New Quinolone-Based Thiosemicarbazones Showing Activity Against Plasmodium falciparum and Mycobacterium tuberculosis
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Pharmacology
3. Materials and Methods
3.1. General Information
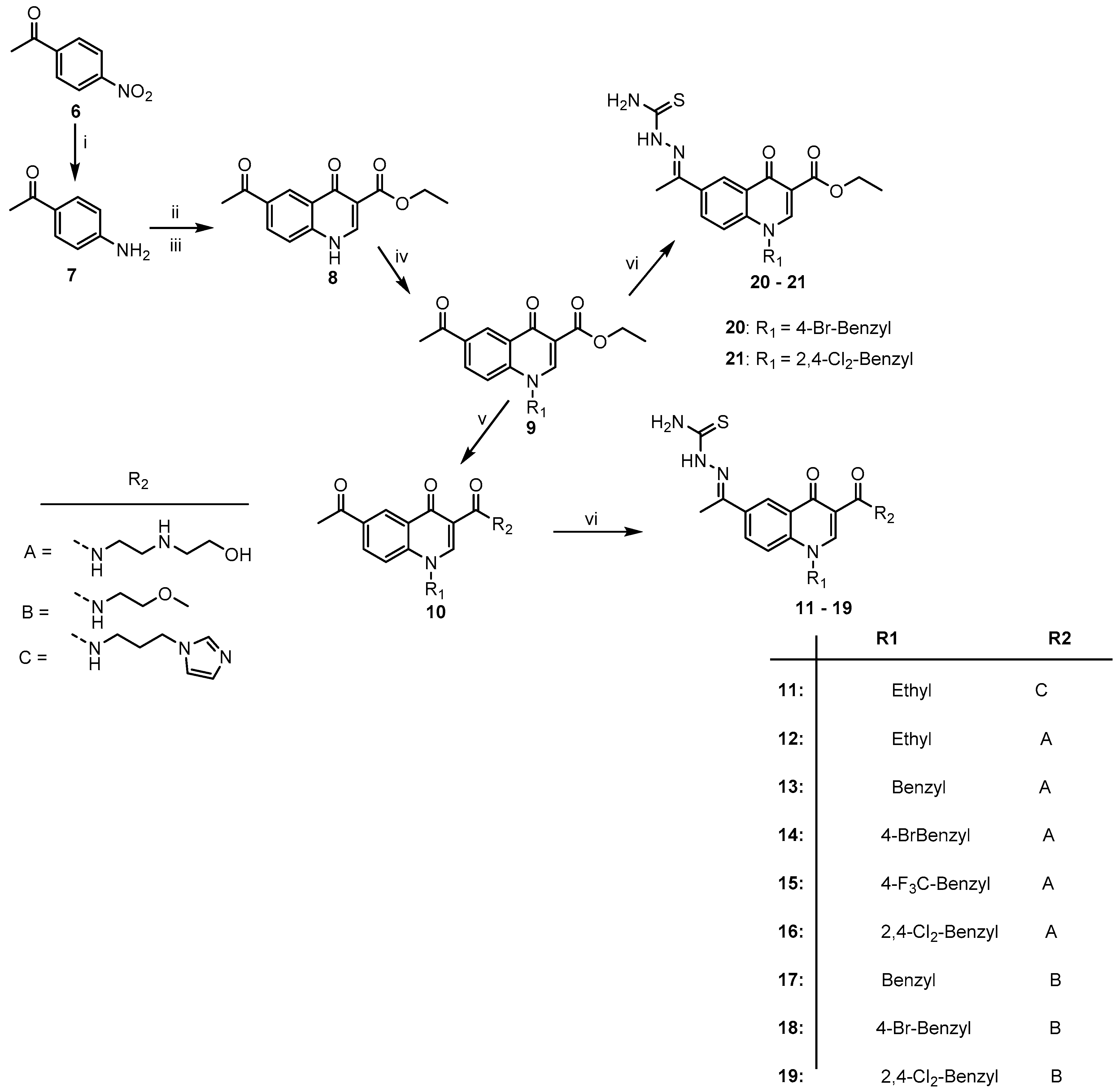
3.2. General Synthetic Procedure for the Quinolone-Thiosemicarbazone Derivatives, 11–21
3.3. In Vitro Anti-Plasmodial Assay
3.4. In Vitro Cytotoxicity Assay
3.5. In Vitro Antimycobacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Delogu, G.; Sali, M.; Fadda, G. The biology of Mycobacterium tuberculosis infection. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013070. [Google Scholar] [CrossRef]
- Bañuls, A.L.; Sanou, A.; Anh, N.T.; Godreuil, S. Mycobacterium tuberculosis: Ecology and evolution of a human bacterium. J. Med. Microbiol. 2015, 64, 1261–1269. [Google Scholar] [CrossRef]
- Ahmad, S. Pathogenesis, immunolog, and diagnosis of latent Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011, 2011, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Flynn, J.-A.L. The end of the binary era: Revisiting the spectrum of tuberculosis. J. Immunol. 2018, 201, 2541–2548. [Google Scholar] [CrossRef]
- Ai, J.-W.; Ruan, Q.-L.; Liu, Q.-H.; Zhang, W.-H. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg. Microbes Infect. 2016, 5, e10. [Google Scholar] [CrossRef] [PubMed]
- Dargie, B.; Tesfaye, G.; Worku, A. Prevalence and associated factors of undernutrition among adult tuberculosis patients in some selected public health facilities of Addis Ababa, Ethiopia: A cross-sectional study. BMC Nutr. 2016, 2, 7. [Google Scholar] [CrossRef]
- Cudahy, P.; Shenoi, S. Diagnostics for pulmonary tuberculosis. Postgrad. Med. J. 2016, 92, 187–193. [Google Scholar] [CrossRef]
- Churchyard, G.; Kim, P.; Shah, N.; Rustomjee, R.; Gandhi, N.; Mathema, B.; Dowdy, D.; Kasmar, A.; Cardenas, V. What we know about tuberculosis transmission: An overview. J. Infect. Dis. 2017, 216, S629–S635. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2018; WHO: Geneva, Switzerland, 2018; ISBN 978-92-4-156564-6. [Google Scholar]
- Lohrasbi, V.; Talebi, M.; Bialvaei, A.; Fattorini, L.; Drancourt, M.; Heidary, M.; Darban-Sarokhalil, D. Trends in the discovery of new drugs for Mycobacterium tuberculosis therapy with a glance at resistance. Tuberculosis 2018, 109, 17–27. [Google Scholar] [CrossRef]
- Auld, S.C.; Shah, N.S.; Mathema, B.; Brown, T.S.; Ismail, N.; Omar, S.V.; Brust, J.C.; Nelson, K.; Allana, S.; Campbell, A.; et al. XDR tuberculosis in South Africa: Genomic evidence supporting transmission in communities. Eur. Respir. J. 2018, 52, 1800246. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2017; WHO: Geneva, Switzerland, 2017; pp. 32–43. [Google Scholar]
