The Effect of Furanocoumarin Derivatives on Induction of Apoptosis and Multidrug Resistance in Human Leukemic Cells
Abstract
1. Introduction
2. Results
2.1. Analysis of Cytotoxicity
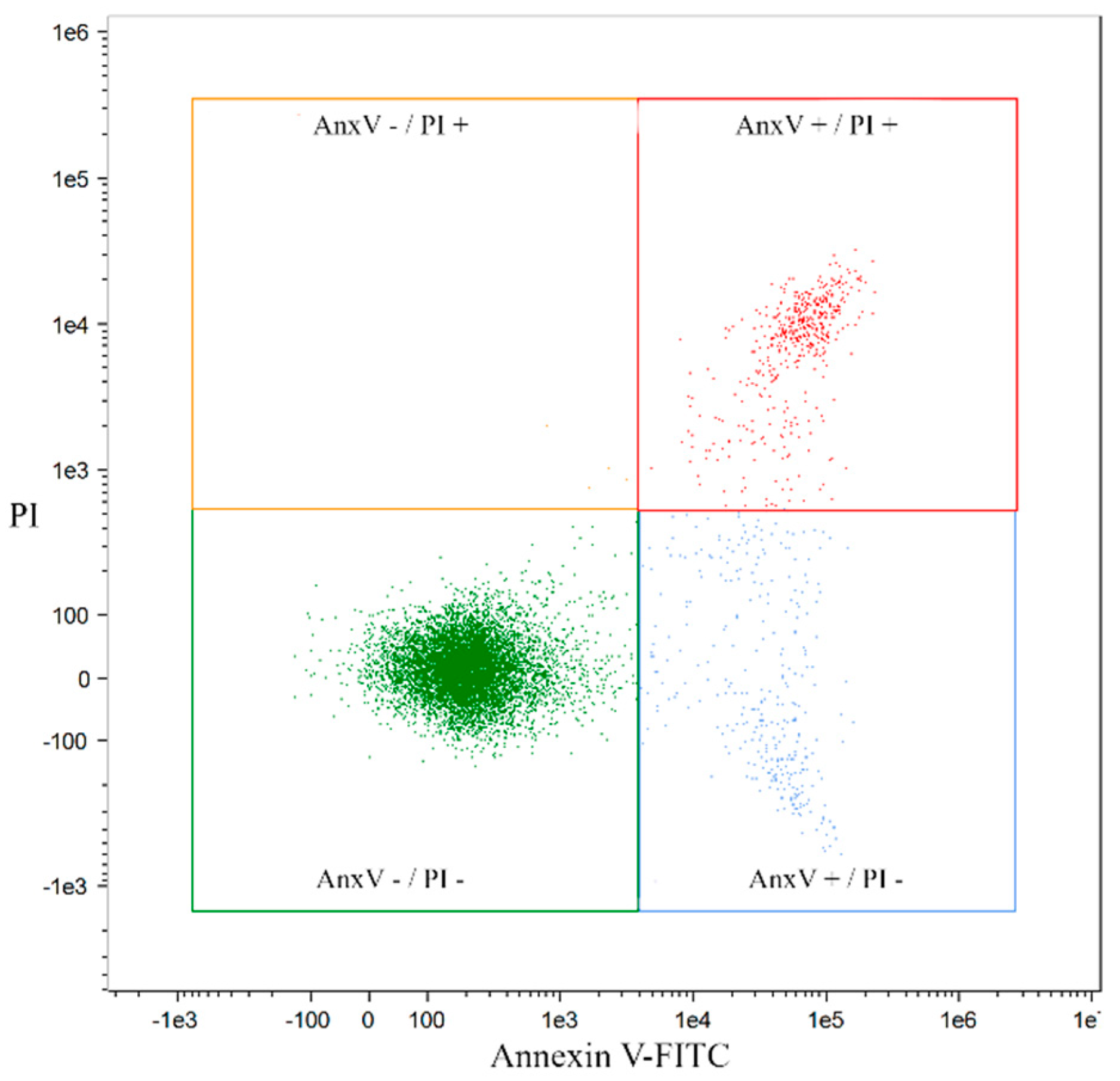
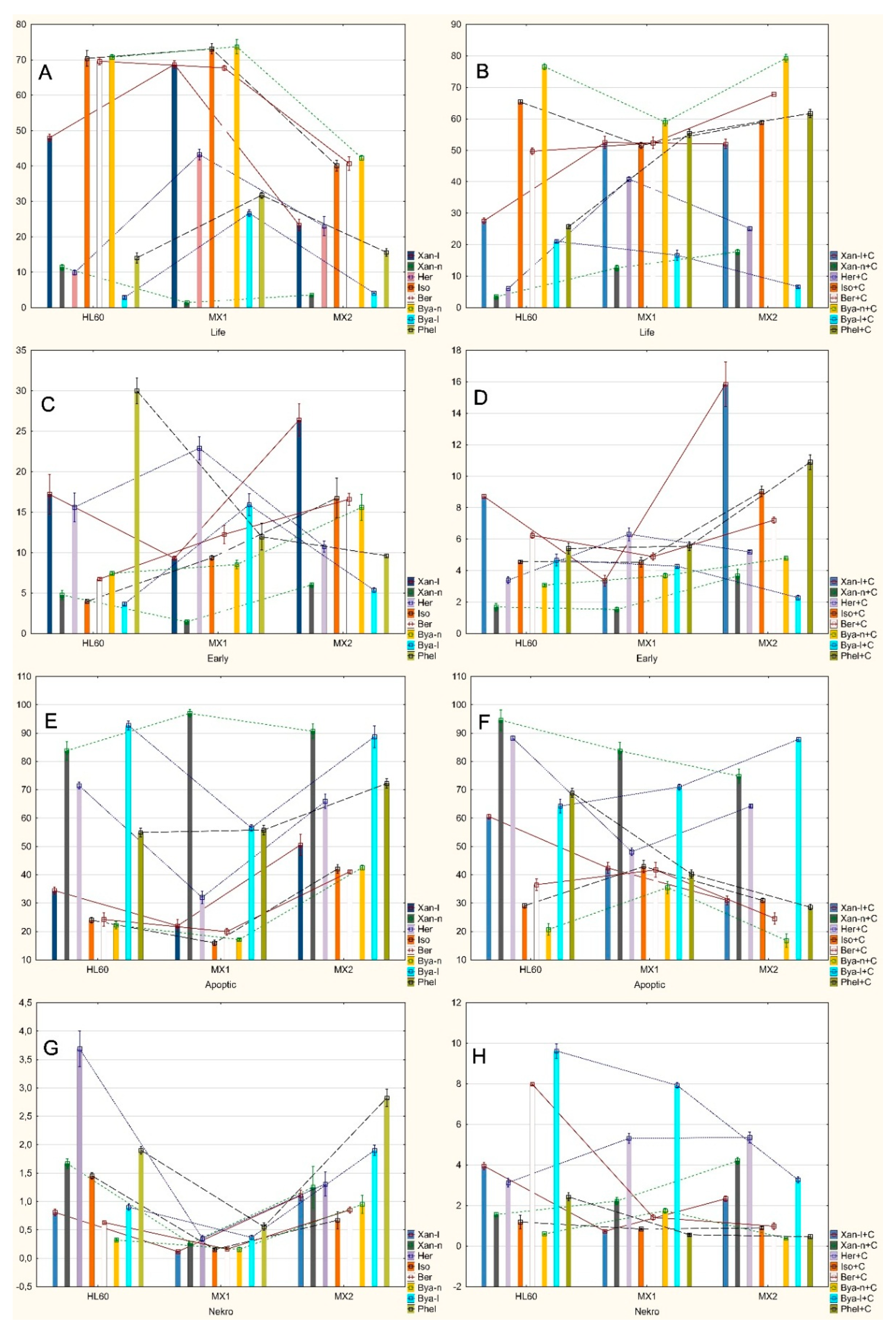
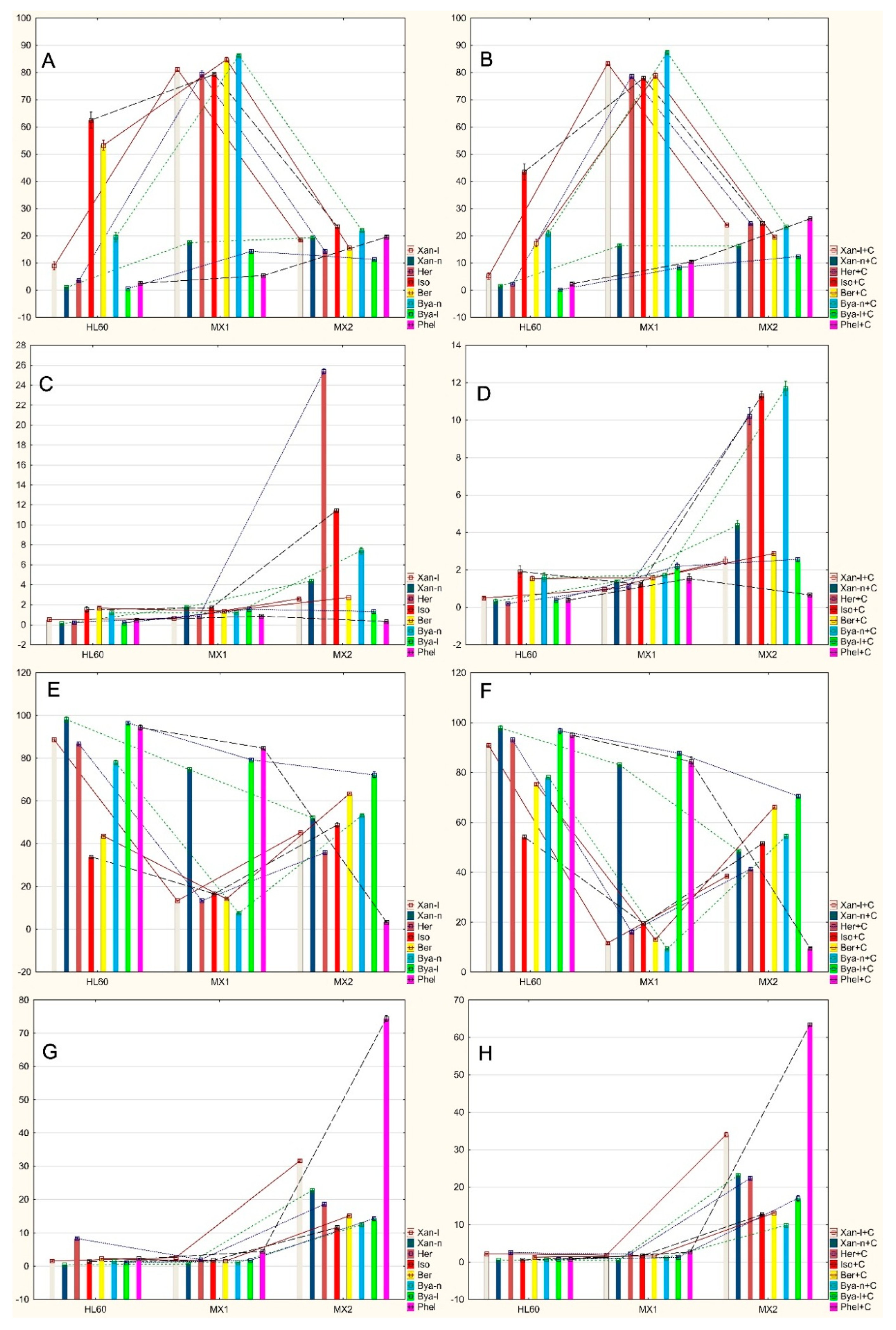
2.2. Analysis of Apoptosis Induced by Coumarin Compounds at Doses of IC50 in the Presence of Mitoxantrone (+C) and without Mitoxantrone Using AnnexinV/IP
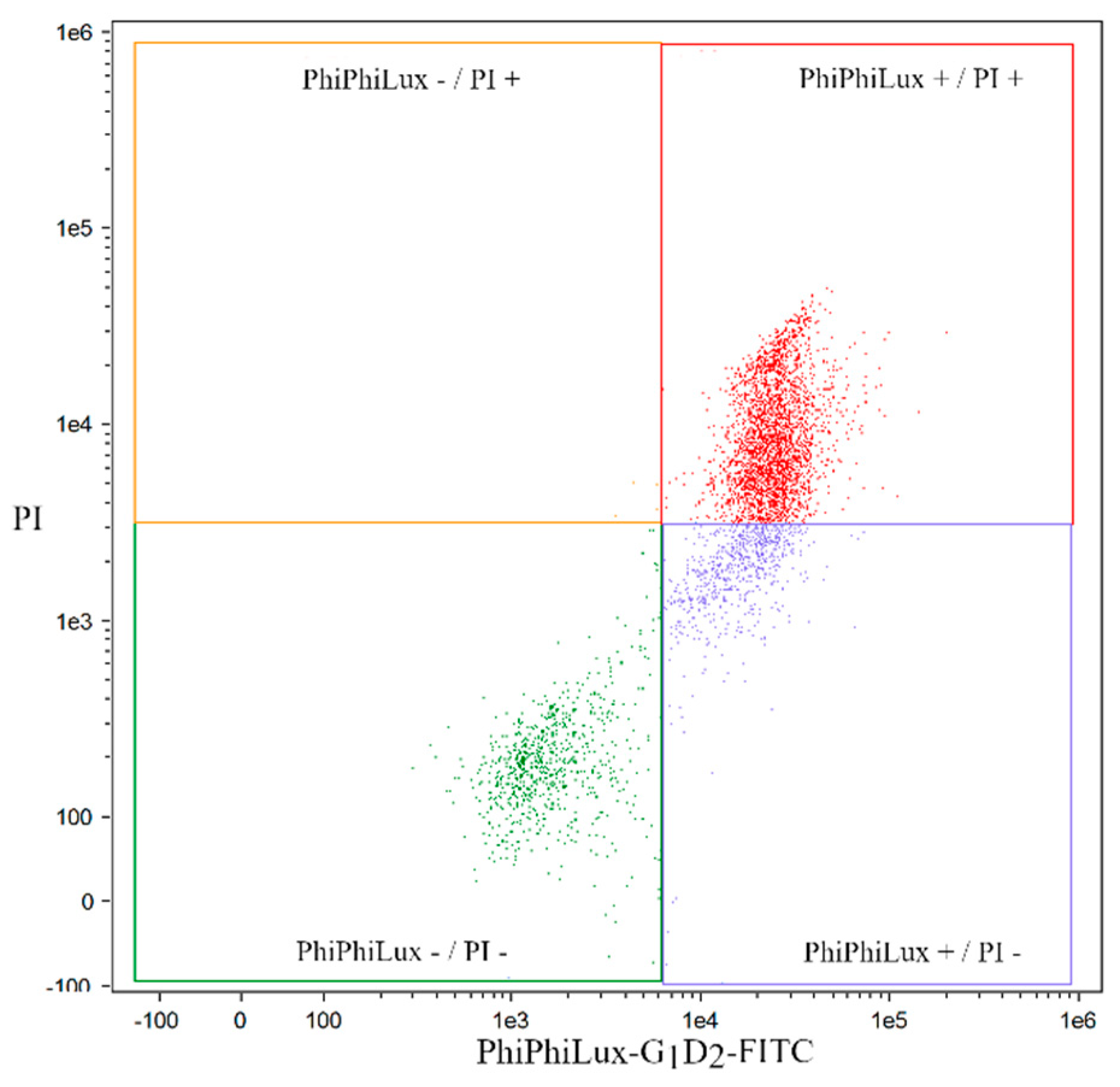
2.3. Analysis of Apoptosis Induced by Coumarin Compounds in an IC50 Dose in the Presence of Mitoxantrone (+C) and without Mitoxantrone Using Caspase 3
3. Discussion
4. Material and Methods
4.1. Cell Lines and Cell Culture
4.2. Analysis of Cell Viability
4.3. Chemicals
4.4. Cell Preparation
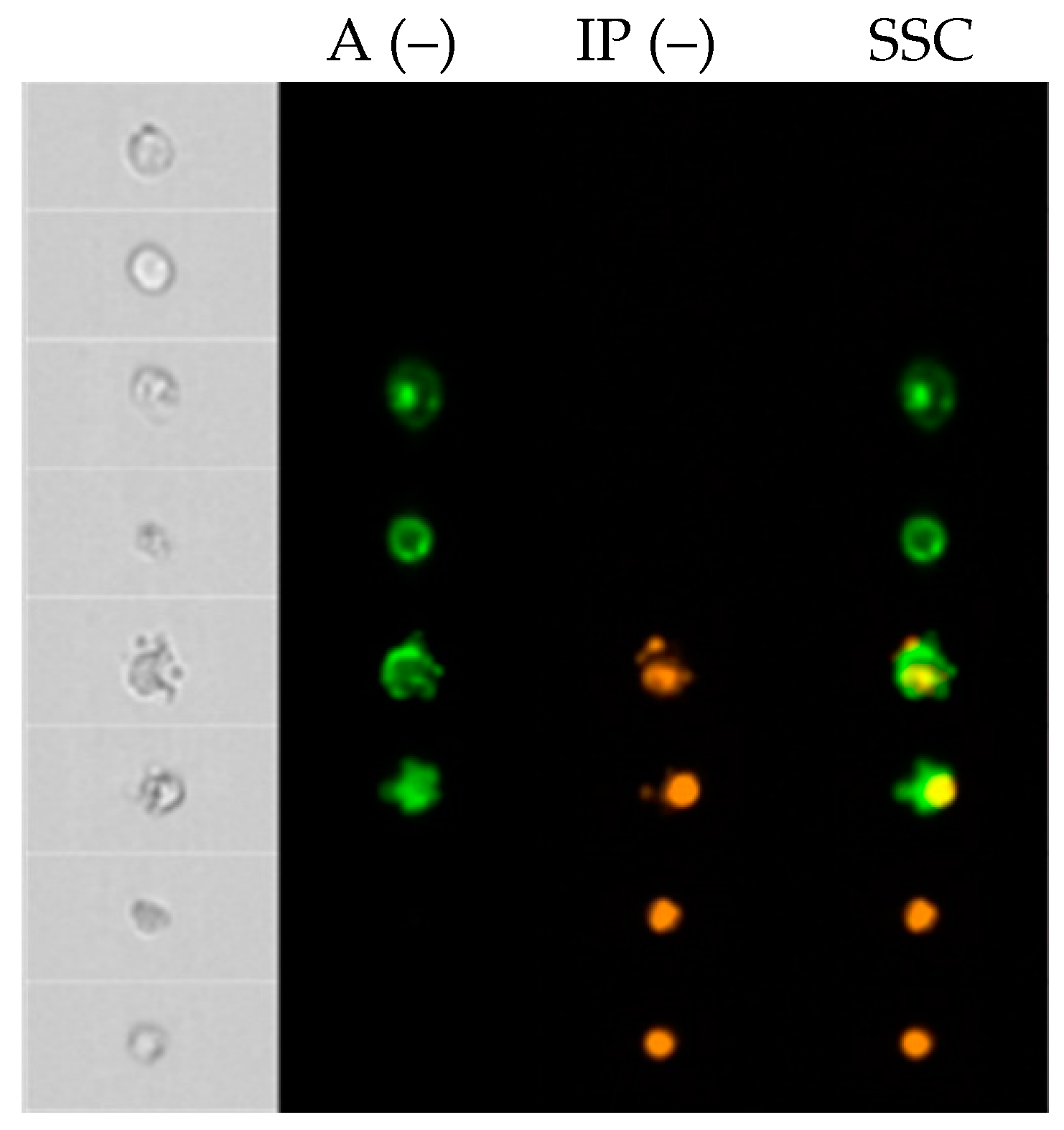
4.5. Quantification of Apoptosis by Annexin V and PI Double Staining
4.6. Quantification of Apoptosis by Caspase 3 (PhiPhiLux-G1D2)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, P.K.; Joshi, H. Coumarin: Chemical and Pharmacological Profile. J. App. Pharm. Sci. 2012, 2, 236–240. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. Biomed Res Int. 2013, 963248, 1–14. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef]
- Hu, X.L.; Xu, Z.; Liu, M.L.; Feng, L.S.; Zhang, G.D. Recent Developments of Coumarin Hybrids as Anti-fungal Agents. Curr. Top. Med. Chem. 2017, 17, 3219–3231. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hou, Z.; Yang, X.; Mou, Y.; Guo, C. Design, Synthesis, and Mechanism of Dihydroartemisinin–Coumarin Hybrids as Potential Anti-Neuroinflammatory Agents. Molecules 2019, 24, 1672. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D.H.; Méndez, J.; Brown, S.A. The natural Coumarins: Occurrence, Chemistry, and Biochemistry; John Willey and Sons LTD: Chichester, England; New York, USA; Brisbane, Australia; Toronto, Canada; Singapore, Asia, 1982; pp. 154–196. [Google Scholar]
