Optimization of Antioxidant Hydrolysate Produced from Tibetan Egg White with Papain and Its Application in Yak Milk Yogurt
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Prediction Capability of the Regression Model
2.2. Response Optimization
2.3. Quality Characteristics of Yak Milk Yogurt
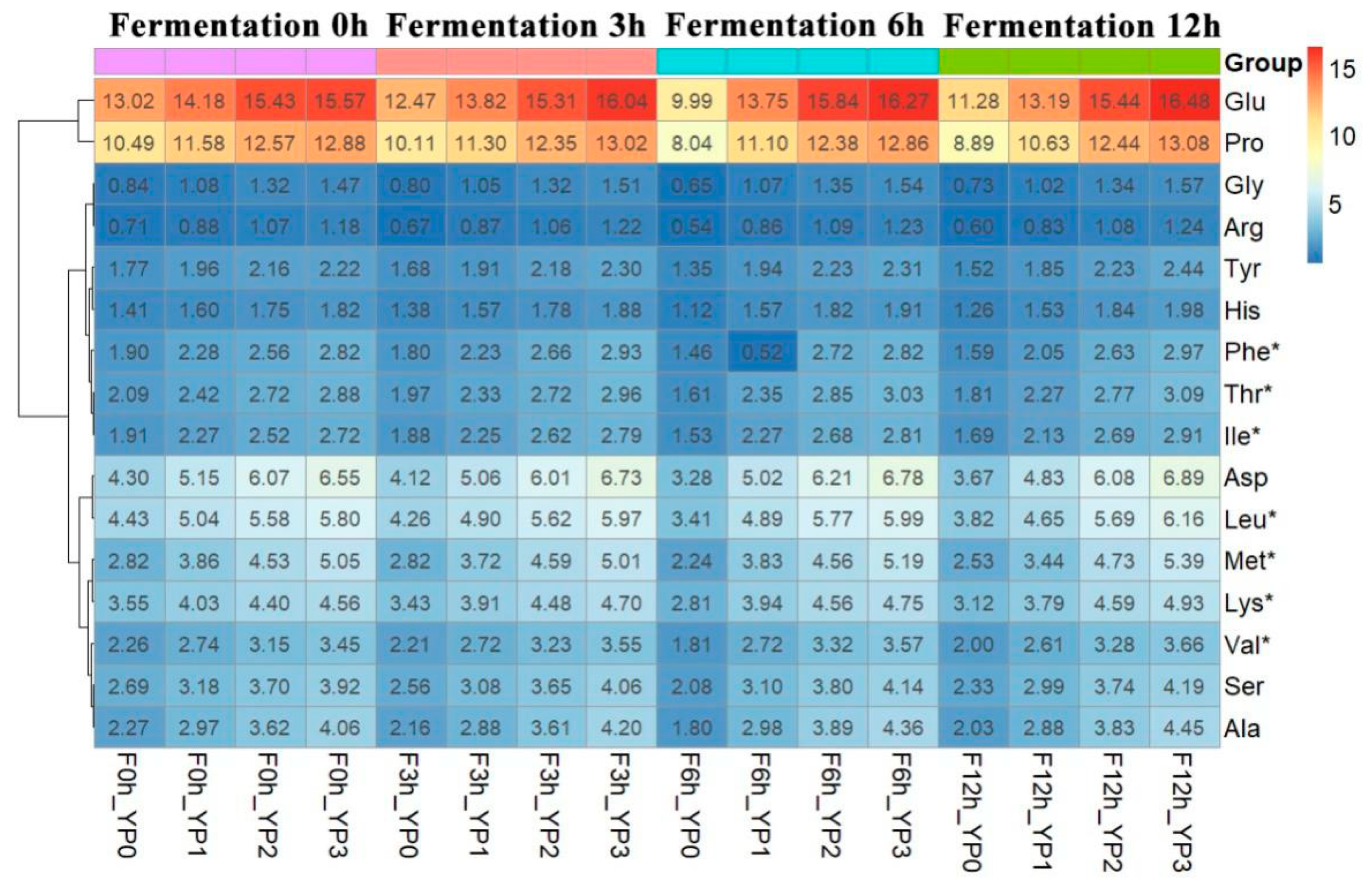
2.4. The Effect of Added PEWH on the Amino Acid Content of Yak Milk Yogurt
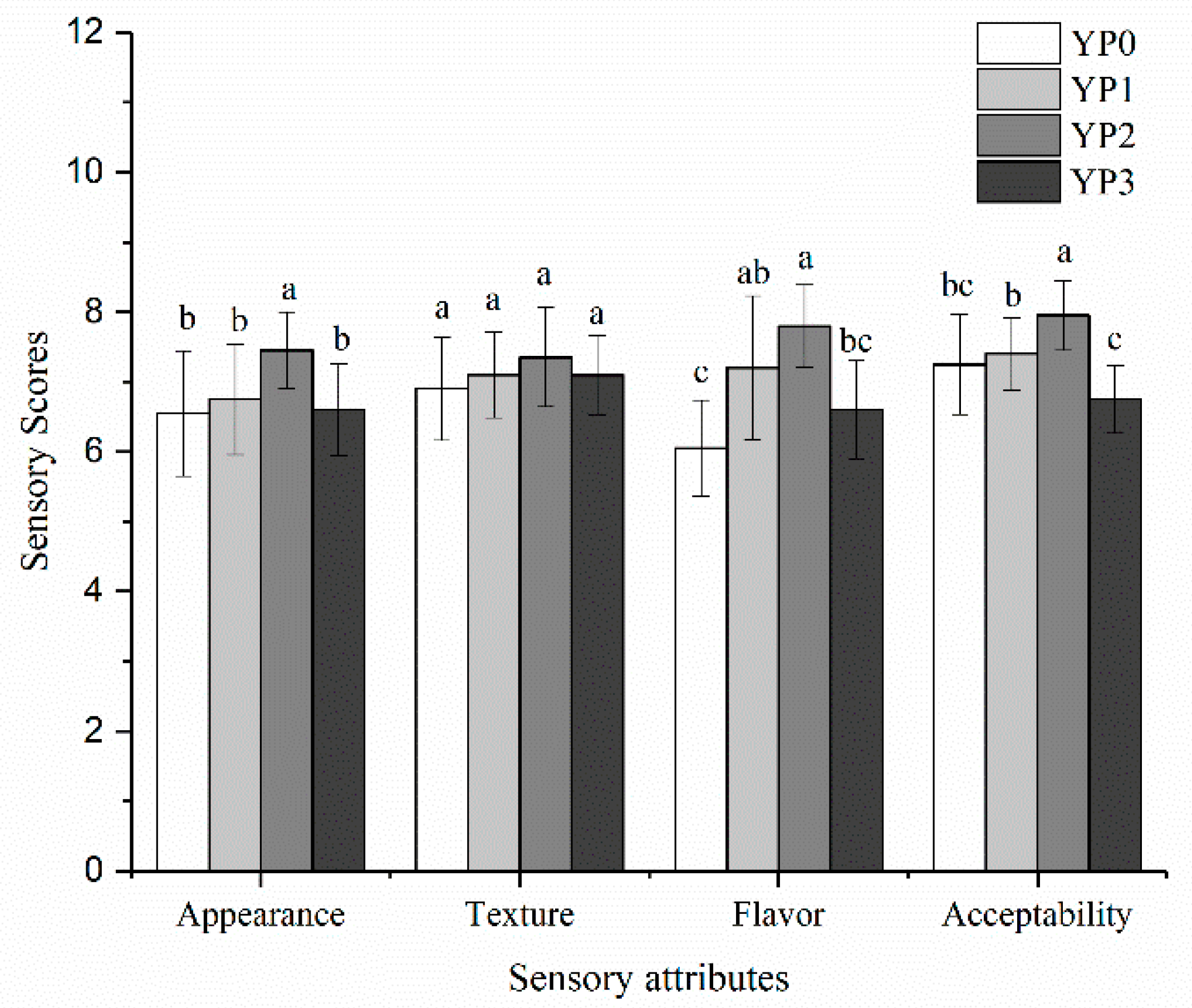
2.5. The Sensory Scores of Yak Milk Yogurt
2.6. Viable Counts of LAB in Yak Milk Yogurt
3. Material and Methods
3.1. Materials
3.2. The Extraction of Egg White Protein from Tibetan Eggs
3.3. The Preparation of PEWH
3.4. O2− Scavenging Activity (%) Assay
3.5. RSM
3.6. The Manufacture of Yak mIlk Yogurt Fortified with PEWH
3.7. Physicochemical Analyses and Sensory Evaluation of the Yak Milk Yogurt
3.8. Viable Counts of LAB
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, C.; Lahti, T.; Tanaka, T.; Nickerson, M.T. Egg proteins: Fractionation, bioactive peptides and allergenicity. J. Sci. Food Agric. 2018, 98, 5547–5558. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Zhang, H.; Jia, C.L.; Ling, Y.; Gou, X.; Deng, X.M.; Wu, C.X. Blood gas, hemoglobin, and growth of Tibetan chicken embryos incubated at high altitude. Poult. Sci. 2007, 86, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.L.; He, L.J.; Li, P.C.; Liu, H.Y.; Wei, Z.H. Effect of egg composition and oxidoreductase on adaptation of Tibetan chicken to high altitude. Poult. Sci. 2016, 95, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-F.; Yang, K.; Zhao, X.-G.; A, Z.; Ren, B.; Dong, P.; Tie, B.; Dai, X.-Q.; Li, Y.-N. Comparative research on the nutrition components of the Tibetan Chicken egg and ordinary egg. J. Southwest Univ. Natl. Nat. Sci. Ed. 2009, 35, 1013–1016. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides from egg: A review. Nutr. Food Sci. 2015, 45, 190–212. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Wang, Y.; Yu, Y.; Liu, J.; Liu, B.; Zhang, T. Identification of antioxidant peptides derived from egg-white protein and its protective effects on H2O2-induced cell damage. Int. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Qiu, N.; Ma, M.; Zhao, L.; Liu, W.; Li, Y.; Mine, Y. Comparative proteomic analysis of egg white proteins under various storage temperatures. J. Agric. Food Chem. 2012, 60, 7746–7753. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, L.; Li, J.; Chi, F. Proteome analysis using iTRAQ reveals the differentiation between Tibetan and ordinary ovalbumin peptides. Int. J. Biol. Macromol. 2019, 132, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Garces-Rimon, M.; Gonzalez, C.; Uranga, J.A.; Lopez-Miranda, V.; Lopez-Fandino, R.; Miguel, M. Pepsin Egg White Hydrolysate Ameliorates Obesity-Related Oxidative Stress, Inflammation and Steatosis in Zucker Fatty Rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Fernandez, S.; Garces-Rimon, M.; Gonzalez, C.; Uranga, J.A.; Lopez-Miranda, V.; Vera, G.; Miguel, M. Pepsin egg white hydrolysate ameliorates metabolic syndrome in high-fat/high-dextrose fed rats. Food Funct. 2018, 9, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-H.; Luo, Z.; Yu, L.; Ren, F.-Z.; Han, B.-Z.; Nout, M.J.R. A survey on composition and microbiota of fresh and fermented yak milk at different Tibetan altitudes. Dairy Sci. Technol. 2009, 89, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Alenisan, M.A.; Alqattan, H.H.; Tolbah, L.S.; Shori, A.B. Antioxidant properties of dairy products fortified with natural additives: A review. J. Assoc. Arab Univ. Basic Appl. Sci. 2018, 24, 101–106. [Google Scholar] [CrossRef]
- Lucas, A.; Sodini, I.; Monnet, C.; Jolivet, P.; Corrieu, G. Probiotic cell counts and acidification in fermented milks supplemented with milk protein hydrolysates. Int. Dairy J. 2004, 14, 47–53. [Google Scholar] [CrossRef]