- WHO. World Malaria Report 2015; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Gomes, A.P.; Vitorino, R.R.; Costa, A.; de Mendonça, E.; Oliveira, M.; Siqueira-Batista, R. Severe Plasmodium falciparum malaria. Rev. Bras. Ter. Intensiva 2011, 23, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P. Mortality associated with severe Plasmodium falciparum malaria increases with age. Clin. Infect. Dis. 2008, 47, 158–160. [Google Scholar] [CrossRef]
- Snow, R.S. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 2015, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Salmanzadeh, S.; Foroutan-Rad, M.; Khademvatan, S.; Moogahi, S.; Bigdeli, S. Significant decline of malaria incidence in southwest of Iran (2001–2014). J. Trop. Med. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Atkinson, P.M.; Snow, R.W. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005, 3, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, A.; Bhattacharjee, S.; Pandharkar, T.; Liu, H.; Estiu, G.; Stahelin, R.; Rizk, S.; Njimoh, D.; Ryan, Y.; Chotivanich, K.; et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 2015, 520, 683–687. [Google Scholar] [CrossRef]
- Mita, T.; Tanabe, K. Evolution of Plasmodium falciparum drug resistance: Implications for the development and containment of artemisinin resistance. Jpn. J. Infect. Dis. 2012, 65, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Pousibet-Puerto, J.; Salas-Coronas, J.; Sánchez-Crespo, A.; Molina-Arrebola, A.; Soriano-Pérez, M.; Giménez-López, M.; Vázquez-Villegas, J.; Cabezas-Fernández, M. Impact of using artemisinin-based combination therapy (ACT) in the treatment of uncomplicated malaria from Plasmodium falciparum in a non-endemic zone. Malar. J. 2016, 15, 339. [Google Scholar] [CrossRef]
- Pasvol, G. The treatment of complicated and severe malaria. Br. Med. Bull. 2005, 75–76, 29–47. [Google Scholar] [CrossRef]
- Cui, L.; Mharakurwa, S.; Ndiaye, D.; Rathod, P.; Rosenthal, P. Antimalarial drug resistance: Literature review and activities and findings of the ICEMR network. Am. J. Trop. Med. Hyg. 2015, 93, 57–68. [Google Scholar] [CrossRef]
- Marriner, G.A.; Nayyar, A.; Uh, E.; Wong, S.; Mukherjee, T.; Via, L.; Carroll, M.; Edwards, R.; Gruber, T.; Choi, I.; et al. The medicinal chemistry of tuberculosis chemotherapy. Top. Med. Chem. 2011, 7, 47–124. [Google Scholar]
- Li, X.-X.; Zhou, X.-N. Co-infection of tuberculosis and parasitic diseases in humans: A systematic review. Parasit. Vectors 2013, 6, 79. [Google Scholar] [CrossRef]
- Murphy, M.E.; Singh, K.P.; Laurenzi, M.; Brown, M.; Gillespie, S.H. Managing malaria in tuberculosis patients on fluoroquinolone-containing regimens: Assessing the risk of QT prolongation. Int. J. Tuberc. Lung Dis. 2012, 16, 144–149. [Google Scholar] [CrossRef]
