Evaluation of the Possibility of Using Hydroponic Cultivations for the Removal of Pharmaceuticals and Endocrine Disrupting Compounds in Municipal Sewage Treatment Plants
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Selected Pharmaceuticals and Endocrine Disrupting Compounds in Treated and Untreated Sewage
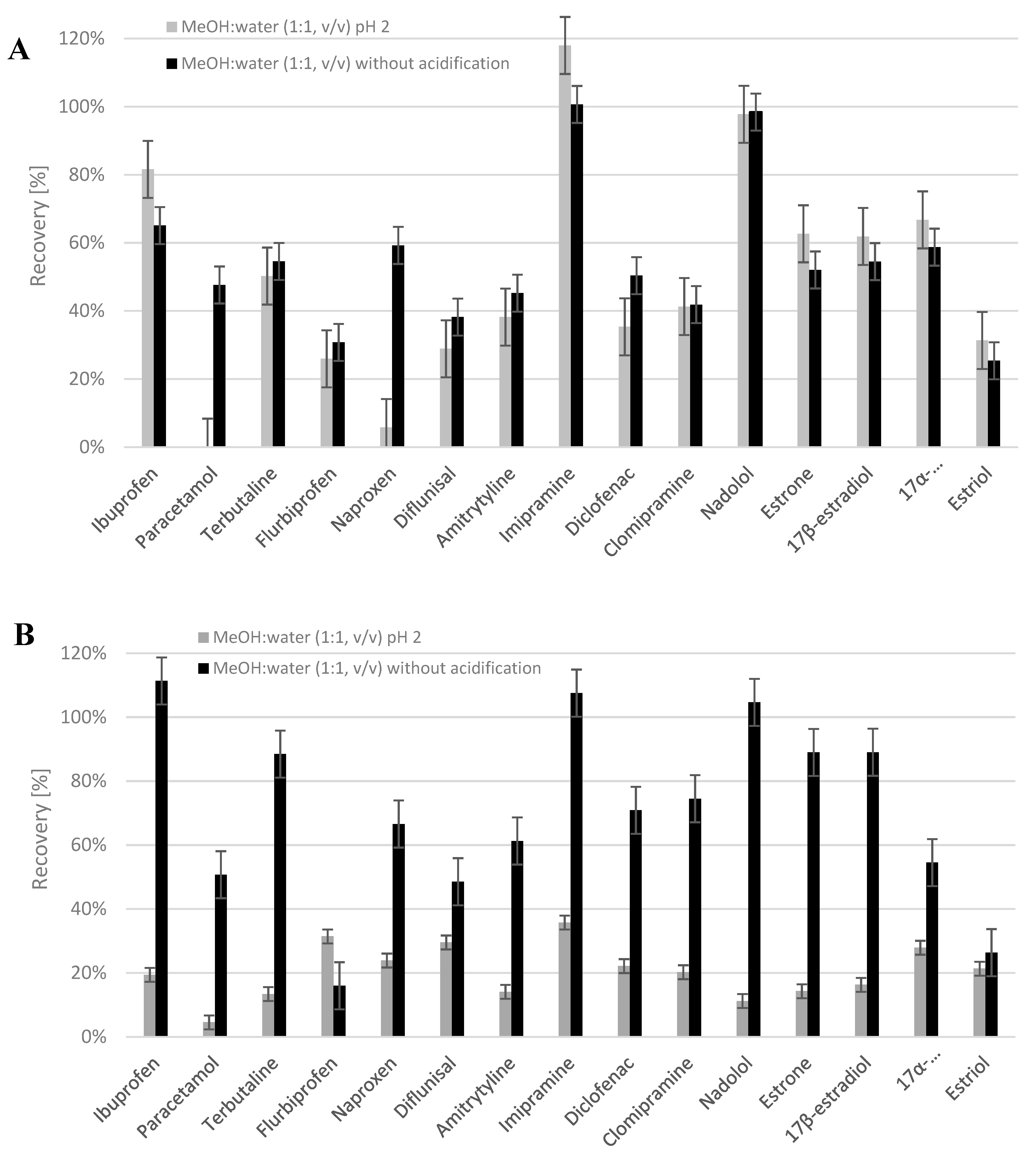
2.2. Evaluation of the Analytical Method for Determining Target Compounds in Plant Materials
2.3. Validation Parameters of the Proposed ASE-SPE-GC-MS(SIM) MetHod for Determining Target Compounds in Plant Materials
2.4. Assessment of Bioaccumulation of Selected Pollutants in Hydroponically Cultivated Plants
2.5. Assessment of the Usefulness of Hydroponically Cultivated Plants for Removing Target Compounds from Sewage Stream
3. Materials and Methods
3.1. Chemicals and Materials
3.2. MWWTP and Constructed Wetlands Characteristic
3.3. Sampling Sewage and Plants from Constructed Wetland
3.4. Determination of Selected Pharmaceuticals and Endocrine Disrupting Compounds in Treated and Untreated Sewage
3.5. Development of the Analytical Method for Determining Target Compounds in Plant Materials
3.6. Validation of the Proposed Method for Determining Target Compounds in Plant Species
3.7. Application of the Proposed Method for Determining Target Compounds in Hydroponically Cultivated Plants
3.8. Evaluation of the Effectiveness of Removing Pharmaceuticals and Endocrine Disrupting Compounds in MWWTP Sochaczew
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uslu, M.O.; Jasim, S.; Arvai, A.; Bewtra, J.; Biswas, N. A Survey of Occurrence and Risk Assessment of Pharmaceutical Substances in the Great Lakes Basin. Ozone Sci. Eng. 2013, 35, 249–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Caban, M.; Lis, E.; Kumirska, J.; Stepnowski, P. Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS(SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci. Total Environ. 2015, 538, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Wagil, M.; Maszkowska, J.; Białk-Bielińska, A.; Caban, M.; Stepnowski, P.; Kumirska, J. Determination of metronidazole residues in water, sediment and fish tissue samples. Chemosphere 2015, 119, A28–A34. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Migowska, N.; Caban, M.; Łukaszewicz, P.; Stepnowski, P. Simultaneous determination of non-steroidal anti-inflammatory drugs and oestrogenic hormones in environmental solid samples. Sci. Total Environ. 2015, 508, 498–505. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Kumirska, J.; Borecka, M.; Caban, M.; Paszkiewicz, M.; Pazdro, K.; Stepnowski, P. Selected analytical challenges in the determination of pharmaceuticals in drinking/marine waters and soil/sediment samples. J. Pharm. Biomed. Anal. 2016, 121, 271–296. [Google Scholar] [CrossRef]
- Golet, E.M.; Strehler, A.; Alder, A.C.; Giger, W. Determination of Fluoroquinolone Antibacterial Agents in Sewage Sludge and Sludge-Treated Soil Using Accelerated Solvent Extraction Followed by Solid-Phase Extraction. Anal. Chem. 2002, 74, 5455–5462. [Google Scholar] [CrossRef]
