Alkaline Extraction, Structural Characterization, and Bioactivities of (1→6)-β-d-Glucan from Lentinus edodes
Abstract
:1. Introduction
2. Results
2.1. Orthogonal Alkaline Extraction of Lentinus Edodes Polysaccharides
2.2. Purification and Chemical Properties of LeP-N2
2.3. Structural Characterization of the β-Glucan LeP-N2
2.3.1. Methylation Analysis
2.3.2. NMR Analysis
2.4. Chain Conformation of the β-Glucan LeP-N2
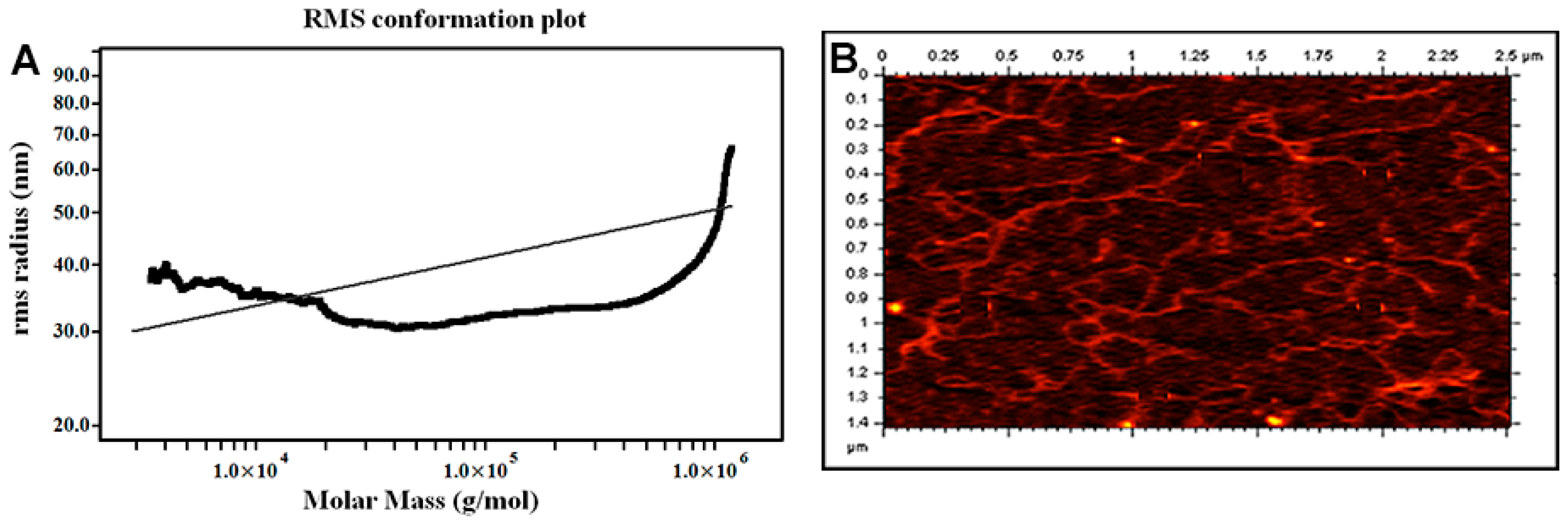
2.4.1. Molecular Weight and Solution Behavior
2.4.2. AFM Intuitive Display
2.5. The Bioactivity Evaluation of the β-Glucan LeP-N2
2.5.1. Assay of Hydroxyl Radical Scavenging Activity
2.5.2. Effects on RAW 264.7 Macrophage Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation and Purification of Lentinus Edodes Polysaccharides
4.3. Chemical Properties and Monosaccharide Composition Analysis
4.4. Methylation Analysis of Lentinus Edodes Polysaccharides
4.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.6. Analysis of Molecular Weights and Chain Conformations
4.7. Atomic Force Microscope (AFM) Visualization
4.8. Hydroxyl Radical Scavenging Activity
4.9. RAW264.7 Cell Line Culture
4.10. Cell Viability Assay
4.11. Nitric Oxide (NO) and Reactive Oxygen Species (ROS) Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pérez-Mendoza, D.; Rodríguez-Carvajal, M.Á.; Romero-Jiménez, L.; Farias, G.D.A.; Lloret, J.; Gallegos, M.T.; Sanjuán, J. Novel mixed-linkage β-glucan activated by c-di-GMP inSinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 2015, 112, E757–E765. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef]
- Misaki, A.; Kakuta, M. Kikurage (Tree-ear) and Shirokikurage (white Jelly-leaf): Auricularia auricula and Tremella fuciformis. Food Res. Int. 1995, 11, 211–218. [Google Scholar] [CrossRef]
- Ohno, N.; Saito, K.; Nemoto, J.; Kaneko, S.; Adachi, Y.; Nishijima, M.; Miyazaki, T.; Yadomae, T. Immunopharmacological characterization of a highly branched fungal (1→3)-β-d-glucan, OL-2, isolated from Omphalia lapidescens. Biol. Pharm. Bull. 1993, 16, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget. 2017, 8, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Samanta, S.; Nandi, A.K.; Sen, I.K.; Paloi, S.; Acharya, K.; Islam, S.S. Structure elucidation and antioxidant properties of a soluble beta-D-glucan from mushroom Entoloma lividoalbum. Int. J. Biol. Macromol. 2014, 63, 140–149. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Maji, P.K.; Paloi, S.; Devi, K.S.; Acharya, K.; Maiti, T.K.; Islam, S.S. Structural, immunological, and antioxidant studies of beta-glucan from edible mushroom Entoloma lividoalbum. Carbohydr. Polym. 2015, 123, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Chihara, G.; Maeda, Y.; Hamuro, J.; Sasaki, T.; Fukuoka, F. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) sing. Nature 1969, 222, 687–688. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. d-glucans from edible mushrooms: A review on the extraction, purification and chemical characterization approaches. Carbohydr. Polym. 2015, 117, 753–761. [Google Scholar] [CrossRef]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm. 2005, 2, 63–80. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Alquini, G.; Tadra-Sfeir, M.Z.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J. Agaricus bisporus and Agaricus brasiliensis (1→6)-β-d-glucans show immunostimulatory activity on human THP-1 derived macrophages. Carbohydr. Polym. 2013, 94, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Y.; Wang, S.; Xu, X.; Zhang, L. Determination of the triple helical chain conformation of beta-glucan by facile and reliable triple-detector size exclusion chromatography. J. Phys. Chem. B. 2014, 118, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef]
- Villares, A.; García-Lafuente, A.; Guillamón, E.; Mateo-Vivaracho, L. Separation and characterization of the structural features of macromolecular carbohydrates from wild edible mushrooms. Bioact. Carbohydr. Diet. Fibre. 2013, 2, 15–21. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Li, Q.; Zhang, Q.; You, R.; Cheng, Y.; Luo, L.; Zhang, Y. Structural differences and conformational characterization of five bioactive polysaccharides from Lentinus edodes. Food Res. Int. 2014, 62, 223–232. [Google Scholar] [CrossRef]
- Ukawa, Y.; Ito, H.; Hisamatsu, M. Antitumor effects of (1→3)-β-d-glucan and (1→6)-β-d-glucan purified from newly cultivated mushroom, Hatakeshimeji (Lyophyllum decastes Sing.). J. Biosci. Bioeng. 2000, 90, 98–104. [Google Scholar] [CrossRef]
- Yan, J.; Han, Z.; Qu, Y.; Yao, C.; Shen, D.; Tai, G.; Cheng, H.; Zhou, Y. Structure elucidation and immunomodulatory activity of a β-glucan derived from the fruiting bodies of Amillariella mellea. Food Chem. 2018, 240, 534–543. [Google Scholar] [CrossRef]
- Jeff, I.B.; Yuan, X.; Sun, L.; Kassim, R.M.; Foday, A.D.; Zhou, Y. Purification and in vitro anti-proliferative effect of novel neutral polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2013, 52, 99–106. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.D.; Monteiro, N.K.; Dekker, R.F.H.; Barbosa, A.M.; Carbonero, E.R.; Silveira, J.L.M.; Sassaki, G.L.; Da Silva, R.; de Lourdes Corradi Da Silva, M. Three exopolysaccharides of the β-(1→6)-d-glucan type and a β-(1→3;1→6)-d-glucan produced by strains of Botryosphaeria rhodina isolated from rotting tropical fruit. Carbohydr. Res. 2008, 343, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y. Method for Separation and Purification of Lentinan for Drug Use. Chinese Patent 2006–10031910, 2006. [Google Scholar]
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1→3)-β-d-glucans is dependent on the triple helix conformation. Carbohydr Res. 2000, 329, 587–596. [Google Scholar] [CrossRef]
