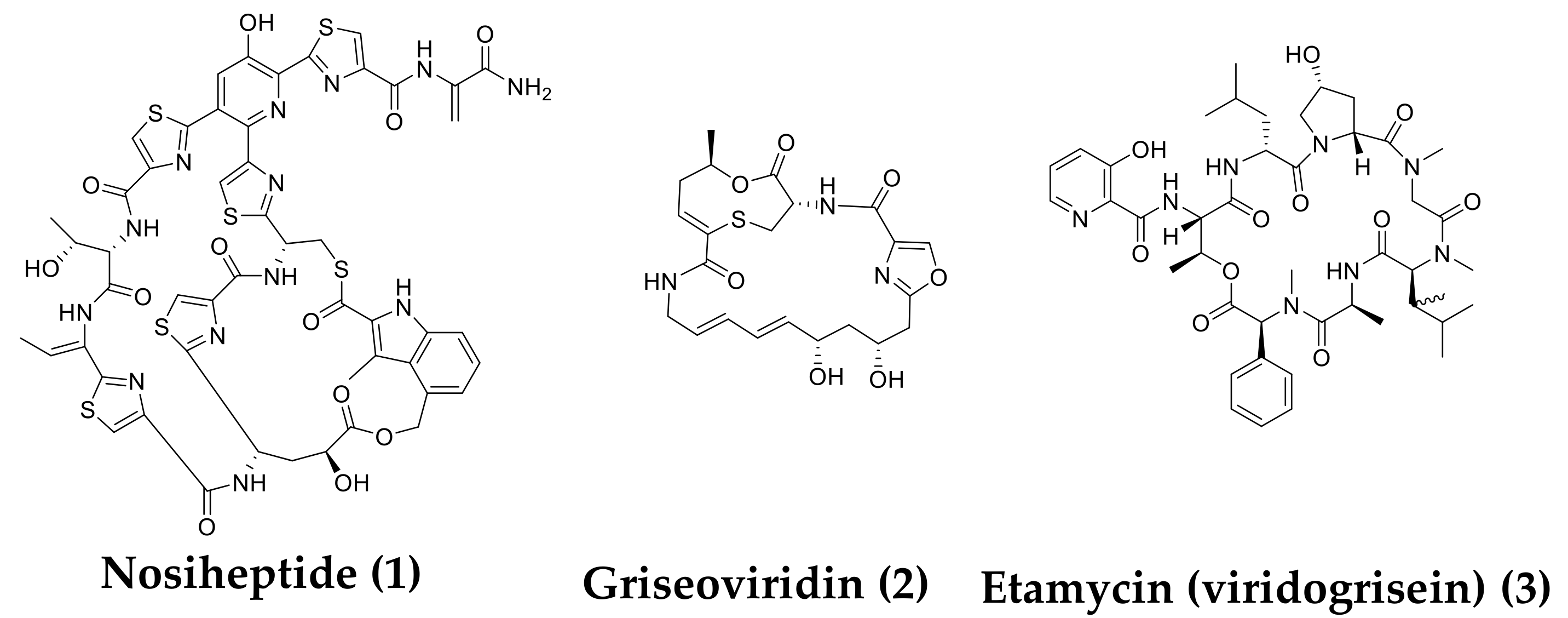
Discovery of Nosiheptide, Griseoviridin, and Etamycin as Potent Anti-Mycobacterial Agents against Mycobacterium avium Complex
Abstract
:1. Introduction
2. Results
2.1. In Vitro Anti-Mycobacterial Activities of 1–3
2.2. Combination Effect of Streptogramins
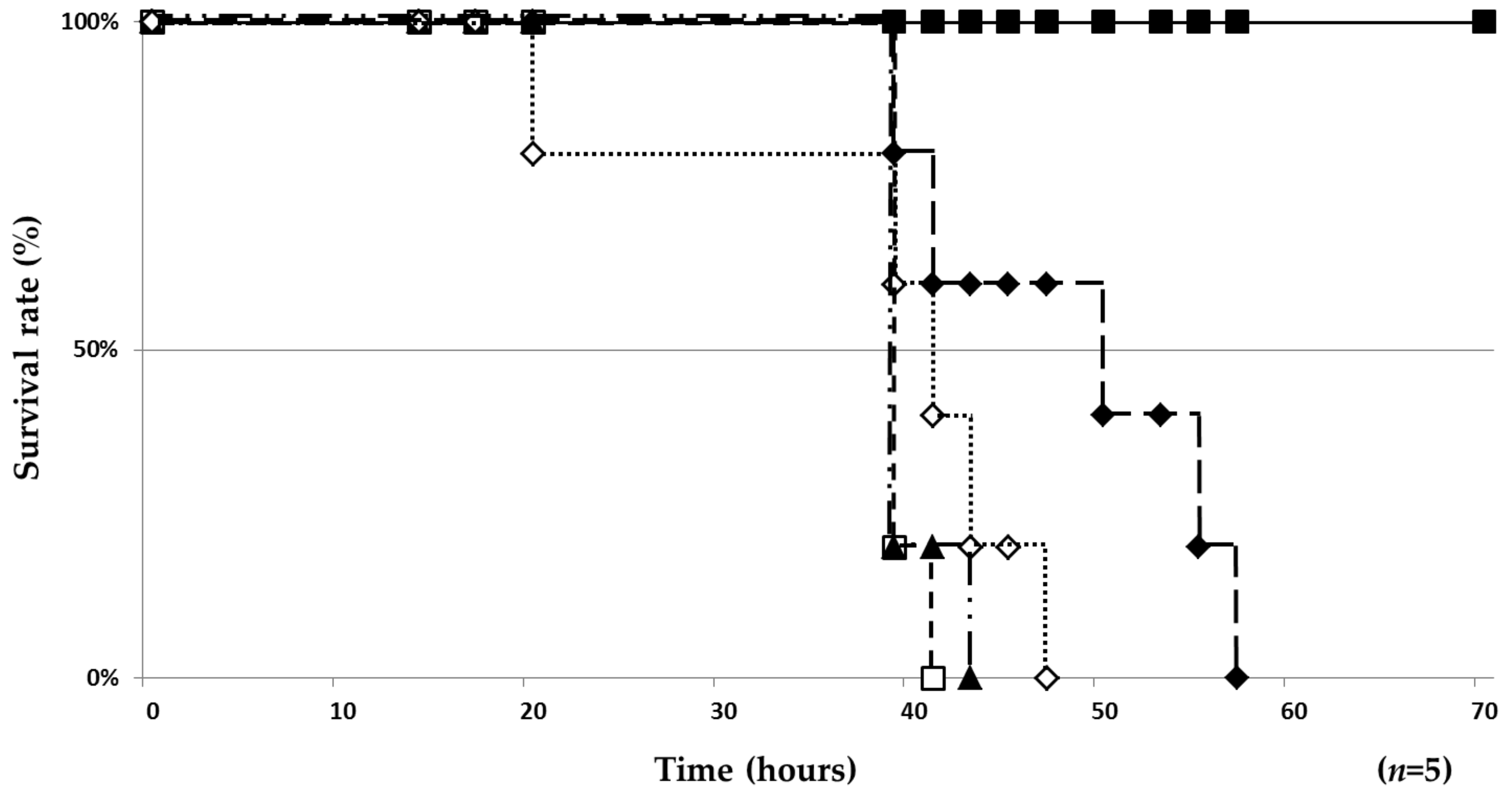
2.3. Therapeutic Effect of 1 and 2/3 in a Silkworm Infection Model
3. Discussion
4. Materials and Methods
4.1. Assay for Anti-Mycobacterial Activity
4.2. Isolation of 1–3
4.3. Measurement of FIC Indexes
4.4. Silkworm Infection Assay with M. smegmatis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care. Med. 2012, 185, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Young, L.S. Nontuberculous Mycobacterial Infections: A Clinical Review. Infection 2004, 32, 257–270. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care. Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Adelman, M.H.; Addrizzo-Harris, D.J. Management of nontuberculous mycobacterial pulmonary disease. Curr. Opin. Pulm. Med. 2018, 24, 212–219. [Google Scholar] [CrossRef]
- Pascard, C.; Ducruix, A.; Lunel, J.; Prangé, T. Highly modified cysteine-containing antibiotics. Chemical structure and configuration of nosiheptide. J. Am. Chem. Soc. 1977, 99, 6418–6423. [Google Scholar] [CrossRef]
- Ames, D.E.; Bowman, R.E.; Cavalla, J.F.; Evans, D.D. Griseoviridin. Part I. J. Chem. Soc. 1955, 0, 4260–4264. [Google Scholar] [CrossRef]