- Borecka, M.; Białk-Bielińska, A.; Siedlewicz, G.; Kornowska, K.; Kumirska, J.; Stepnowski, P.; Pazdro, K. A new approach for the estimation of expanded uncertainty of results of an analytical method developed for determining antibiotics in seawater using solid-phase extraction disks and liquid chromatography coupled with tandem mass spectrometry technique. J. Chromatogr. A 2013, 1304, 138–146. [Google Scholar] [CrossRef]
- Caban, M.; Szaniawska, A.; Stepnowski, P. Screening of 17α-ethynylestradiol and non-steroidal anti-inflammatory pharmaceuticals accumulation in Mytilus edulis trossulus (Gould, 1890) collected from the Gulf of Gdańsk. Oceanol. Hydrobiol. Stud. 2016, 45, 605–614. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Zhou, Z.; Sharma, V.K. Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in waste water—A review from global views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Petrie, B.; McAdam, E.J.; Scrimshaw, M.D.; Lester, J.N.; Cartmell, E. Fate of drugs during wastewater treatment. Trends Anal. Chem. 2013, 49, 145–159. [Google Scholar] [CrossRef]
- Repice, C.; Grande, M.D.; Maggi, R.; Pedrazzani, R. Licit and illicit drugs in a wastewater treatment plant in Verona, Italy. Sci. Total Environ. 2013, 463–464, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernández-Alba, A.R. Degradation of fifteen emerging contaminants at μg L-1initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res. 2010, 44, 545–554. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Reif, R.; Garrido-Baserba, M.; Hernández-Sancho, F.; Omil, F.; Poch, M.; Sala-Garrido, R. Economic valuation of environmental benefits of removing pharmaceutical and personal care products from WWTP effluents by ozonation. Sci. Total Environ. 2013, 461–462, 409–415. [Google Scholar] [CrossRef]
- Trinh, T.; van den Akker, B.; Stuetz, R.M.; Coleman, H.M.; Le-Clech, P.; Khan, S.J. Removal of trace organic chemical contaminants by a membrane bioreactor. Water Sci. Technol. 2012, 66, 1856–1863. [Google Scholar] [CrossRef]
- Naddeo, V.; Belgiorno, V.; Ricco, D.; Kassinos, D. Degradation of diclofenac during sonolysis, ozonation and their simultaneous application. Ultrason. Sonochem. 2009, 16, 790–794. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. How efficient are constructed wetlands in removing pharmaceuticals from untreated and treated urban wastewaters? A review. Sci. Total Environ. 2014, 470–471, 1281–1306. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Kumar, S.; Sharma, C. Constructed wetlands: An option for pulp and paper mill wastewater treatment. Electron. J. Environ. Agric. Food Chem. 2011, 10, 3023–3037. [Google Scholar]
- Davies, L.C.; Pedro, I.S.; Ferreira, R.A.; Freire, F.G.; Novais, J.M.; Martins-Dias, S. Constructed wetland treatment system in textile industry and sustainable development. Water Sci. Technol. 2008, 58, 2017. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Rousseau, D.P.L.; Morató, J.; Lesage, E.; Matamoros, V.; Bayona, J.M. Contaminant Removal Processes in Subsurface-Flow Constructed Wetlands: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 561–661. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Sundaravadivel, M.; Vigneswaran, S. Constructed Wetlands for Wastewater Treatment. Crit. Rev. Environ. Sci. Technol. 2001, 31, 351–409. [Google Scholar] [CrossRef]