- Fang, X.; Xie, N.; Chen, X.; Yu, H.; Chen, J. Optimization of antioxidant hydrolysate production from flying squid muscle protein using response surface methodology. Food Bioprod. Process. 2012, 90, 676–682. [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Sato, H.H. A response surface approach on optimization of hydrolysis parameters for the production of egg white protein hydrolysates with antioxidant activities. Biocatal. Agric. Biotechnol. 2015, 4, 55–62. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT-Food Sci. Technol. 2008, 41, 1624–1632. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L. Preparation of reactive oxygen scavenging peptides from tilapia (Oreochromis niloticus) skin gelatin: Optimization using response surface methodology. J. Food Sci. 2011, 76, C483–C489. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.N.; Gong, Q.X.; Yang, Q.L.; Sun, J.; Bi, J.; Zhang, C.S. Technology optimization on preparation of peanut polypeptide using Alcalase. In Advanced Materials Research; Trans. Tech. Publications Ltd.: Stafa-Zurich, Switzerland, 2011; pp. 2586–2593. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Sin, H.N.; Yusof, S.; Sheikh Abdul Hamid, N.; Rahman, R.A. Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng. 2006, 73, 313–319. [Google Scholar] [CrossRef]
- Dave, R.; Shah, N. Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J. Dairy Sci. 1998, 81, 2804–2816. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Conte Junior, C.A.; Moraes, J.; Costa, M.P.; Raices, R.S.L.; Franco, R.M.; Cruz, A.G.; Silva, A.C.O. Physicochemical evaluation of sheep milk yogurts containing different levels of inulin. J. Dairy Sci. 2016, 99, 4160–4168. [Google Scholar] [CrossRef]
- Li, Q.; Kang, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. Microbial succession and metabolite changes during traditional serofluid dish fermentation. LWT 2017, 84, 771–779. [Google Scholar] [CrossRef]
