Abstract
Type 2 diabetic patients possess a two to four-fold-increased risk for cardiovascular diseases (CVD). Hyperglycemia, oxidative stress associated with endothelial dysfunction and dyslipidemia are regarded as pro-atherogenic mechanisms of CVD. In this study, high-fat diet-induced diabetic and non-diabetic vervet monkeys were treated with 90 mg/kg of aspalathin-rich green rooibos extract (Afriplex GRT) for 28 days, followed by a 1-month wash-out period. Supplementation showed improvements in both the intravenous glucose tolerance test (IVGTT) glycemic area under curve (AUC) and total cholesterol (due to a decrease of the low-density lipoprotein [LDL]) values in diabetics, while non-diabetic monkeys benefited from an increase in high-density lipoprotein (HDL) levels. No variation of plasma coenzyme Q10 (CoQ10) were found, suggesting that the LDL-lowering effect of Afriplex GRT could be related to its ability to modulate the mevalonate pathway differently from statins. Concerning the plasma oxidative status, a decrease in percentage of oxidized CoQ10 and circulating oxidized LDL (ox-LDL) levels after supplementation was observed in diabetics. Finally, the direct correlation between the amount of oxidized LDL and total LDL concentration, and the inverse correlation between ox-LDL and plasma CoQ10 levels, detected in the diabetic monkeys highlighted the potential cardiovascular protective role of green rooibos extract. Taken together, these findings suggest that Afriplex GRT could counteract hyperglycemia, oxidative stress and dyslipidemia, thereby lowering fundamental cardiovascular risk factors associated with diabetes.
1. Introduction
Diabetes mellitus is a global pandemic afflicting 425 million adults worldwide, with trends suggesting the rate will continue to rise, reaching 642 million in 2040 []. Almost 90% of diabetic patients are affected by the lifestyle-related sub-type, type 2 diabetes (T2DM) [], a progressive metabolic disease characterized by insulin resistance and eventual functional failure of pancreatic beta cells [].
It is known that T2DM is associated with higher cardiovascular morbidity and mortality. In particular, type 2 diabetic patients have a two to four-fold increased risk of incident coronary heart disease, ischemic stroke and a 1.5 to 3.6-fold increase in mortality [].
Hyperglycemia is a key cardiovascular risk factor for patients with type 2 diabetes []. Increased glucose flux through the polyol pathway, intracellular formation of advanced glycation end products (AGE) and increased expression of its receptors [], as well as activation of protein kinase C (PKC) [] and increased glucose flux through the hexosamine pathway [,,] represent the five pro-atherogenic mechanisms of diabetes associated with hyperglycemia. Several lines of evidence indicate that these mechanisms are activated by mitochondrial reactive oxygen species (ROS) overproduction [], produced by proton leakage at the mitochondrial electron transport chain, resulting in increased production of superoxide []. In turn, these mechanisms ultimately lead to further increases of free radical formation. Moreover, increased oxidative stress underlies endothelial dysfunction, caused by decreased bioavailability of nitric oxide (NO), a critical signaling molecule mediating vasoactive activity. In fact, high levels of ROS promotes NO oxidation, leading to the production of peroxynitrite, a highly reactive molecule responsible for extensive oxidative damage in the endothelium [].
Besides hyperglycemia and oxidative stress, diabetic dyslipidemia—characterized by elevated plasma triglyceride concentrations, low-density lipoprotein (LDL) concentrations, and reduced high density lipoprotein (HDL) levels—represents one of the most common cardiovascular risk factors, with a prevalence of 72–85% in T2DM [,].
Further, macrophages have no affinity for non-oxidized LDL; even at high concentrations, non-oxidized LDL has little or no atherogenic-promoting properties. However, oxidized LDL contributes to the atherogenic processes in the arterial wall, driven by inflammation and the formation of lipid-laden foam cells that lead to plaque formation [].
Accordingly, in order to prevent its oxidation, low plasma LDL levels should be maintained, particularly in clinical conditions characterized by elevated oxidative stress—such as in diabetes. Therefore, guidelines for primary and secondary prevention of cardiovascular disease in diabetes include hypocholesterolemic therapies for the reduction of plasma LDL []. Pharmacological targets endorsed by the American Diabetic Association refer to LDL plasma levels below 100 mg/dL in the primary prevention of individuals with diabetes [], compared to 130 mg/dL in non-diabetic patients.
The Old World non-human primate species Chlorocebus aethiops (vervet monkey), endemic to Southern Africa, shares the same subfamily with the macaque (Cercopithecinae) and are phylogenetically close to humans []. Vervet monkeys are omnivorous and readily consume experimental diets, including high-fat diets, which has proven to be particularly useful for cardiovascular and metabolic disease research [,,,,,,]. In particular, LDL concentrations correlate with the type and amount of fat in the diet []. A strong correlation was established within our vervet colony between dietary lipid intake, LDL cholesterol and atherogenic aortic lipid deposition []. An inherent susceptibility of this species to cholesterolemia and development of atherosclerotic lesions that correspond to the human classification types I–VII has also been confirmed [,]. In addition, these vervet monkeys are responsive to both dietary and pharmacological intervention strategies [,,,,,,]. Although the normal plasma LDL:HDL ratio is lower in vervet monkeys [,] compared to humans [], their LDL responses to Westernized human diets are similar and therefore relevant to this study [].
Aspalathus linearis, also known as rooibos, is a shrub-like leguminous bush native to the Western and Northern Cape regions of South Africa. Commercially, rooibos is processed to produce unfermented (green) or fermented (red) rooibos. While fermentation results in oxidation of the plant polyphenols, leading to a decrease in the total antioxidant content, they remain preserved in green rooibos—particularly aspalathin, which represents the major bioactive compound [].
In addition to its proven antioxidant properties, several studies demonstrated that rooibos tea has important anticancer [], antihemolytic [], antimutagenic [,] and anti-inflammatory [] properties.
In the last years, many in vitro and ex vivo studies have focused on the antidiabetic effects of rooibos. In particular, Mazibuko et al. [] confirmed the capacity of rooibos to reduce insulin resistance in C2C12 muscle cells, while other studies demonstrated a hypoglycemic effect of aspalathin in different murine type 2 diabetic models [,]. Finally, Kamakura et al. [] used an aspalathin-rich green rooibos extract to promote glucose uptake in L6 myotubes and to counteract the increase in fasting blood glucose levels in type 2 diabetic model KK-Ay mice. The antidiabetic activity of rooibos is attributed to both the ability of aspalathin to increase GLUT 4 translocation to the plasma membrane via adenosine 5′ monophosphate-activated protein kinase (AMPK) and activation of Akt in skeletal muscle [,], and to reduce the gene expression of hepatic enzymes related to glucose production and lipogenesis [].