- Vymazal, J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009, 35, 1–17. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Reyes-Contreras, C.; Domínguez, C.; Bécares, E.; Bayona, J.M. Behaviour of pharmaceuticals and personal care products in constructed wetland compartments: Influent, effluent, pore water, substrate and plant roots. Chemosphere 2016, 145, 508–517. [Google Scholar] [CrossRef]
- Matamoros, V.; Nguyen, L.X.; Arias, C.A.; Salvadó, V.; Brix, H. Evaluation of aquatic plants for removing polar microcontaminants: A microcosm experiment. Chemosphere 2012, 88, 1257–1264. [Google Scholar] [CrossRef]
- Ranieri, E.; Verlicchi, P.; Young, T.M. Paracetamol removal in subsurface flow constructed wetlands. J. Hydrol. 2011, 404, 130–135. [Google Scholar] [CrossRef]
- Li, X.; Manman, C.; Anderson, B.C. Design and performance of a water quality treatment wetland in a public park in Shanghai, China. Ecol. Eng. 2009, 35, 18–24. [Google Scholar] [CrossRef]
- Vystavna, Y.; Frkova, Z.; Marchand, L.; Vergeles, Y.; Stolberg, F. Removal efficiency of pharmaceuticals in a full scale constructed wetland in East Ukraine. Ecol. Eng. 2017, 108, 50–58. [Google Scholar] [CrossRef]
- Nuel, M.; Laurent, J.; Bois, P.; Heintz, D.; Wanko, A. Seasonal and ageing effect on the behaviour of 86 drugs in a full-scale surface treatment wetland: Removal efficiencies and distribution in plants and sediments. Sci. Total Environ. 2018, 615, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, T.; Kelly, B.C.; Gin, K.Y.-H. Bioaccumulation behaviour of pharmaceuticals and personal care products in a constructed wetland. Chemosphere 2019, 222, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Park, N.; Vanderford, B.J.; Snyder, S.A.; Sarp, S.; Kim, S.D.; Cho, J. Effective controls of micropollutants included in wastewater effluent using constructed wetlands under anoxic condition. Ecol. Eng. 2009, 35, 418–423. [Google Scholar] [CrossRef]
- Zarate, F.M.; Schulwitz, S.E.; Stevens, K.J.; Venables, B.J. Bioconcentration of triclosan, methyl-triclosan, and triclocarban in the plants and sediments of a constructed wetland. Chemosphere 2012, 88, 323–329. [Google Scholar] [CrossRef]
- Bartha, B.; Huber, C.; Schröder, P. Uptake and metabolism of diclofenac in Typha latifolia—How plants cope with human pharmaceutical pollution. Plant Sci. 2014, 227, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Giebułtowicz, J.; Nałęcz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. [Google Scholar] [CrossRef]
- Wolecki, D.; Caban, M.; Pazdro, K.; Mulkiewicz, E.; Stepnowski, P.; Kumirska, J. Simultaneous determination of non-steroidal anti-inflammatory drugs and natural estrogens in the mussels Mytilus edulis trossulus. Talanta 2019, 200, 316–323. [Google Scholar] [CrossRef]