- Bartz, Q.R.; Standiford, J.; Mold, J.D.; Johannessen, D.W.; Ryder, A.; Maretzki, A.; Haskell, T.H. Griseoviridin and viridogrisein: isolation and characterization. Antibiotics. Annual. 1955, 2, 777–783. [Google Scholar]
- Kairo, S.K.; Bedwell, J.; Tyler, P.C.; Carter, A.; Corbel, M.J. Development of a tetrazolium salt assay for rapid determination of viability of BCG vaccines. Vaccine 1999, 17, 2423–2328. [Google Scholar] [CrossRef]
- Noeske, J.; Huang, J.; Olivier, N.B.; Giacobbe, R.A.; Zambrowski, M.; Cate, J.H. Synergy of Streptogramin Antibiotics Occurs Independently of Their Effects on Translation. Antimicrob. Agents. Chemother. 2014, 58, 5269–5279. [Google Scholar] [CrossRef]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining Fractional Inhibitory Concentration Index Cutoffs for Additive Interactions Based on Self-Drug Additive Combinations, Monte Carlo Simulation Analysis, and In Vitro-In Vivo Correlation Data for Antifungal Drug Combinations against Aspergillus fumigatus. Antimicrob. Agents. Chemother. 2010, 54, 602–609. [Google Scholar] [PubMed]
- Yagi, A.; Uchida, R.; Hamamoto, H.; Sekimizu, K.; Kimura, K.; Tomoda, H. Anti-Mycobacterium activity of microbial peptides in a silkworm infection model with Mycobacterium smegmatis. J. Antibiot. 2017, 70, 685–690. [Google Scholar]