Apart from a limited human study by Marnewick et al. [], showing that consumption of rooibos tea (six cups/day) by participants at increased cardiovascular risk significantly decreased serum LDL and triglycerides and lowered plasma markers of lipid peroxidation, the effects of rooibos on other cardiovascular risk factors associated with diabetic pathology have been not studied.
This study aimed to evaluate the biological activity of standardized, pharmaceutical-grade, aspalathin-rich green rooibos extract (Afriplex, GRT) containing 12.8% aspalathin in improving the oxidative status and lipid profile of high-fat diet-fed diabetic and healthy non-human primates (Chlorocebus pygerythrus).
2. Results
2.1. Lipid Profile
Diabetic vervet monkeys showed significantly elevated plasma total cholesterol levels in comparison to normal monkeys (+164%; p < 0.01) at baseline, but also following treatment (14 days +101%; 28 days +123%; after wash-out +136%, p < 0.01) (Figure 1A). This is mainly due to different amounts of LDL (Figure 1B) between both experimental groups. Conversely, HDL levels are significantly different only at the start of the study (non-diabetics 1.59 ± 0.16 mmol/L, diabetics 2.44 ± 0.21 mmol/L; p = 0.010) (Figure 1C).
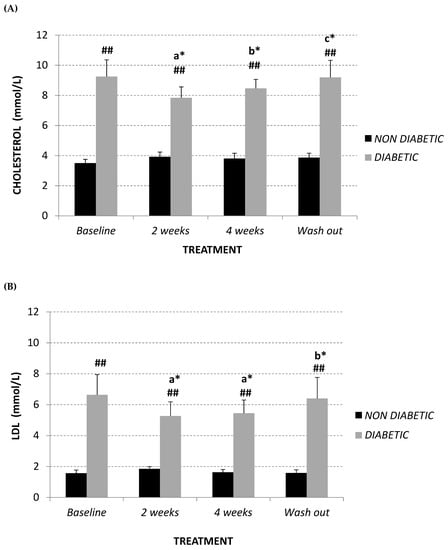
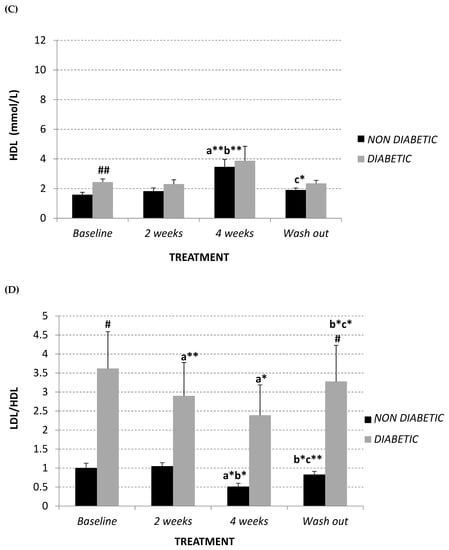
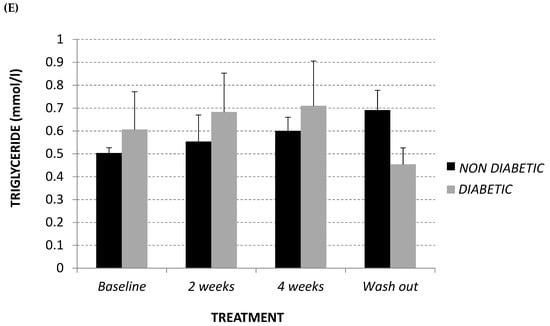
Figure 1.
Plasma total cholesterol (A), LDL (B), HDL (C) (mmol/L), LDL/HDL ratio (D) and triglyceride (mmol/L) (E) at baseline, after 2 and 4 weeks of Afriplex GRT treatment, and following 4 weeks wash-out in non-diabetic (black) and diabetic (grey) vervet monkeys. * p < 0.05, ** p < 0.01 comparing different time points within each experimental group (a = baseline, b = 2 weeks-treatment, c = 4 weeks-treatment); # p < 0.05 and ## p < 0.01 comparing both population groups for the same experimental point. a = baseline, b = 2 weeks-treatment, c = 4 weeks-treatment.
Two weeks of treatment with Afriplex GRT was sufficient to significantly decrease plasma LDL levels (baseline 6.64 ± 1.31 mmol/L; 14 days 5.27 ± 0.91 mmol/L, p = 0.015) (Figure 1B) and, consequently, total cholesterol (Figure 1A) (baseline 9.25 ± 1.11 mmol/L; 14 days 7.84 ± 0.73 mmol/L, p = 0.02) in diabetic monkeys. Moreover, after 4 weeks of treatment, LDL levels of diabetic monkeys remained unchanged (Figure 1B), while the total cholesterol significantly increased in comparison following 2 weeks treatment (Figure 1A) (+8%, p = 0.032). This is probably related to an increase, although not significant, of HDL levels in the same experimental group (Figure 1C) (+69%, p = 0.2).
In relation to non-diabetic vervet monkeys, total cholesterol and LDL levels remained unchanged at each experimental point (Figure 1A,B), while 4 weeks treatment promoted a highly significant increase of plasma HDL content in comparison with baseline (+118%, p = 0.012) and after 2 weeks of treatment (+90%, p = 0.008) (Figure 1C).
Summarizing, both diabetic and non-diabetic vervet monkeys showed a significant decrease in LDL:HDL ratio after supplementation with Afriplex GRT (Figure 1D), reducing the significant difference showed at baseline (non-diabetics 1.00 ± 0.12; diabetics 3.62 ± 0.97, p = 0.04). After the 4 weeks of wash-out, these values tended to revert back to the baseline level (Figure 1A–D).
In contrast, plasma triglyceride content remained unchanged in both studied populations (Figure 1E).
2.2. Glycemic Parameters
In order to evaluate insulin-response, fasting glucagon and glycemia levels, an intravenous glucose tolerance test (IVGTT) and a glucose-stimulated insulin secretion test (GSIST) were used.