- Caban, M.; Lis, H.; Kobylis, P.; Stepnowski, P. The triple-sorbents solid-phase extraction for pharmaceuticals and estrogens determination in wastewater samples. Microchem. J. 2019, 149, 103965. [Google Scholar] [CrossRef]
- Breitholtz, M.; Näslund, M.; Stråe, D.; Borg, H.; Grabic, R.; Fick, J. An evaluation of free water surface wetlands as tertiary sewage water treatment of micro-pollutants. Ecotoxicol. Environ. Saf. 2012, 78, 63–71. [Google Scholar] [CrossRef]
- Dordio, A.; Carvalho, A.J.P.; Teixeira, D.M.; Dias, C.B.; Pinto, A.P. Removal of pharmaceuticals in microcosm constructed wetlands using Typha spp. and LECA. Bioresour. Technol. 2010, 101, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; Matamoros, V.; Domingo, V.; Bayona, J.M.; García, J. Water quality improvement in a full-scale tertiary constructed wetland: Effects on conventional and specific organic contaminants. Sci. Total Environ. 2009, 407, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; García, J.; Bayona, J.M. Organic micropollutant removal in a full-scale surface flow constructed wetland fed with secondary effluent. Water Res. 2008, 42, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Salvadó, V. Evaluation of the seasonal performance of a water reclamation pond-constructed wetland system for removing emerging contaminants. Chemosphere 2012, 86, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D.; Al Aukidy, M.; Zambello, E. Removal of selected pharmaceuticals from domestic wastewater in an activated sludge system followed by a horizontal subsurface flow bed—Analysis of their respective contributions. Sci. Total Environ. 2013, 454–455, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Reyes, C.; Bayona, J.M.; García, J. Emerging organic contaminant removal depending on primary treatment and operational strategy in horizontal subsurface flow constructed wetlands: Influence of redox. Water Res. 2013, 47, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Conkle, J.L.; White, J.R.; Metcalfe, C.D. Reduction of pharmaceutically active compounds by a lagoon wetland wastewater treatment system in Southeast Louisiana. Chemosphere 2008, 73, 1741–1748. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Matamoros, V.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Comprehensive assessment of the design configuration of constructed wetlands for the removal of pharmaceuticals and personal care products from urban wastewaters. Water Res. 2010, 44, 3669–3678. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Matamoros, V.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Assessment of full-scale natural systems for the removal of PPCPs from wastewater in small communities. Water Res. 2010, 44, 1429–1439. [Google Scholar] [CrossRef]
- Lee, S.; Kang, S.-I.; Lim, J.-L.; Huh, Y.J.; Kim, K.-S.; Cho, J. Evaluating controllability of pharmaceuticals and metabolites in biologically engineered processes, using corresponding octanol–water distribution coefficient. Ecol. Eng. 2011, 37, 1595–1600. [Google Scholar] [CrossRef]
