino Acid Derivatives from the Paraguayan Crude Drug Adenocalymma marginatum (ysypó hû)" Molecules 25, no. 1: 180. https://doi.org/10.3390/molecules25010180
APA StyleSchmeda-Hirschmann, G., Burgos-Edwards, A., Jiménez-Aspee, F., Mieres-Castro, D., Theoduloz, C., Pormetter, L., Fogel, R., Céspedes, C., Soria, N., & Valdez, S. (2020). Iridoids and Amino Acid Derivatives from the Paraguayan Crude Drug Adenocalymma marginatum (ysypó hû). Molecules, 25(1), 180. https://doi.org/10.3390/molecules25010180