- Su, N.; Ren, L.; Ye, H.; Sui, Y.; Li, J.; Ye, M. Antioxidant activity and flavor compounds of hickory yogurt. Int. J. Food Prop. 2016, 20, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, N.; Liu, C.; Jin, J. Effect of Silkworm Pupae Peptide on the Fermentation and Quality of Yogurt. J. Food Process. Preserv. 2017, 41, e12893. [Google Scholar] [CrossRef]
- Ye, M.; Ren, L.; Wu, Y.; Wang, Y.; Liu, Y. Quality characteristics and antioxidant activity of hickory-black soybean yogurt. LWT-Food Sci. Technol. 2013, 51, 314–318. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Zhao, M.Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef]
- Lin, W.H.; Hwang, C.F.; Chen, L.W.; Tsen, H.Y. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Food Microbiol. 2006, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
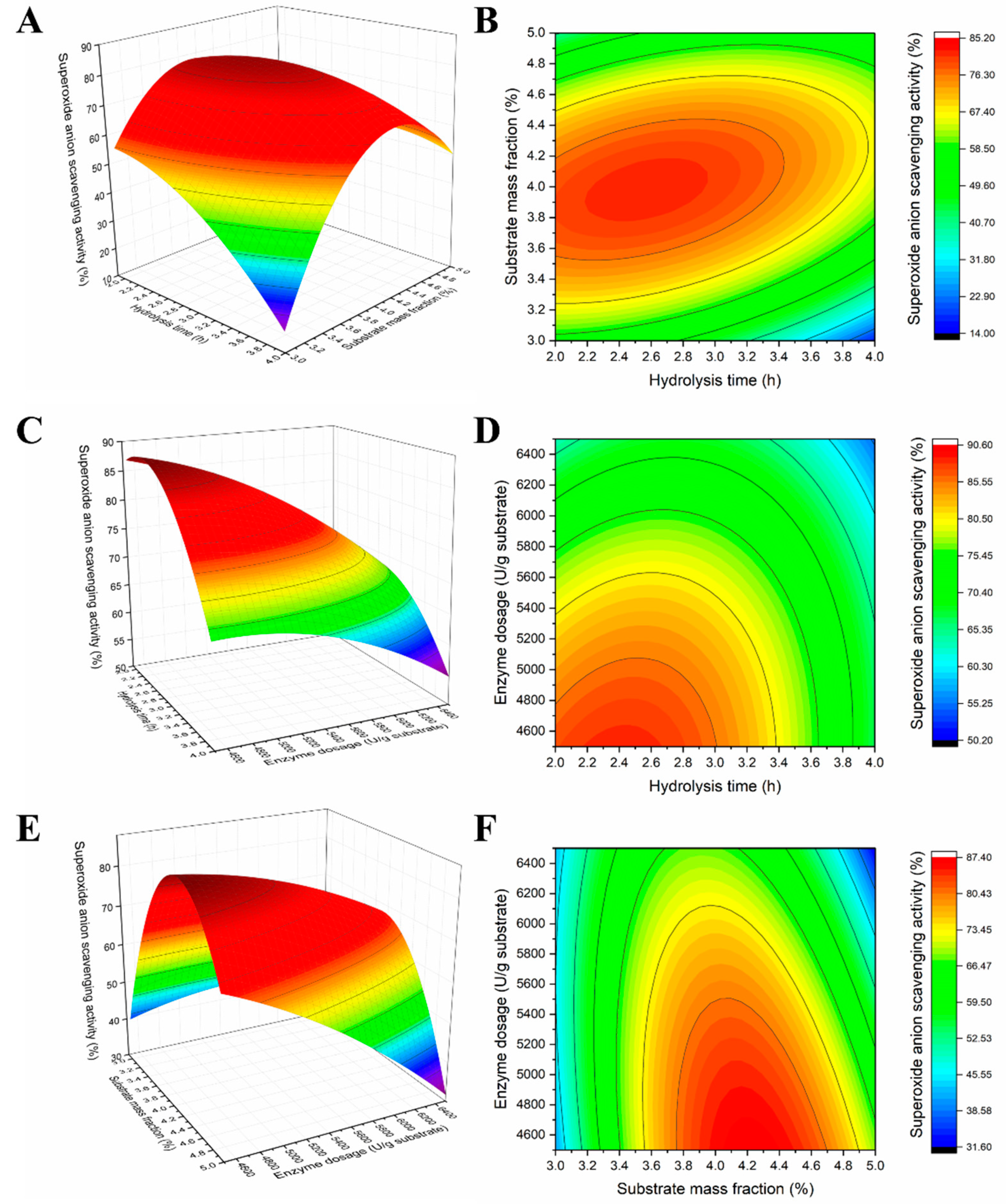


| Run Number | Experimental Values | Y: The O2− Scavenging Activity (%) | ||
|---|---|---|---|---|
| X1: Hydrolysis Time (h) | X2: Substrate Mass Fraction (%) | X3: Enzyme Dosage (U/g Substrate) | ||
| 1 | 4 (1) | 3 (−1) | 5500 (0) | 14.58 |
| 2 | 2 (−1) | 5 | 5500 | 42.34 |
| 3 | 4 | 5 (1) | 5500 | 47.75 |
| 4 | 3 (0) | 4 (0) | 5500 | 80.26 |
| 5 | 3 | 3 | 4500 (−1) | 43.55 |
| 6 | 3 | 5 | 4500 | 71.47 |
| 7 | 3 | 4 | 5500 | 76.55 |
| 8 | 3 | 4 | 5500 | 81.56 |
| 9 | 2 | 4 | 4500 | 82.11 |
| 10 | 4 | 4 | 4500 | 66.55 |
| 11 | 3 | 3 | 6500 (1) | 39.94 |
| 12 | 3 | 4 | 5500 | 84.71 |
| 13 | 4 | 4 | 6500 | 59.68 |
| 14 | 2 | 3 | 5500 | 58.75 |
| 15 | 3 | 5 | 6500 | 29.13 |
| 16 | 2 | 4 | 6500 | 63.70 |
| 17 | 3 | 4 | 5500 | 78.73 |
| Factor | SS | DF | MS | F-Value | P-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 6803.09 | 9 | 755.9 | 38.64 | <0.0001 |
| X1 | 425.44 | 1 | 425.44 | 21.75 | 0.0023 |