- Sasaki, T.; Takasuka, N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Chen, C.; Wu, W.; Xu, X.; Zhang, L.; Liu, Y.; Wang, K. Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 2014, 105, 308–316. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.; Zhang, L.; Fang, Y.; Jiang, F.; Phillips, G.O. Structure and chain conformation of water-soluble heteropolysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2011, 86, 844–851. [Google Scholar] [CrossRef]
- Wu, D.T.; Meng, L.Z.; Wang, L.Y.; Lv, G.P.; Cheong, K.L.; Hu, D.J.; Guan, J.; Zhao, J.; Li, S.P. Chain conformation and immunomodulatory activity of a hyperbranched polysaccharide from Cordyceps sinensis. Carbohydr. Polym. 2014, 110, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Zhang, L. Thermally Induced Conformation Transition of Triple-Helical Lentinan in NaCl Aqueous Solution. J. Phys. Chem. B. 2008, 112, 10343–10351. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Miao, S.; Zhang, Y.; Lin, S.; Jian, Y.; Tian, Y.; Zheng, B. Isolation, preliminary structural characterization and hypolipidemic effect of polysaccharide fractions from Fortunella margarita (Lour.) Swingle. Food Hydrocolloid. 2016, 52, 126–136. [Google Scholar] [CrossRef]
- Zhao, W.; Chai, D.; Li, H.; Chen, T.; Tang, Y. Significance of metal ion supplementation in the fermentation medium on the structure and anti-tumor activity of Tuber polysaccharides produced by submerged culture of Tuber melanosporum. Process Biochem. 2014, 49, 2030–2038. [Google Scholar] [CrossRef]
- Nandi, A.K.; Samanta, S.; Maity, S.; Sen, I.K.; Khatua, S.; Devi, K.S.; Acharya, K.; Maiti, T.K.; Islam, S.S. Antioxidant and immunostimulant beta-glucan from edible mushroom Russula albonigra (Krombh.) Fr. Carbohydr. Polym. 2014, 99, 774–782. [Google Scholar] [CrossRef]
- Pattanayak, M.; Samanta, S.; Maity, P.; Sen, I.K.; Nandi, A.K.; Manna, D.K.; Mitra, P.; Acharya, K.; Islam, S.S. Heteroglycan of an edible mushroom Termitomyces clypeatus: Structure elucidation and antioxidant properties. Carbohydr. Res. 2015, 413, 30–36. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Leng, B.; Liu, X.D.; Chen, Q.X. Inhibitory effects of anticancer peptide from Mercenaria on the BGC-823 cells and several enzymes. FEBS Lett. 2005, 579, 1187–1190. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Xue, C.; Dong, P.; Sheng, W.; Yu, G.; Chai, W. Sequence determination of a non-sulfated glycosaminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandem mass spectrometry and NMR spectroscopy. Glycoconj. J. 2008, 25, 481–492. [Google Scholar] [CrossRef]
- Anumula, K.R.; Taylor, P.B. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 1992, 203, 101–108. [Google Scholar] [CrossRef]
- The CCRC Spectral Database for PMAA’s. Available online: https://www.ccrc.uga.edu/specdb/ms/pmaa/pframe.html. (accessed on 18 April 2019).