As demonstrated in Figure 2A,B, at baseline diabetic animals showed significantly higher levels of glycaemia and insulin with respect to the non-diabetic monkeys (+63%, p = 0.03 and +125%, p = 0.04, respectively), while, in terms of fasting glucagon level, the difference recorded did not reach statistical significance (Figure 2C). Afriplex GRT did not affect the insulin or glucagon levels; despite this, after 2 weeks of treatment, Afriplex GRT had a noticeable hypoglycemic effect in the diabetic group, resulting in a significant decline of glycemia that persisted also after the wash-out period. More importantly, after 4 weeks of Afriplex GRT treatment, blood glycemia was reduced both in comparison to the baseline (from 487 ± 12 to 416 ± 4, p = 0.04) and the 2-weeks treatment (468 ± 7 to 416 ± 4, p = 0.03), also in the non-diabetic group.
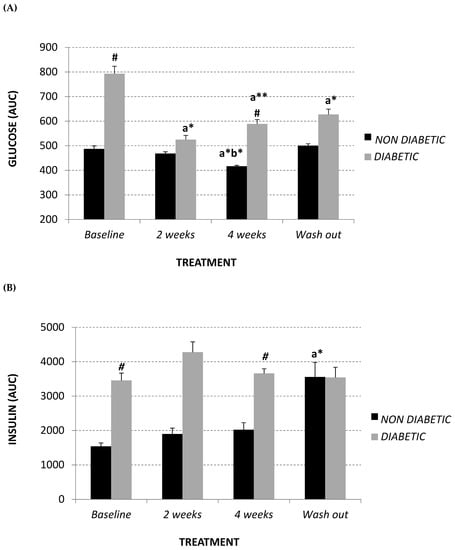
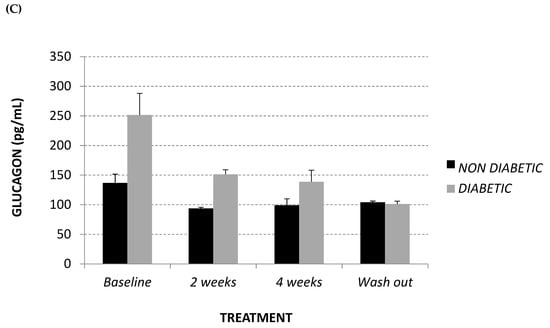
Figure 2.
Intravenous glucose tolerance test (area under curve: AUC) (A), glucose stimulated insulin secretion test (AUC) (B) and fasting glucagon (pg/mL) (C) at baseline, after 2 and 4 weeks of Afriplex GRT treatment, and following 4 weeks wash-out in non-diabetic (black) and diabetic (grey) vervet monkeys. * p < 0.05, ** p < 0.01 comparing different experimental time points for each group; # p < 0.05 comparing both population groups for the same experimental time point. a = baseline, b = 2 weeks-treatment.
2.3. Total Plasma Coenzyme Q10 (CoQ10) Level and Oxidative Status
CoQ10 plasma content was assessed in order to evaluate if the lipid-lowering effect of Afriplex GRT, highlighted in the diabetic group, also influenced CoQ10 synthesis via the mevalonate pathway. Interestingly, treatment did not produce any variation in plasma CoQ10 content in the diabetic monkeys, while non-diabetic monkeys showed a trend towards a decline in plasma CoQ10, which was restored after the wash-out period (Figure 3A).
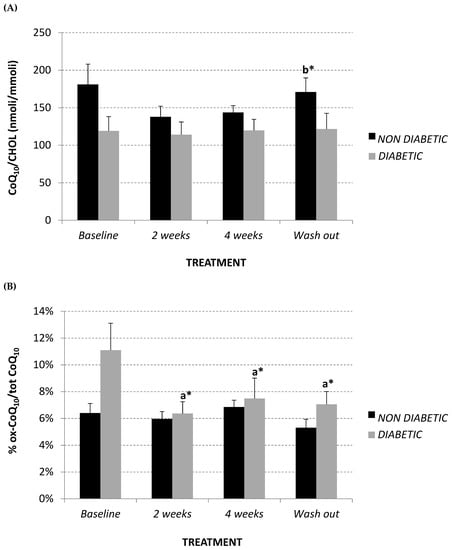
Figure 3.
Plasma CoQ10/Cholesterol levels (nmol/mmol) (A), and % oxidized CoQ10/total (B) at baseline, after 2 and 4 weeks of Afriplex GRT treatment, and following 4 weeks wash-out in non-diabetic (black) and diabetic (grey) vervet monkeys. * p < 0.05 comparing different experimental points for each group. a = baseline, b = 2 weeks-treatment.
Percentage of oxidized CoQ10 was also evaluated as a plasma oxidative marker in the monkeys. At baseline, the diabetic group showed a trend towards significantly higher levels of oxidized CoQ10 in comparison to the non-diabetics (Figure 3B) (non-diabetics 6.40% ± 0.01; diabetics 11.10% ± 0.02, p = 0.08). However, after Afriplex GRT treatment, the diabetic monkeys showed a significant decrease in the percentage of oxidized CoQ10; this effect also persisted after the wash-out period (Figure 3B).
2.4. Circulating Oxidized LDL
Circulating oxidized LDL (ox-LDL) was measured in plasma using an enzyme-linked immunosorbent assay (ELISA) kit. The diabetic monkeys showed significantly higher ox-LDL levels at each experimental time point, in comparison with the non-diabetic monkeys (Figure 4A). After 4 weeks of treatment, the diabetic monkeys showed a trend towards a decrease in this parameter (baseline 67.2 ± 14.8 U/L; 4-week treatment 53.8 ± 14.3 U/L), which returned to the baseline level after wash-out. However, a significant positive correlation was observed between ox-LDL and total LDL (R2 = 0.388, p = 0.017) (Figure 4B), while a significant inverse correlation was observed between oxidized LDL and lipoprotein CoQ10 content (mM CoQ/nM cholesterol) (R2 = 0.529, p = 0.002) (Figure 4C) in the diabetic monkey group.