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.-T.; Zhou, T.-T.; Liu, B.; Bao, J.-K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, Y.; Ding, W.; Xu, J.; Chen, R.; Xie, J.; Zhu, W.; Jia, L.; Ma, T. Anticancer activity and DNA-binding investigations of the Cu(II) and Ni(II) complexes with coumarin derivative. Chem. Biol. Drug Des. 2015, 85, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Bacso, Z.; Everson, R.B.; Eliason, J.F. The DNA of Annexin V-binding apoptotic cells is highly fragmented. Cancer Res. 2000, 60, 4623–4628. [Google Scholar]
- Darzynkiewicz, Z.; Bruno, S.; Del Bino, G.; Gorczyca, W.; Hotz, M.A.; Lassota, P.; Traganos, F. Features of apoptotic cells measured by flow cytometry. Cytometry 1992, 13, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Denecker, G.; Vercammen, D.; Declercq, W.; Vandenabeele, P. Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol. Life Sci. 2001, 58, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, L.; Lazebnik, Y. Caspases disrupt the nuclear-cytoplasmic barrier. J. Cell Biol. 2000, 151, 951–959. [Google Scholar] [CrossRef]
- Kubrak, T.; Bogucka-Kocka, A.; Komsta, Ł.; Załuski, D.; Bogucki, J.; Galkowski, D.; Kaczmarczyk, R.; Feldo, M.; Cioch, M.; Kocki, J. Modulation of multidrug resistance gene expression by coumarin derivatives in human leukemic cells. Oxid. Med. Cell Longev. 2017, 5647281. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef]