- Zhang, X.; Zhao, X.L.; Lang, Y.Z.; Li, Q.Y.; Liu, X.X.; Cai, C.; Hao, J.J.; Li, G.Y.; Yu, G.L. Low anticoagulant heparin oligosaccharides as inhibitors of BACE-1, the Alzheimer’s beta-secretase. Carbohydr. Polym. 2016, 151, 51–59. [Google Scholar] [CrossRef]
- Zhao, X.L.; Lv, Y.J.; Li, M.M.; Li, G.S.; Wang, Y.F.; Yu, G.L. Extraction Process of Polysaccharides from Pyrus betulifolia Bge. and its free radical scavenging Activity. J. Food Sci. Biotechnol. 2012, 31, 276–282. [Google Scholar]
- Yang, Y.; Zhao, X.L.; Li, J.; Jiang, H.; Shan, X.D.; Wang, Y.; Ma, W.B.; Hao, J.J.; Yu, G.L. A β-glucan from Durvillaea Antarctica has immunomodulatory effects on RAW264.7 macrophages via toll-like receptor 4. Carbohydr. Polym. 2018, 191, 255–265. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound LeP-N2 is available from the authors. |





| Methylated Alditol Acetates Derivative a | Retention Time (min) | Type of Linkage | Relative Abundance (%) | Mass Fragments (m/z) (Relative Abundance, %) |
|---|---|---|---|---|
| 2,3,4,6-Me4-Glc | 14.2 | Glcp-(1→ | 13.2 | 59,71,87,102,118,129,145,162,175,205 |
| 2,4,6-Me3-Glc | 15.1 | →3)-Glcp-(1→ | 10.9 | 59,71,87,101,118,129, 161,174,191,234 |
| 2,3,4-Me3-Glc | 15.7 | →6)-Glcp-(1→ | 63.1 | 59,71,87,99,102,118,129,162, 189,233 |
| 2,4-Me2-Glc | 18.1 | →3,6)-Glcp-(1→ | 12.8 | 59,74,87,101,118,129,139,160,189,234 |
| Residues | δ13C a/1H b (ppm) | |||||
|---|---|---|---|---|---|---|
| C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 | |
| A β-d-Glcp (→1 | 104.08/4.36 | 73.72/3.06 | 76.55/3.17 | 70.02/3.01 | 76.42/3.16 | 60.85/3.42, 3.65 |
| B →3)-β-d-Glcp-(1→ | 103.42/4.34 | 73.09/3.19 | 87.25/3.35 | 68.9/3.15 | 76.55/3.11 | 59.73/3.35, 3.48 |
| C →6)-β-d-Glcp(1→ | 103.42/4.22 | 73.69/2.98 | 76.55/3.14 | 70.02/3.12 | 75.73/3.29 | 68.82/3.55, 3.95 |
| D →3, 6)-β-d-Glcp-(1→ | 103.26/4.32 | 72.91/3.20 | 87.1/3.39 | 68.26/3.27 | 75.73/3.30 | 68.82/3.55, 3.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cai, C.; Zheng, M.; Hao, J.; Wang, Y.; Hu, M.; Fan, L.; Yu, G. Alkaline Extraction, Structural Characterization, and Bioactivities of (1→6)-β-d-Glucan from Lentinus edodes. Molecules 2019, 24, 1610. https://doi.org/10.3390/molecules24081610
Li J, Cai C, Zheng M, Hao J, Wang Y, Hu M, Fan L, Yu G. Alkaline Extraction, Structural Characterization, and Bioactivities of (1→6)-β-d-Glucan from Lentinus edodes. Molecules. 2019; 24(8):1610. https://doi.org/10.3390/molecules24081610
Chicago/Turabian StyleLi, Jia, Chao Cai, Mengmeng Zheng, Jiejie Hao, Ya Wang, Minghua Hu, Luodi Fan, and Guangli Yu. 2019. "Alkaline Extraction, Structural Characterization, and Bioactivities of (1→6)-β-d-Glucan from Lentinus edodes" Molecules 24, no. 8: 1610. https://doi.org/10.3390/molecules24081610
APA StyleLi, J., Cai, C., Zheng, M., Hao, J., Wang, Y., Hu, M., Fan, L., & Yu, G. (2019). Alkaline Extraction, Structural Characterization, and Bioactivities of (1→6)-β-d-Glucan from Lentinus edodes. Molecules, 24(8), 1610. https://doi.org/10.3390/molecules24081610