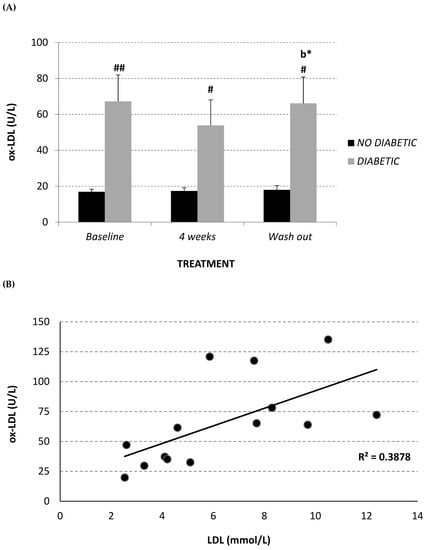
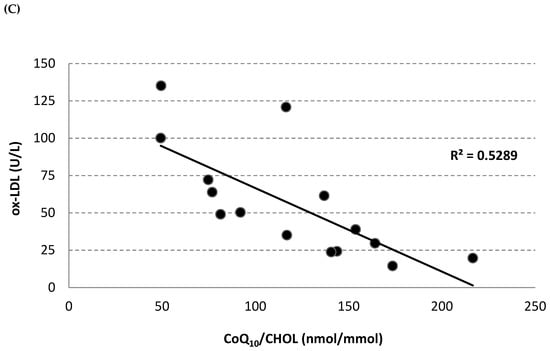
Figure 4.
Plasma oxidized LDL level (U/L) (A) at baseline, after 2 and 4 weeks of Afriplex GRT treatment, and following 1 month-wash-out in non-diabetic (black) and diabetic (grey) vervet monkeys. Correlation between plasma oxidized LDL level and both total LDL (B) and CoQ10/Chol level (C) in diabetic monkeys in baseline, after 4 weeks of supplementation and wash-out period. * p < 0.05 comparing different experimental points for each group; # p < 0.05 ## p < 0.01 comparing both groups for the same experimental time point. b = 4 weeks-treatment.
3. Discussion
The present study focused on the effects of standardized pharmaceutical grade aspalathin-rich green rooibos extract (Afriplex GRT) in counteracting selected cardiovascular risk factors underlying diabetic pathology. Specifically, gluco-lipidic and oxidative indexes in high-fat diet-fed diabetic and non-diabetic vervet monkeys (Chlorocebus pygerythrus) were assessed prior to the onset of treatment (baseline), and after 2 and 4 weeks of treatment with 90 mg/kg of body weight of Afriplex GRT extract containing ca. 12.8% aspalathin. Moreover, analysis was repeated following 4 weeks of wash-out.
Clinically, the high-fat diet induced insulin resistance, glucose intolerance and, in susceptible monkeys, diabetic changes to the pancreatic islets; including reduced beta-cell mass and islet-associated amyloid deposits, exacerbating parameters for cardiovascular risk []. Although the monkeys on the high-fat diet (HFD) did not develop overt obesity in this colony, they displayed glucose intolerance with associated hyperinsulinemia and hyperglucagonemia, consistent with T2DM. In relation to glycemic parameters, a significant decrease of glycaemia was observed after just 2 weeks of treatment with Afriplex GRT and this effect persisted after 4 weeks of wash-out without any dietary interventions. It is important to note that there were no significant changes in the total calorie intake for the monkeys on their respective diets during the course of the study when compared to data recorded prior to the commencement of the study. This data, observed for the first time in non-human primates, confirms the hypoglycemic potential of green rooibos extract and its major phenolic compound, aspalathin, previously demonstrated in different murine type 2 diabetic models [,,]. Additionally, the treatment effects were similar for males and females.
Interestingly however, insulin and glucagon levels remained unchanged in both the diabetic and non-diabetic animals. These results highlight that the potential antidiabetic role of Afriplex GRT could predominantly be related to an increase in insulin sensitivity, through promotion of glucose uptake, rather than its ability to stimulate the synthesis or secretion of the respective hormones. In this regard, Kamakura et al. [] showed that the stimulatory effect of aspalathin on insulin secretion was less potent than the effect on glucose uptake in L6 myotubes; L6 myotubes responded to aspalathin concentrations 100 times lower than that which was effective in the beta cells.
In this study, besides the confirmatory effects on glucose usage in diabetic models, and in line with other reports in the literature, we also showed a marked improvement on the plasma lipid profile. In both the diabetic and non-diabetic vervet monkeys, the LDL:HDL ratio, a cardiovascular risk indicator with greater predictive value than isolated indexes used independently, were improved []. Vervet monkeys on a high-fat diet have an inherent susceptibility to developing LDL-cholesterolemia and atherosclerosis comparable to that found in humans [,,].
Specifically, treated non-diabetic monkeys benefited from an increase of plasma HDL levels, whose anti-atherogenic properties are widely demonstrated [], while diabetic animals mainly benefited from a decrease in LDL plasma levels that reached significant levels in only 2 weeks of treatment. In a previous study, treating high-fat fed monkeys with etofibrate for 20 months lowered LDL and demonstrated an anti-atherogenic effect []. In addition to lowering LDL, etofibrate has been demonstrated to protect LDL against oxidation in human subjects, and thereby lower cardiovascular risk in diabetic patients []. In a human study, Marnewick et al. demonstrated a decrease of 15% in circulating LDL and an increase of 33% in HDL as well as redox status following consumption of six cups of rooibos tea daily for six weeks []. These findings validate the relevance of this diet-induced vervet monkey model in predicting treatment efficacy of human metabolic disease.
This remarkable ability of rooibos flavonoids to improve lipid profiles could play an important role in cardiovascular protection of diabetic patients. In fact, it is known that the main pharmacological approaches used in the primary and secondary prevention of cardiovascular diseases associated with diabetic dyslipidemia aim to lower circulating LDL levels. In particular, 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductases inhibitors, also known as statins, are the most common and consolidated therapy used for this purpose. However, even though their efficiency to reduce cardiovascular morbidity and mortality in diabetes has been widely demonstrated [], statins can cause adverse side effects. Primarily, they may promote hyperglycemia by increasing the calcium concentration in pancreatic β islet cells, leading to a decrease in insulin release, or by decreasing GLUT 4-mediated peripheral glucose uptake []. Secondarily, multiple studies have shown that statins can decrease serum coenzyme Q10 levels [], affecting an early step in the mevalonate pathway. In fact, mevalonate is a common precursor for cholesterol and CoQ10 synthesis. CoQ10, also known as ubiquinone, is an endogenous quinone with a key role in mitochondrial bioenergetics; in its reduced form (ubiquinol), it represents one of the most important lipophilic antioxidants []. Observational studies have reported that plasma CoQ10 concentration is an independent predictor of mortality in patients with congestive heart failure [], whereby its decline associated with statin therapy may be related not only to muscle disorders but also to increased risk of cardiovascular diseases. These indications support the hypothesis that statins, while efficiently minimizing some CVD related risk factors, may be detrimental to cardiovascular health in diabetic patients by promoting hyperglycemia and decreasing CoQ10 biosynthesis.