- Reif, R.; Besancon, A.; Le Corre, K.; Jefferson, B.; Lema, J.M.; Omil, F. Comparison of PPCPs removal on a parallel-operated MBR and AS system and evaluation of effluent post-treatment on vertical flow reed beds. Water Sci. Technol. 2011, 63, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Pedescoll, A.; Matamoros, V.; Bayona, J.M.; García, J. Capacity of a horizontal subsurface flow constructed wetland system for the removal of emerging pollutants: An injection experiment. Chemosphere 2010, 81, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Plenis, A.; Łukaszewicz, P.; Caban, M.; Migowska, N.; Białk-Bielińska, A.; Czerwicka, M.; Stepnowski, P. Chemometric optimization of derivatization reactions prior to gas chromatography-mass spectrometry analysis. J. Chromatogr. A 2013, 1296, 164–178. [Google Scholar] [CrossRef] [PubMed]
- De Bièvre, P. The 2012 International Vocabulary of Metrology: “VIM”. Accredit. Qual. Assur. 2012, 17, 231–232. [Google Scholar] [CrossRef]
- Migowska, N.; Caban, M.; Stepnowski, P.; Kumirska, J. Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography-mass spectrometry and gas chromatography with electron capture detection. Sci. Total Environ. 2012, 441, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Migowska, N.; Stepnowski, P.; Kwiatkowski, M.; Kumirska, J. Matrix effects and recovery calculations in analyses of pharmaceuticals based on the determination of β-blockers and β-agonists in environmental samples. J. Chromatogr. A 2012, 1258, 117–127. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Pharmaceuticals | Concentration in Untreated Sewage | Concentration in Treated Sewage |
|---|---|---|
| (mean ± SD) [ng/L] | ||
| Ibuprofen | 2695 ± 916 | 8 ± 1 |
| Paracetamol | 2130 ± 298 | <MDL |
| Terbutaline | 154 ± 8 | <MDL |
| Flurbiprofen | 51 ± 12 | <MDL |
| Naproxen | 3420 ± 342 | 38 ± 1 |
| Diflunisal | 67 ± 12 | 43 ± 7 |
| Amitriptyline | 1676 ± 184 | 613 ± 38 |
| Imipramine | <MDL | <MDL |
| Diclofenac | <MDL | <MDL |
| Clomipramine | 169 ± 29 | 50 ± 5 |
| Nadolol | <MDL | <MDL |
| Estrone | 52 ± 3 | 19 ± 2 |
| 17β-estradiol | 110 ± 13 | 4 ± 0 |
| 17α-ethinylestradiol | 1622 ± 260 | 122 ± 18 |
| Estriol | 273 ± 27 | 13 ± 3 |
| Type of Solvents/ Temperature/ Pharmaceuticals | EtOH:H2O (1:1, v/v) | ACN:H2O (1:1, v/v) | MeOH:H2O (1:1, v/v) | |||
|---|---|---|---|---|---|---|
| 80 °C | 50 °C | 80 °C | 50 °C | 80 °C | 50 °C | |
| Value of Recovery [% ± SD (%)] | ||||||
| Ibuprofen | 105 ± 22 | 154 ± 5 | 105 ± 16 | 194 ± 13 | 65 ± 5 | 111 ± 9 |
| Paracetamol | 26 ± 11 | 36 ± 7 | 10 ± 2 | 25 ± 3 | 42 ± 5 | 74 ± 9 |
| Terbutaline | 16 ± 5 | 25 ± 6 | 19 ± 4 | 26 ± 3 | 48 ± 3 | 51 ± 5 |
| Flurbiprofen | 89 ± 15 | 120 ± 18 | 80 ± 14 | 140 ± 8 | 52 ± 2 | 89 ± 10 |
| Naproxen | 91 ± 9 | 118 ± 6 | 80 ± 13 | 139 ± 7 | 54 ± 3 | 89 ± 11 |
| Diflunisal | 90 ± 18 | 115 ± 7 | 90 ± 11 | 159 ± 7 | 55 ± 3 | 88 ± 12 |
| Amitriptyline | 27 ± 8 | 58 ± 11 | 52 ± 11 | 10 ± 3 | 25 ± 4 | 26 ± 5 |
| Imipramine | 29 ± 8 | 88 ± 25 | 74 ± 10 | 16 ± 2 | 59 ± 2 | 55 ± 8 |