- Yang, L.L.; Min-Chieh, W.; Lih-Geeng, C.; Ching-Chiung, W. Cytotoxic activity of coumarins from the fruits of Cnidium monnieri on leukemia cell lines. Planta Med. 2003, 69, 1091–1095. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef]
- Musa, M.A.; Badisa, V.L.; Latinwo, L.M.; Cooperwood, J.; Sinclair, A.; Abdullah, A. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Anticancer Res. 2011, 31, 2017–2022. [Google Scholar]
- Klenkar, J.; Molnar, M. Natural and synthetic coumarins as potential anticancer agents. J. Chem. Pharm. Res. 2015, 7, 1223–1238. [Google Scholar]
- Patil, J.R.; Jayaprakasha, G.K.; Kim, J.; Chidambara Murthy, K.N.; Chetti, M.B.; Nam, S.J.; Patil, B.S. 5-Geranyloxy-7-Methoxycoumarin inhibits colon cancer (SW480) cells growth by inducing apoptosis. Planta Med. 2013, 79, 219–226. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Palma, M.G.; Bartella, V.; Rago, V.; Maggiolini, M.; Sisci, D.; Lanzino, M.; De Amicis, F.; Andò, S. Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr. Cancer Drug Targets 2009, 9, 469–481. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Mastroianni, F.; Palma, M.G.; Bartella, V.; Carpino, A.; Aquila, S.; Andò, S. Breast cancer cell survival signal is affected by bergapten combined with an ultraviolet irradiation. FEBS Lett. 2010, 584, 2321–2326. [Google Scholar] [CrossRef]
- Santoro, M.; Guido, C.; De Amicis, F.; Sisci, D.; Cione, E.; Vincenza, D.; Donà, A.; Panno, M.L.; Aquila, S. Bergapten induces metabolic reprogramming in breast cancer cells. Oncol. Rep. 2016, 35, 568–576. [Google Scholar] [CrossRef]
- Rasul, A.; Khan, M.; Yu, B.; Ma, T.; Yang, H. Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells. Asian Pac. J. Cancer Prev. 2011, 12, 1219–1223. [Google Scholar]