In our study, while the treatment with 90 mg/day/kg of body weight of pharmaceutical grade aspalathin-enriched green rooibos extract (Afriplex GRT) was effective in lowering serum LDL levels in diabetic animals, no gross side effects described for statin treatment was observed. On the contrary, no variation in terms of plasma CoQ10 normalized for cholesterol was observed during all experimental phases in diabetic monkeys. This suggests that the LDL-lowering effect of aspalathin could be related to its ability to modulate the mevalonate pathway differently from statins, or to affect other biochemical mechanisms not affecting CoQ10 biosynthesis, therefore preserving its cardioprotective role.
In fact, several studies showed that some flavonoids directly affect cholesterol metabolism at different steps: Curcumin is able to increase the excretion of bile acid by upregulating the expression of cholesterol 7α-hydroxylase []; tocotrienols inhibit HMG-CoA reductase [,] similarly to statins; and flavonoids of green tea upregulate the LDL receptor [,]. Beltrán-Debón et al. [] demonstrated a hypolipidemic effect of rooibos in LDLr-/-mice fed a high-fat Western-type diet, but this effect was stringently dependent on diet type. In these high-fat fed animals, rooibos extract significantly lowered serum cholesterol, triglyceride and free fatty acid concentrations. However, mechanisms underlying LDL-lowering effect of Afriplex GRT are not reported in literature.
Finally, in order to evaluate the antioxidant role of Afriplex GRT treatment, the plasma oxidative status of CoQ10 was analyzed. In fact, several studies demonstrated that the reduced form of CoQ10, a key and ubiquitous lipophilic antioxidant, could represent a sensitive marker of oxidative stress in vivo [,,,]. At baseline, diabetic animals showed a higher—although not significant—percentage of oxidized CoQ10 in comparison to non-diabetic monkeys. Only 2 weeks of treatment with Afriplex GRT was sufficient to abolish this difference, significantly decreasing the percentage of oxidized CoQ10. Remarkably, this antioxidant effect persisted after 4 weeks of wash-out. In line with the improvement of plasma oxidative status, a slight, although not significant, decrease of circulating ox-LDL was observed following treatment in the diabetic group, although after 4 weeks of wash-out these were restored to baseline values. The significant correlation between the amount of circulating ox-LDL and total LDL concentration observed in the diabetic population confirms, in a pro-oxidant condition characterizing diabetic pathology, the high susceptibility of lipoproteins to oxidation and the relevance of hypocholesterolemic therapy. However, the significant reverse correlation between circulating ox-LDL and plasma CoQ10 levels detected in the same population suggests that CoQ10 could play an important role in protecting LDL from oxidation, as is widely demonstrated in literature [,,,].
4. Material and Methods
4.1. Vervet Monkeys: Ethics
In-house bred vervet monkeys (Chlorocebus pygerythrus) were cared for and managed according to the documented standard operating procedures of the Primate Unit and Animal Centre (PUDAC) of the South African Medical Council, and according to the SAMRC Guidelines for the Use of Animals in Research and Training, the National Code for Animal Use in Research, Education, Diagnosis and Testing of Drugs and Related Substances in South Africa, and the Veterinary and Para-Veterinary Professions Act of 1997. Ethical approval numbers 12/03 and 01/16 were obtained from the Ethics Committee for Research on Animals (ECRA) of the South African Medical Research Council.
4.2. Aspalathin-Rich Green Rooibos Extract
In this study, a pharmaceutical certified grade, aspalathin-rich, unfermented rooibos (Afriplex GRT™) extract produced by Afriplex Pharmaceuticals PTY (LTD) (Paarl, South Africa), product code: CPE—03287, Batch Number: 730330, manufactured date: September 2015 and expiry date: August 2017, was used. The extract was previously chemically characterized for its phenolic content and was shown to contain approximately 12% aspalathin []. The selected dose of 90 mg/kg BW was calculated from a dose range (25–300 mg/kg) shown to be effective in rats and extrapolated to monkeys using the method described by Reagan-Shaw et al. [].
4.3. Experimental Design
Eight vervet monkeys (Chlorocebus pygerythrus) (4 male/4 female, age 18 ± 2, BMI 5.0 ± 0.3) on a high-fat, diabetogenic diet (1527 kJ per monkey per day with 14% energy from protein, 43% from fat derived from animal and plant sources (99 mg/day cholesterol with a polyunsaturated/saturated [P/S] fat ratio of 0.3 and 43% from carbohydrates) for at least 5 years prior to selection and six normal controls (4 male/2 female, age 16 ± 1, BMI 4.3 ± 0.3) fed a maize based control diet (1378 kJ per monkey per day with 15% energy from protein, 5% fat as energy (19.7 mg/day cholesterol with a P/S fat ratio of 3.40) and 80% from carbohydrate) were selected and maintained at the Primate Unit (PUDAC) of SAMRC. They had access to water ad lib via an automatic watering device. The respective diets were continued for the duration of the study, including the wash-out period.
Both diabetic and non-diabetic monkeys were treated for four weeks with Afriplex GRT, fed orally to the monkeys at a dose of 90 mg/kg BW with a 30 g bolus of maize three times daily, followed by 1-month wash-out. Their demographic data are presented in Table 1. Blood was collected by femoral venipuncture under ketamine anesthesia at 10 mg/kg body weight intramuscular injection after overnight fasting of at least 14 h. Blood was collected at baseline (before treatment), and during treatment at 2- and 4-weeks, and following a 4 weeks wash-out period. Analysis included the plasma lipid profile, fasting plasma glucose and intravenous glucose tolerance test (IVGTT), glucose stimulated insulin secretion test (GSIST), fasting glucagon levels, plasma CoQ10 levels, CoQ10 oxidative status and oxidized-LDL levels.

Table 1.
Demographic data of vervet monkeys.
4.4. Lipid Profile
Lipid profiles, in terms of total cholesterol, LDL, HDL and triglyceride levels, were determined in plasma by Pathcare Laboratories (N1 City, Cape Town, South Africa) using a Beckman AU5800 analyzer for lipid analyses. Specifically, an enzymatic method (using cholesterol esterase) was used to determine cholesterol concentrations, a coupled enzymatic reaction (using ATP as an agent) was used for triglyceride determination, an enzyme chromogen reaction was used for HDL determination, and a cholesterol esterase/cholesterol oxidize method was used for LDL determination. Results are expressed as mmol/L and as an LDL:HDL ratio.