- Grzegorzewicz, A.E.; Korduláková, J.; Jones, V.; Born, S.; Belardinelli, J.; Vaquié, A.; Gundi, V.; Madacki, J.; Slama, N.; Laval, F.; et al. A Common mechanism of inhibition of the Mycobacterium tuberculosis mycolic acid biosynthetic pathway by Isoxyl and Thiacetazone. J. Biol. Chem. 2012, 287, 38434–38441. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.; Mackey, Z.; Hansell, E.; Doyle, P.; Gut, J.; Caffrey, C.R.; Lehman, J.; Rosenthal, P.J.; McKerrow, J.H.; Chibale, K. Synthesis and structure activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against Plasmodium falciparum, Trypanosoma brucei and Trypanasoma cruzi. J. Med. Chem. 2004, 47, 3212–3219. [Google Scholar] [CrossRef]
- Akhtar, R.; Yousaf, M.; Naqvi, S.; Irfan, M.; Zahoor, A.; Hussain, A.; Chatha, S. Synthesis of ciprofloxacin-based compounds: A review. Syn. Commun. 2016, 46, 1849–1879. [Google Scholar] [CrossRef]
- Dixit, S.; Mishra, N.; Sharma, M.; Singh, S.; Agarwal, A.; Awasthi, S.; Bhasin, V. Synthesis and in vitro antiplasmodial activities of fluoroquinolone analogs. Eur. J. Med. Chem. 2012, 51, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Beteck, R.M.; Seldon, R.; Jordaan, A.; Warner, D.F.; Hoppe, H.C.; Laming, D.; Legoabe, L.J.; Khanye, S.D. Quinolone-isoniazid hybrids: Synthesis and preliminary in vitro cytotoxicity and anti-tuberculosis evaluation. Medchemcomm 2019, 10, 326–331. [Google Scholar] [CrossRef]
- Neelarapu, R.; Maignan, J.; Lichorowic, C.; Monastyrskyi, A.; Mutka, T.; LaCrue, A.; Blake, L.; Casandra, D.; Mashkouri, S.; Burrows, J.; et al. Design and synthesis of orally bioavailable piperazine substituted 4(1H)-quinolones with potent antimalarial activity: Structure-activity and structure-property relationship studies. J. Med. Chem. 2018, 61, 1450–1473. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Pierens, G.K.; Reutens, D.C. Synthesis, NMR structural characterization and molecular modeling of substituted thiosemicarbazones and semicarbazones using DFT calculations to prove the syn/anti isomer formation. Magn. Reson. Chem. 2014, 52, 98–105. [Google Scholar] [CrossRef]
- Beteck, R.M.; Isaacs, M.; Hoppe, H.C.; Khanye, S.D. Sysnthesis, in vitro cytotoxicity and trypanocidal evaluation of novel 1,3,6-substituted non-fluorquinolones. S. Afr. J. Chem. 2018, 71, 188–195. [Google Scholar] [CrossRef]
- Gumbo, M.; Beteck, R.M.; Mandizvo, T.; Seldon, R.; Warner, D.F.; Hoppe, H.C.; Isaacs, M.; Laming, D.; Tam, C.C.; Cheng, L.W.; et al. Cinnamoyl-oxaborole amides: Synthesis and their in vitro biological activity. Molecules 2018, 23, 2038. [Google Scholar] [CrossRef] [PubMed]