| X2 | 143.4 | 1 | 143.4 | 7.33 | 0.0303 |
| X3 | 634.21 | 1 | 634.21 | 32.42 | 0.0007 |
| X1X2 | 614.54 | 1 | 614.54 | 31.41 | 0.0008 |
| X1X3 | 33.29 | 1 | 33.29 | 1.7 | 0.2333 |
| X2X3 | 375 | 1 | 375 | 19.17 | 0.0032 |
| X12 | 323.09 | 1 | 323.09 | 16.51 | 0.0048 |
| X22 | 3980.6 | 1 | 3980.6 | 203.46 | <0.0001 |
| X32 | 54.33 | 1 | 54.33 | 2.78 | 0.1396 |
| Residual | 136.95 | 7 | 19.56 | ||
| Lack of fit | 99.41 | 3 | 33.14 | 3.53 | 0.1273 |
| Pure error | 37.55 | 4 | 9.39 | ||
| Total variation | 6940.04 | 16 | |||
| R2 = 0.9803; Adj R2 = 0.9549; C.V.% = 7.36 | |||||
| Items | Values |
|---|---|
| Independent variables | |
| X1: Hydrolysis time (h) | 2.51 |
| X2: Substrate mass fraction (%) | 4.13 |
| X3: Enzyme dosage (U/g substrate) | 4500 |
| Response values: O2− scavenging activity | |
| Predicted values | 89.06% |
| Actual values | 88.05 ± 1.2% |
| Level | Parameter | ||
|---|---|---|---|
| X1: Hydrolysis Time (h) | X2: Substrate Mass Fraction (%) | X3: Enzyme Dosage (U/g Substrate) | |
| −1 | 2 | 3 | 4500 |
| 0 | 3 | 4 | 5500 |
| 1 | 4 | 5 | 6500 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, F.; Liu, T.; Liu, L.; Tan, Z.; Gu, X.; Yang, L.; Luo, Z. Optimization of Antioxidant Hydrolysate Produced from Tibetan Egg White with Papain and Its Application in Yak Milk Yogurt. Molecules 2020, 25, 109. https://doi.org/10.3390/molecules25010109
Chi F, Liu T, Liu L, Tan Z, Gu X, Yang L, Luo Z. Optimization of Antioxidant Hydrolysate Produced from Tibetan Egg White with Papain and Its Application in Yak Milk Yogurt. Molecules. 2020; 25(1):109. https://doi.org/10.3390/molecules25010109
Chicago/Turabian StyleChi, Fumin, Ting Liu, Liu Liu, Zhankun Tan, Xuedong Gu, Lin Yang, and Zhang Luo. 2020. "Optimization of Antioxidant Hydrolysate Produced from Tibetan Egg White with Papain and Its Application in Yak Milk Yogurt" Molecules 25, no. 1: 109. https://doi.org/10.3390/molecules25010109