4.5. Total Plasma CoQ10 Levels and Oxidative Status
CoQ10 content and its oxidized form in plasma were assayed by high performance liquid chromatography (HPLC) with electro-chemical detector (ECD) by Shiseido Co. Ltd. (Tokyo, Japan) as described by Orlando et al. []. Plasma levels of CoQ10 were normalized for total cholesterol, and expressed as nmol of CoQ10/mmol of cholesterol, representing the CoQ10 in plasma mainly associated with LDL. The oxidative status of CoQ10 was reported as percentage of oxidized CoQ10/total CoQ10.
4.6. Circulating Oxidized-LDL
Circulating oxidized-LDL (ox-LDL) was measured in plasma by a sandwich ELISA using mAb-4E6 as a specific monoclonal antibody. The assay was conducted according to the custom protocol (Oxidized LDL ELISA kit, Mercodia, Sweden), and the results are expressed as U/L.
4.7. Glycemic Responses
After drawing the baseline blood samples, 50% dextrose (750 mg/kg BW) was infused via the saphenous vein. Blood samples for glucose were collected into sodium fluoride/potassium oxalate tubes at 0, 5, 10, 15, 20, 40 and 60 min. To assess the glucose stimulated (GS)-insulin response, blood samples were collected at 0, 5, 10, 15, 30 and 60 min into serum separating tubes (SST), and centrifuged at 2000× g for 10 min in a refrigerated centrifuge after clotting for 30 min. Blood for assessing fasting glucagon were collected into ethylenediaminetetraacetic acid (EDTA) tubes. Results are expressed as area under curve (AUC) for IVGTT and GSIST, and as pg/mL for glucagon.
4.8. Statistical Analysis
For data analysis, mean value, standard deviation and standard error of means (SEM) were calculated. All values were presented as means ± SEM. The normal distribution of the data was verified using the Shapiro–Wilk test on the pooled data for each parameter. Homoscedasticity was verified using the Bartlett test, thereby comparing the variance within each group. Both assumptions were verified and therefore a parametric test statistical analysis of the data was performed. The significance of differences between mean values obtained from both experimental groups and before and following Afriplex GRT treatment were evaluated using one way ANOVA with Dunnett post hoc test if significant. A p-value < 0.05 was considered statistically significant, and p < 0.01 was considered highly significant. Pearson’s correlation coefficient was used to evaluate the correlations between ox-LDL and both LDL and CoQ10/Chol plasma levels. Area under the curve values were calculated in GraphPad Prism version 6 using the trapezoid rule.
5. Conclusions
In conclusion, the present study demonstrated that treatment of diabetic vervet monkeys with 90 mg/day/kg of body weight of pharmaceutical-grade aspalathin-enriched green rooibos extract containing 12.8% aspalathin could counteract hyperglycemia, oxidative stress and dyslipidemia, which represent the main cardiovascular risk factors in diabetic pathology. In addition, the cardioprotective role of Afriplex GRT is emphasized by its ability to lower serum LDL levels, preserving CoQ10 biosynthesis and therefore maintaining its important cardiovascular role.
In light of these results, further studies are needed to investigate the mechanism by which aspalathin lowers serum lipids, and to test its efficacy in association with statins for the purposes of achieving a pharmacological target of plasma LDL at lower doses, thereby minimizing the potential toxic side effects of these widely used drugs.
Author Contributions
All the authors have contributed substantially to the present work. In particular: Conceptualization, J.L. and C.J.F.M.; Data curation, P.O.; Formal analysis, P.O.; Investigation, N.C.; Methodology, P.O. and N.C.; Project administration, C.J.F.M.; Software, I.C.; Supervision, J.L.; Validation, L.T. and P.D.; Visualization, L.T.; Writing—original draft, P.O.; Writing—review & editing, C.J.F.M., N.C. and E.J.
Funding
This research was funded by the South African National Research Foundation and the Italian Ministry of Foreign Affairs, under the South Africa-Italy Joint Science and Technology Research Cooperation grant number 92724.
Acknowledgments
This work was supported by the Biomedical Research and Innovation Platform of the South African Medical Research Council (SAMRC) baseline funding and the South African National Research Foundation International Science and Technology Agreements (NRF; grant number 92724). The grant holders acknowledge that opinions, findings, and conclusions or recommendations expressed in any publication generated by the SAMRC or NRF supported research are those of the authors, and that the SAMRC and NRF accepts no liability whatsoever in this regard.
Conflicts of Interest
The authors report no conflicts of interest. All authors are responsible for the content and writing of the paper.
Data Availability
All data used to support the findings of this study are available from the corresponding author upon request.
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas 8th Edition. Available online: http://www.diabetesatlas.org/ (accessed on 2 April 2019).