- Bogucka-Kocka, A.; Smolarz, H.; Cioch, M.; Dmoszyńska, A.; Kocki, J. Xantotoxin-induced apoptosis in Chronic Myeleogenous Leukemia. Pol. J. Environ. Stud. 2005, 14, 453–454. [Google Scholar]
- Zhang, Y.-Y.; Zhang, Q.-Q.; Song, J.-L.; Zhang, L.; Jiang, C.-S.; Zhang, H. Design, synthesis, and antiproliferative evaluation of novel coumarin/2-cyanoacryloyl hybrids as apoptosis inducing agents by activation of caspase-dependent pathway. Molecules 2018, 23, 1972. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Dicato, M.; Diederich, M. Hybrid curcumin compounds: A new strategy for cancer treatment. Molecules. 2014, 19, 20839–20863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure-Activity Relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015-2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |

| LIFE | EARLY APOPTOTIC | LATE APOPTOTIC | NECROTIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | ||
| Xan-l | Z | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | |
| Xan-n | Z | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 2.683282 | 1.341641 | 1.341641 | 2.385139 | 0.745356 | 1.639783 |
| P | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.021871 | 0.539137 | 0.539137 | 0.051218 | 1.000000 | 0.303151 | |
| Her | Z | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 |
| p | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | |
| Iso | Z | 1.043498 | 1.490712 | 2.534210 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 2.683282 | 1.341641 | 1.341641 |
| p | 0.890153 | 0.408111 | 0.033810 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.021871 | 0.539137 | 0.539137 | |
| Ber | Z | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | |
| Bya-n | Z | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | |
| Bya-l | Z | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.385139 | 0.745356 | 1.639783 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.051218 | 1.000000 | 0.303151 | 0.539137 | 0.539137 | 0.021871 | |
| Phel | Z | 2.385139 | 0.745356 | 1.639783 | 1.341641 | 2.683282 | 1.341641 | 0.447214 | 2.236068 | 1.788854 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.051218 | 1.000000 | 0.303151 | 0.539137 | 0.021871 | 0.539137 | 1.000000 | 0.076042 | 0.220915 | 0.539137 | 0.539137 | 0.021871 | |
| LIFE | EARLY APOPTOTIC | LATE APOPTOTIC | NECROTIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | ||
| Xan-l+C | Z | 2.236068 | 1.788854 | 0.447214 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 |
| p | 0.076042 | 0.220915 | 1.000000 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | |
| Xan-n+C | Z | 1.341641 | 2.683282 | 1.341641 | 0.447214 | 1.788854 | 2.236068 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| P | 0.539137 | 0.021871 | 0.539137 | 1.000000 | 0.220915 | 0.076042 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Her+C | Z | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.788854 | 2.236068 | 0.447214 |
| p | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.220915 | 0.076042 | 1.000000 | |
| Iso+C | Z | 2.683282 | 1.341641 | 1.341641 | 0.149071 | 1.937926 | 2.086997 | 2.683282 | 1.341641 | 1.341641 | 1.490712 | 0.745356 | 0.745356 |
| p | 0.021871 | 0.539137 | 0.539137 | 1.000000 | 0.157897 | 0.110665 | 0.021871 | 0.539137 | 0.539137 | 0.408111 | 1.000000 | 1.000000 | |
| Ber+C | Z | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | |
| Bya-n+C | Z | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.490712 | 1.043498 | 2.534210 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.408111 | 0.890153 | 0.033810 | 0.539137 | 0.539137 | 0.021871 | |
| Bya-l+C | Z | 1.341641 | 2.683282 | 1.341641 | 0.745356 | 2.385139 | 1.639783 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.539137 | 0.021871 | 0.539137 | 1.000000 | 0.051218 | 0.303151 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Phel+C | Z | 1.341641 | 2.683282 | 1.341641 | 0.447214 | 2.236068 | 1.788854 | 1.341641 | 2.683282 | 1.341641 | 1.639783 | 2.385139 | 0.745356 |
| p | 0.539137 | 0.021871 | 0.539137 | 1.000000 | 0.076042 | 0.220915 | 0.539137 | 0.021871 | 0.539137 | 0.303151 | 0.051218 | 1.000000 | |
| LIFE | EARLY APOPTOTIC | LATE APOPTOTIC | NECROTIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | ||
| Xan-l | Z | 2.68328 | 1.34164 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.02187 | 0.53913 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Xan-n | Z | 1.34164 | 2.68328 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| P | 0.53913 | 0.02187 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Her | Z | 2.68328 | 1.34164 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.02187 | 0.53913 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | |
| Iso | Z | 1.34164 | 1.34164 | 2.683282 | 0.447214 | 2.236068 | 1.788854 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.53913 | 0.53913 | 0.021871 | 1.000000 | 0.076042 | 0.220915 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | |
| Ber | Z | 1.34164 | 1.34164 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.53913 | 0.53913 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | |
| Bya-n | Z | 2.68328 | 1.34164 | 1.341641 | 0.447214 | 2.236068 | 1.788854 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.02187 | 0.53913 | 0.539137 | 1.000000 | 0.076042 | 0.220915 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | |
| Bya-l | Z | 2.68328 | 1.34164 | 1.341641 | 2.385139 | 1.639783 | 0.745356 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.02187 | 0.53913 | 0.539137 | 0.051218 | 0.303151 | 1.000000 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Phel | Z | 1.34164 | 2.68328 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.53913 | 0.02187 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| LIFE | EARLY APOPTOTIC | LATE APOPTOTIC | NECROTIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | HL60/ MX1 | HL60/ MX2 | MX1/ MX2 | ||
| Xan-l+C | Z | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | |
| Xan-n+C | Z | 2.086997 | 1.937926 | 0.149071 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 |
| P | 0.110665 | 0.157897 | 1.000000 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | |
| Her+C | Z | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 1.341641 | 2.683282 |
| p | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | |
| Iso+C | Z | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Ber+C | Z | 2.683282 | 1.341641 | 1.341641 | 0.745356 | 2.385139 | 1.639783 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.021871 | 0.539137 | 0.539137 | 1.000000 | 0.051218 | 0.303151 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Bya-n+C | Z | 2.683282 | 1.341641 | 1.341641 | 0.447214 | 2.236068 | 1.788854 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.021871 | 0.539137 | 0.539137 | 1.000000 | 0.076042 | 0.220915 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Bya-l+C | Z | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