| Diclofenac | 103 ± 12 | 10 5± 3 | 68 ± 8 | 76 ± 9 | 98 ± 6 | 105 ± 5 |
| Clomipramine | 16 ± 7 | 44 ± 19 | 60 ± 8 | 6 ± 1 | 31 ± 6 | 16 ± 4 |
| Nadolol | 73 ± 6 | 91 ± 12 | 59 ± 4 | 97 ± 17 | 59 ± 3 | 67 ± 9 |
| Estrone | 63 ± 13 | 80 ± 15 | 49 ± 6 | 67 ± 5 | 38 ± 3 | 49 ± 6 |
| 17β-estradiol | 77 ± 10 | 86 ± 15 | 71 ± 11 | 100 ± 5 | 45 ± 4 | 61 ± 8 |
| 17α-ethinylestradiol | 125 ± 16 | 130 ± 10 | 235 ± 14 | 219 ± 12 | 101 ± 9 | 108 ± 10 |
| Estriol | 65 ± 7 | 93 ± 14 | 64 ± 7 | 88 ± 7 | 50 ± 4 | 71 ± 7 |
| Validation Parameters | Calibration Curves | R2 | Measurement Intermediate Precision (RSD%) | Mean Recovery | ME | MQL | MDL |
|---|---|---|---|---|---|---|---|
| Compound | (%) | % | (ng/g d.w.) | ||||
| Ibuprofen | 68698 (±635) x + 1208.4 (±648.4) | 0.9995 | 0.44–7.61 | 80–102 | 10 | 0.4 | 0.1 |
| Paracetamol | 74010 (±4738) x − 8672.6 (±4835.8) | 0.9760 | 2.46–20.25 | 100 | 19 | 0.5 | 0.2 |
| Terbutaline | 364393 (±2459) x − 1622.6 (±2510.1) | 0.9997 | 0.39–8.75 | 99–100 | −31 | 0.8 | 0.3 |
| Flurbiprofen | 67739 (±632) x − 737.2 (621.9) | 0.9996 | 0.46–10.86 | 95–98 | −20 | 0.4 | 0.1 |
| Naproxen | 67687 (±829) x − 1444.7 (±880.5) | 0.9994 | 3.73–13.65 | 99–101 | 43 | 0.4 | 0.1 |
| Diflunisal | 114510 (±2633) x − 4994.1 (±2590.4) | 0.9970 | 0.05–9.33 | 97–102 | 15 | 0.4 | 0.1 |
| Amitriptyline | 339801 (±3704) x + 30042.5 (3642.7) | 0.9994 | 0.44–16.20 | 94–102 | −9 | 2 | 0.7 |
| Imipramine | 33490 (±333) x − 752.78 (±339.8) | 0.9994 | 1.08–14.00 | 97–100 | 12 | 0.7 | 0.2 |
| Diclofenac | 18224 (±287) x − 673.8 (±292.7) | 0.9990 | 3.62–21.60 | 89–101 | 25 | 0.4 | 0.1 |
| Clomipramine | 17286 (±128) x − 26.9 (±125.7) | 0.9997 | 0.56–17.09 | 99–100 | −8 | 2 | 0.7 |
| Nadolol | 550924(±13936) x − 31,586 (±13706.5) | 0.9970 | 1.82–9.86 | 91–102 | 28 | 0.6 | 0.2 |
| Estrone (E1) | 24502(±587) x + 284.4 (±599.1) | 0.9970 | 2.03–14.88 | 99–100 | −2 | 0.8 | 0.3 |
| 17β-estradiol (E2) | 44380 (±1275) x − 276.4 (1302.1) | 0.9950 | 0.18–10.78 | 80–101 | −3 | 0.6 | 0.2 |
| 17α-ethinylestradiol (EE2) | 12263 (±246) x + 560.9 (±251.5) | 0.9980 | 0.16–12.16 | 80–102 | −11 | 0.4 | 0.1 |
| Estriol (E3) | 9240 (±265) x + 724.7 (±303.3) | 0.9970 | 1.00–15.78 | 99–100 | 31 | 0.5 | 0.2 |
| Pharmaceuticals | Cyperus papyru (Papyrus) | Lysimachia nemorum (Yellow Pimpernel) | Euonymus europaeus (European Spindle) | EE |
|---|---|---|---|---|
| (mean ± SD) [ng/g Dry Weight] | % | |||
| Ibuprofen | 700 ± 28 | <MDL | 1616 ± 124 | 99.7 |
| Paracetamol | <MDL | <MDL | <MDL | >99.9 |
| Terbutaline | <MDL | 5323 ± 1869 | <MDL | >99.9 |
| Flurbiprofen | 2107 ± 92 | <MDL | <MDL | >99.9 |
| Naproxen | <MDL | 1422 ± 216 | <MDL | 98.9 |
| Diflunisal | 1569 ± 321 | 5569 ± 1298 | 260 ± 4 | 35.8 |
| Amitriptyline | 404 ± 5 | 1399 ± 20 | 435 ± 5 | 63.4 |
| Imipramine | 3533 ± 198 | <MDL | <MDL | -1 |
| Diclofenac | <MDL | <MDL | <MDL | -1 |
| Clomipramine | <MDL | <MDL | <MDL | 70.4 |
| Nadolol | <MDL | <MDL | <MDL | -1 |
| Estrone (E1) | <MDL | <MDL | <MDL | 63.5 |
| 17β-estradiol (E2) | <MDL | <MDL | <MDL | 96.4 |