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Pistrosch, F.; Natali, A.; Hanefeld, M. Is Hyperglycemia a Cardiovascular Risk Factor? Diabetes Care 2011, 34, S128–S131. [Google Scholar] [CrossRef] [PubMed]
- Das Evcimen, N.; King, G.L. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Nadler, J.L. Inflammatory mechanisms of diabetic complications. Curr. Diab. Rep. 2007, 7, 242–248. [Google Scholar] [CrossRef]
- Natarajan, R.; Nadler, J.L. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1542–1548. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 615–625. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Griendling, K.K.; FitzGerald, G.A. Oxidative stress and cardiovascular injury: Part I: Basic mechanisms and in vivo monitoring of ROS. Circulation 2003, 108, 1912–1916. [Google Scholar] [CrossRef]
- Turner, R.C.; Millns, H.; Neil, H.A.; Stratton, I.M.; Manley, S.E.; Matthews, D.R.; Holman, R.R. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998, 316, 823–828. [Google Scholar] [CrossRef]
- Doucet, J.; Le Floch, J.P.; Bauduceau, B.; Verny, C. GERODIAB: Glycaemic control and 5-year morbidity/mortality of type 2 diabetic patients aged 70 years and older: 1. Description of the population at inclusion. Diabetes Metab. 2012, 38, 523–530. [Google Scholar] [CrossRef]
- Burchardt, P.; Żurawski, J.; Zuchowski, B.; Kubacki, T.; Murawa, D.; Wiktorowicz, K.; Wysocki, H. Low-density lipoprotein, its susceptibility to oxidation and the role of lipoprotein-associated phospholipase A2 and carboxyl ester lipase lipases in atherosclerotic plaque formation. Arch. Med. Sci. 2013, 9, 151–158. [Google Scholar] [CrossRef]
- Graham, I.; Atar, D.; Borch-Johnsen, K.; Boysen, G.; Burell, G.; Cifkova, R.; Dallongeville, J.; De Backer, G.; Ebrahim, S.; Gjelsvik, B.; et al. ESC Committee for Practice Guidelines. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary. Atherosclerosis 2007, 194, 1–45. [Google Scholar] [CrossRef]
- Eldor, R.; Raz, I. American Diabetes Association Indications for Statins in Diabetes Is there evidence? Diabetes Care 2009, 32 (Suppl. 2), S384–S391. [Google Scholar] [CrossRef]
- Gibbs, R.A.; Rogers, J.; Katze, M.G.; Bumgarner, R.; Weinstock, G.M.; Mardis, E.R.; Remington, K.A.; Strausberg, R.L.; Venter, J.C.; Wilson, R.K.; et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science 2007, 316, 222–234. [Google Scholar]
- Rudel, L.L.; Reynolds, J.A.; Bullock, B.C. Nutritional effects on blood lipid and HDL-cholesterol concentrations in two subspecies of African green monkeys (Cercopithecus aethiops). J. Lipid Res. 1981, 22, 278–286. [Google Scholar]
- Weight, M.J.; Benade, A.J.S.; Lombard, C.J.; Fincham, J.E.; Marais, M.; Dando, B.; Seier, J.V.; Kritchevsky, D. Low density lipoprotein kinetics in African Green monkeys showing variable cholesterolaemic responses to diets realistic for westernized people. Atherosclerosis 1988, 73, 1–11. [Google Scholar] [CrossRef]
- Martin, S.; Palmour, R.M.; Goldwater, R.; Gutkowsa, J.; Hughes, C.; Hamet, P.; Ervin, F.R. Characterization of a primate model of hypertension. The response of hypertensive and normotensive male vervets (Cercopithecus aethiops) to cold pressor stress, captopril administration, and acute bolus of atrial natriuretic factor. Am. J. Hypertens. 1990, 3, 27–32. [Google Scholar] [CrossRef]
- Suckling, K.; Jackson, B. Animal models of human lipid metabolism. Prog. Lipid Res. 1993, 32, 1–24. [Google Scholar] [CrossRef]
- Fincham, J.E.; Quack, G.; Wuelfroth, O.P.; Benade, A.J. Confirmation of efficacy of etofibrate in nonhuman primates which model human lesion types I-IV. Arzneim.-Forsch./Drug Res. 1996, 46, 519–525. [Google Scholar]
- Fairbanks, L. Alternative old world primate models for non-AIDS research: African green monkeys (vervets). In: Rhesus monkey demands in biomedical research: a workshop report. ILAR J. 2003, 44, 222–235. [Google Scholar]
- Wallace, J.M.; Schwarz, M.; Coward, P.; Houze, J.; Sawyer, J.K.; Kelley, K.L.; Chai, A.; Rudel, L.L. Effects of peroxisome proliferator-activated receptor alpha/delta agonists on HDL-cholesterol in vervet monkeys. J. Lipid Res. 2005, 46, 1009–1016. [Google Scholar] [CrossRef]
- Kruger, M.; Smuts, C.M.; Benadé, A.J.S.; Fincham, J.E.; Lombard, C.J.; Albertse, E.A.; van der Merwe, K.J. Comparison of the effect of the amount and degree of unsaturation of dietary fat on plasma low density lipoproteins in vervet monkeys. Lipids 1992, 27, 733–739. [Google Scholar] [CrossRef]
- Fincham, J.E.; Benade, J.S.; Kruger, M.; Smuts, C.M.; Gobregts, E.; Chalton, D.O.; Kritchevsky, D. Atherosclerosis: Aortic lipid changes induced by diets suggest diffuse disease with focal severity in primates that model human atheromas. Nutrition 1998, 14, 17–22. [Google Scholar] [CrossRef]
- St. Clair, R.W.; Wood, L.L.; Clarkson, T.B. Effect of sucrose polyester on plasma lipids and cholesterol absorption in African Green monkeys with variable hypercholesterolaemic response to dietary cholesterol. Metabolism 1981, 30, 176–183. [Google Scholar] [CrossRef]
- Smuts, C.M.; Kruger, M.; van Jaarsveld, P.J.; Fincham, J.E.; Schall, R.; van der Merwe, K.J.; Benade, A.J.S. The influence of fish oil supplementation on plasma lipoprotein and arterial lipids in vervet monkeys with established atherosclerois. Prostaglandins Leukot. Essent. Fat. Acids 1992, 47, 129–138. [Google Scholar] [CrossRef]
- Kavanagh, K.; Fairbanks, L.A.; Bailey, J.N.; Jorgensen, M.J.; Wilson, M.; Zhang, L.; Rudel, L.L.; Wagner, J.D. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obes. Silver Spring Md. 2007, 15, 1666–1674. [Google Scholar] [CrossRef]
- Van Jaarsveld, P.J.; Benadé, A.J.S. Effect of palm olein oil in a moderate-fat diet on low-density lipoprotein composition in non-human primates. Asia Pac. J. Clin. Nutr. 2002, 11, S416–S423. [Google Scholar] [CrossRef]
- Papanicolas, I.; Woskie, R.L.; Jha, A.K. Health Care Spending in the United States and Other High-Income Countries. JAMA 2018, 319, 1024–1039. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Marnewick, J.; Joubert, E.; Joseph, S.; Swanevelder, S.; Swart, P.; Gelderbolm, W. Inhibition of tumor promotion in mouse skin by extracts of Rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique Southern African herbal teas. Cancer Lett. 2005, 224, 193–202. [Google Scholar] [CrossRef]