| Phel+C | Z | 1.341641 | 2.683282 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 1.341641 | 2.683282 | 1.341641 | 1.341641 | 2.683282 | 1.341641 |
| p | 0.539137 | 0.021871 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | 0.539137 | 0.021871 | 0.539137 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubrak, T.; Czop, M.; Kołodziej, P.; Ziaja-Sołtys, M.; Bogucki, J.; Makuch-Kocka, A.; Aebisher, D.; Kocki, J.; Bogucka-Kocka, A. The Effect of Furanocoumarin Derivatives on Induction of Apoptosis and Multidrug Resistance in Human Leukemic Cells. Molecules 2019, 24, 1824. https://doi.org/10.3390/molecules24091824
Kubrak T, Czop M, Kołodziej P, Ziaja-Sołtys M, Bogucki J, Makuch-Kocka A, Aebisher D, Kocki J, Bogucka-Kocka A. The Effect of Furanocoumarin Derivatives on Induction of Apoptosis and Multidrug Resistance in Human Leukemic Cells. Molecules. 2019; 24(9):1824. https://doi.org/10.3390/molecules24091824
Chicago/Turabian StyleKubrak, Tomasz, Marcin Czop, Przemysław Kołodziej, Marta Ziaja-Sołtys, Jacek Bogucki, Anna Makuch-Kocka, David Aebisher, Janusz Kocki, and Anna Bogucka-Kocka. 2019. "The Effect of Furanocoumarin Derivatives on Induction of Apoptosis and Multidrug Resistance in Human Leukemic Cells" Molecules 24, no. 9: 1824. https://doi.org/10.3390/molecules24091824
APA StyleKubrak, T., Czop, M., Kołodziej, P., Ziaja-Sołtys, M., Bogucki, J., Makuch-Kocka, A., Aebisher, D., Kocki, J., & Bogucka-Kocka, A. (2019). The Effect of Furanocoumarin Derivatives on Induction of Apoptosis and Multidrug Resistance in Human Leukemic Cells. Molecules, 24(9), 1824. https://doi.org/10.3390/molecules24091824