- Mbaba, M.; Mabhula, A.N.; Boelb, N.; Edkinsb, A.L.; Isaacs, M.; Hoppe, H.C.; Khanye, S.D. Ferrocenyl and organic novobiocin derivatives: Synthesis and their in vitro biological activity. J. Inorg. Biochem. 2017, 172, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Oderinlo, O.O.; Tukulula, M.; Isaacs, M.; Hoppe, H.C.; Taylor, D.; Smith, V.J.; Khanye, S.D. New thiazolidine-2,4-dione derivatives combined with organometallic ferrocene: Synthesis, structure and antiparasitic activity. Appl. Organomet. Chem. 2018, 32, e4385. [Google Scholar] [CrossRef]
- Abrahams, K.A.; Cox, J.G.; Spivey, V.L.; Loman, N.J.; Pallen, M.J.; Constantinindou, C.; Fernández, R.; Alemparte, C.; Remuñuinán, M.J.; Barros, D.; et al. Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB. PLoS ONE 2012, 7, e52951. [Google Scholar] [CrossRef] [PubMed]
- De Voss, J.J.; Rutter, K.; Schroeder, B.G.; Su, H.; Zhu, Y.; Barry, C.E., III. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. PNAS 2000, 97, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the compounds are available from the authors. |

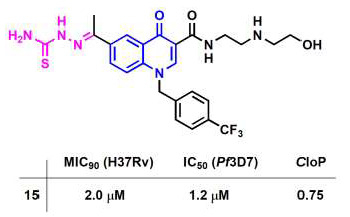
| Compound | MIC90 (μM) | IC50 (μM) | ClogP a |
|---|---|---|---|
| H37Rv | Pf 3D7 | ||
| 11 | ˃125 | 3.7 | −0.02 |
| 12 | 73.4 | na | −1.17 |
| 13 | 2.7 | 4.1 | 0.18 |
| 14 | 10.3 | 4.7 | 0.95 |
| 15 | 2.0 | 1.2 | 0.75 |
| 16 | 4.8 | 3.1 | 1.38 |
| 17 | 44.2 | 24.6 | 1.07 |
| 18 | 15.0 | 9.9 | 1.84 |
| 19 | 10.2 | na | 2.27 |
| 20 | 102.5 | na | 2.55 |
| 21 | 31.6 | na | 3.52 |
| CQ | - | 0.012 | - |
| RF | 0.062 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beteck, R.M.; Seldon, R.; Jordaan, A.; Warner, D.F.; Hoppe, H.C.; Laming, D.; Khanye, S.D. New Quinolone-Based Thiosemicarbazones Showing Activity Against Plasmodium falciparum and Mycobacterium tuberculosis. Molecules 2019, 24, 1740. https://doi.org/10.3390/molecules24091740
Beteck RM, Seldon R, Jordaan A, Warner DF, Hoppe HC, Laming D, Khanye SD. New Quinolone-Based Thiosemicarbazones Showing Activity Against Plasmodium falciparum and Mycobacterium tuberculosis. Molecules. 2019; 24(9):1740. https://doi.org/10.3390/molecules24091740
Chicago/Turabian StyleBeteck, Richard M., Ronnett Seldon, Audrey Jordaan, Digby F. Warner, Heinrich C. Hoppe, Dustin Laming, and Setshaba D. Khanye. 2019. "New Quinolone-Based Thiosemicarbazones Showing Activity Against Plasmodium falciparum and Mycobacterium tuberculosis" Molecules 24, no. 9: 1740. https://doi.org/10.3390/molecules24091740
APA StyleBeteck, R. M., Seldon, R., Jordaan, A., Warner, D. F., Hoppe, H. C., Laming, D., & Khanye, S. D. (2019). New Quinolone-Based Thiosemicarbazones Showing Activity Against Plasmodium falciparum and Mycobacterium tuberculosis. Molecules, 24(9), 1740. https://doi.org/10.3390/molecules24091740