| 17α-ethinylestradiol (EE2) | 4126 ± 821 | 3136 ± 599 | 214 ± 3 | 92.5 |
| Estriol (E3) | <MDL | <MDL | <MDL | 95.2 |
| Plant Species | Cyperus papyru | Lysimachia nemorum | Euonymus europaeus |
|---|---|---|---|
| ∑Selected pharmaceuticals and ECDs | [ng/g dry weight] | ||
| 12,439 | 16,849 | 2525 | |
| Factor | Results for Treated Wastewater | Results for Untreated Wastewater | The Highest Acceptable Concentration | Removal Efficiency |
|---|---|---|---|---|
| BOD5 | 3.4 mg/L O2 | 460 mg/L O2 | 15 mg/L O2 | 99.3% |
| CODCr | 32.7 mg/L O2 | 1144.7 mg/L O2 | 125 mg/L O2 | 97.1% |
| Ntotal | 8.8 mg/L | 96.7 mg/L | 15 mg/L | 90.5% |
| Ptotal | 0.2 mg/L | 11.9 mg/L | 2 mg/L | 98.8% |
| Suspensions | 8.6 mg/L | 567.5 mg/L | 35 mg/L | 98.5% |
| pH | 7.2 | 7.9 | - | - |
| Identification Parameters/ Pharmaceuticals | Retention Time (Rt) [min] | Characteristic Ions (m/z) (Quantitative and Confirmation Ions) | Time Windows [min] |
|---|---|---|---|
| Ibuprofen | 10.39 | 160; 263(18); 278(10) | 10.01–13.52 |
| Paracetamol | 10.64 | 206; 280(90); 295(60) | |
| Terbutaline | 16.20 | 86; 356(47) | 13.52–19.88 |
| Flurbiprofen | 17.01 | 180; 165(85); 301(25) | |
| Naproxen | 18.36 | 185; 243(80) | |
| Diflunisal | 18.88 | 379; 380(30) | |
| Amitriptyline | 20.64 | 58; 215(30) | 19.88–22.50 |
| Imipramine | 21.09 | 243; 58(60); 195(40) | |
| Diclofenac | 21.73 | 214; 242(50); 352(12); 367(25) | |
| Clomipramine | 23.90 | 268; 314(25) | 22.50–25.63 |
| Nadolol | 24.53 | 86; 73(8); 510(33) | |
| Estrone (E1) | 26.48 | 342; 257(50); 285(36); 327(10) | 25.63–27.97 |
| 17β-estradiol (E2) | 27.11 | 416; 285(90) | |
| 17α-ethinylestradiol (EE2) | 28.59 | 425; 440(35) | 27.97–29.30 |
| Estriol (E3) | 29.77 | 504; 311(81) | 29.30–30.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolecki, D.; Caban, M.; Pazda, M.; Stepnowski, P.; Kumirska, J. Evaluation of the Possibility of Using Hydroponic Cultivations for the Removal of Pharmaceuticals and Endocrine Disrupting Compounds in Municipal Sewage Treatment Plants. Molecules 2020, 25, 162. https://doi.org/10.3390/molecules25010162
Wolecki D, Caban M, Pazda M, Stepnowski P, Kumirska J. Evaluation of the Possibility of Using Hydroponic Cultivations for the Removal of Pharmaceuticals and Endocrine Disrupting Compounds in Municipal Sewage Treatment Plants. Molecules. 2020; 25(1):162. https://doi.org/10.3390/molecules25010162
Chicago/Turabian StyleWolecki, Daniel, Magda Caban, Magdalena Pazda, Piotr Stepnowski, and Jolanta Kumirska. 2020. "Evaluation of the Possibility of Using Hydroponic Cultivations for the Removal of Pharmaceuticals and Endocrine Disrupting Compounds in Municipal Sewage Treatment Plants" Molecules 25, no. 1: 162. https://doi.org/10.3390/molecules25010162
APA StyleWolecki, D., Caban, M., Pazda, M., Stepnowski, P., & Kumirska, J. (2020). Evaluation of the Possibility of Using Hydroponic Cultivations for the Removal of Pharmaceuticals and Endocrine Disrupting Compounds in Municipal Sewage Treatment Plants. Molecules, 25(1), 162. https://doi.org/10.3390/molecules25010162