- Simon, M.; Horvoska, L.; Greksak, M.; Dusinsky, R.; Nakano, M. Antihemolytic effect of Rooibos tea (Aspalathus linearis) on red cells of Japanese quails. Gen. Physiol. Biophys. 2000, 19, 365–371. [Google Scholar]
- Standley, L.; Winterton, P.; Marnewick, J.L.; Gelderblom, W.C.; Joubert, E.; Britz, I.J. Influence of processing stages on antimutagenic and antioxidant potentials of rooibos tea. J. Agric. Food Chem. 2001, 49, 114–117. [Google Scholar] [CrossRef]
- Snijman, P.W.; Swanevelder, S.; Joubert, E.; Green, I.R.; Gelderblom, W.C. The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): Some dose-response effects on mutagen activation-flavonoid interactions. Mutat. Res. 2007, 631, 111–123. [Google Scholar] [CrossRef]
- Na, H.K.; Mossanda, K.S.; Lee, J.Y.; Surh, Y.J. Inhibition of phorbol ester-induced COX-2 expression by some edible African plants. Biofactors 2004, 21, 149–153. [Google Scholar] [CrossRef]
- Mazibuko, S.E.; Muller, C.J.; Joubert, E.; de Beer, D.; Johnson, R.; Opoku, A.R.; Louw, J. Amelioration of palmitate-induced insulin resistance in C₂C₁₂ muscle cells by rooibos (Aspalathus linearis). Phytomedicine 2013, 20, 813–819. [Google Scholar] [CrossRef]
- Kawano, A.; Nakamura, H.; Hata, S.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 2009, 16, 437–443. [Google Scholar] [CrossRef]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef]
- Kamakura, M.J.S.; de Beer, D.; Joubert, E.; Miura, Y.; Yagasaki, K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-Ay mice. Cytotechnology 2015, 67, 699–710. [Google Scholar] [CrossRef]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef]
- Louw, J.; Woodroof, C.; Seier, J.; Wolfe-Coote, S.A. The effect of diet on the Vervet monkey endocrine pancreas. J. Med. Primatol. 1997, 26, 307–311. [Google Scholar] [CrossRef]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Barter, P. The role of HDL-cholesterol in preventing atherosclerotic disease. Eur. Heart J. Suppl. 2005, 7, F4–F8. [Google Scholar] [CrossRef]
- Wülfroth, P.; Richter, C.M.; Burkard, M. Etofibrate treatment alters low-density lipoprotein susceptibility to lipid peroxidation. Drugs Exp. Clin. Res. 1992, 18, 469–474. [Google Scholar]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Kearney, P.M.; Blackwell, L.; Collins, R.; Keech, A.; Simes, J.; Peto, R.; Armitage, J.; Baigent, C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar]
- Sukhija, R.; Prayaga, S.; Marashdeh, M.; Bursac, Z.; Kakar, P.; Bansal, D.; Sachdeva, R.; Kesan, S.H.; Mehta, J.L. Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J. Investig. Med. 2009, 57, 495–499. [Google Scholar] [CrossRef]
- Littarru, G.P.; Langsjoen, P. Coenzyme Q10 and statins: Biochemical and clinical implications. Mitochondrion 2007, 7, S168–S174. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L.; Belardinelli, R.; Watts, G.F. Coenzyme Q(10), endothelial function, and cardiovascular disease. Biofactors 2011, 37, 366–373. [Google Scholar] [CrossRef]
- Molyneux, S.L.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef]
- Sun, W.; Yan, Y.; Dong, F. Progression of tocotrienols. J. Hyg. Res. 2004, 33, 243–245. [Google Scholar]
- Iqbal, J.; Minhajuddin, M.; Beg, Z.H. Suppression of 7,12-dimethylbenz[alpha]anthracene-induced carcinogenesis and hypercholesterolaemia in rats by tocotrienol-rich fraction isolated from rice bran oil. Eur. J. Cancer Prev. 2003, 12, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.A.; Abbey, M.; Roach, P.D. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and up-regulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis 2007, 193, 86–93. [Google Scholar] [CrossRef]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Beltrãn-Debãn, R.; Rull, A.; Rodríguez-Sanabria, F.; Iswaldi, I.; Herranz-López, M.; Aragonès, G.; Camps, J.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V.; et al. Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice. Phytomedicine 2011, 18, 414–424. [Google Scholar]
- Lagendijk, J.; Ubbink, J.B.; Vermaak, W.J.J. Measurement of the ratio between the reduced and oxidized forms of coenzyme Q10 in human plasma as a possible marker of oxidative stress. Lipid Res. 1996, 37, 67–75. [Google Scholar]
- Menke, T.; Niklowitz, P.; Adam, S.; Weber, M.; Schlüter, B.; Andler, W. Simultaneous detection of ubiquinol-10, ubiquinone-10, and tocopherols in human plasma microsamples and macrosamples as a marker of oxidative damage in neonates and infants. Anal. Biochem. 2000, 282, 209–217. [Google Scholar] [CrossRef]
- Weber, C.; Sejersgård Jakobsen, T.; Mortensen, S.A.; Paulsen, G.; Hølmer, G. Antioxidative effect of dietary coenzyme Q10 in human blood plasma. Int. J. Vitam. Nutr. Res. 1994, 64, 311–315. [Google Scholar]
- Yamamoto, Y.; Yamashita, S. Plasma ratio of ubiquinol and ubiquinone as a marker of oxidative stress. Mol. Asp. Med. 1997, 18, S79–S84. [Google Scholar] [CrossRef]
- Stocker, R.; Bowry, V.W.; Frei, B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does a-tocopherol. Proc. Natl. Acad. Sci. USA 1991, 88, 1646–1650. [Google Scholar] [CrossRef]
- Ernster, L.; Forsmark-Andrèe, P. Ubiquinol: An endogenous antioxidant in aerobic organisms. Clin. Investig. 1993, 71, S60–S65. [Google Scholar] [CrossRef]
- Esterbauer, H.; Wag, G.; Puhl, H. Lipid peroxidation and its role in atherosclerosis. Br. Med. Bull. 1993, 49, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Bowry, V.W.; Stanley, K.K.; Stocker, R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. USA 1992, 89, 10316–10320. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.; Muller, C.; Joubert, E.; Louw, J.; Rosenkranz, B.; Awortwe, C. Inhibitory Interactions of Aspalathus linearis (rooibos) extracts and compounds, aspalathin and z-2-(β-d-Glucopyranosyloxy)-3-phenylpropenoic acid, on cytochromes metabolizing hypoglycemic and hypolipidemic drugs. Molecules 2016, 21, 1515. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Orlando, P.; Silvestri, S.; Brugè, F.; Tiano, L.; Kloting, I.; Falcioni, G.; Polidori, C. High-fat diet-induced met-hemoglobin formation in rats prone (WOKW) or resistant (DA) to the metabolic syndrome: Effect of CoQ10 supplementation. Biofactors 2014, 40, 603–609. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).