- Haste, N.M.; Thienphrapa, W.; Tran, D.N.; Loesgen, S.; Sun, P.; Nam, S.J.; Jensen, P.R.; Fenical, W.; Sakoulas, G.; Nizet, V.; et al. Activity of the thiopeptide antibiotic nosiheptide against contemporary strains of methicillin-resistant Staphylococcus aureus. J. Antibiot. 2012, 65, 593–598. [Google Scholar] [CrossRef]
- Tadeusz, K.; Zuzanna, K.G.; Wlodzimierz, K. Antibiotics: Origin, Nature and Properties; Elsevier: Amsterdam, The Netherlands, 1967; Volume 1, pp. 351–356. ISBN 9781483223056. [Google Scholar]
- Hamamoto, H.; Kurokawa, K.; Kaito, C.; Kamura, K.; Manitra, R.I.; Kusuhara, H.; Santa, T.; Sekimizu, K. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents. Chemother. 2004, 48, 774–779. [Google Scholar] [CrossRef]
- Benazet, F.; Cartier, J.R. Effect of Nosiheptide as a Feed Additive in Chicks on the Quantity, Duration, Prevalence of Excretion, and Resistance to Antibacterial Agents of Salmonella typhimurium; On the Proportion of Escherichia coli and other Coliforms Resistant to Antibacterial Agents; and on Their Degree and Spectrum of Resistance. Poult. Sci. 1980, 59, 1405–1415. [Google Scholar]
- Allington, D.R.; Rivey, M.P. Quinupristin/dalfopristin: A therapeutic review. Clin. Ther. 2001, 23, 24–44. [Google Scholar] [CrossRef]
- Koyama, N.; Kojima, S.; Nonaka, K.; Masuma, R.; Matsumoto, M.; Omura, S.; Tomoda, H. Calpinactam, a new anti-mycobacterial agent, produced by Mortierella alpina FKI-4905. J. Antibiot. 2010, 63, 183–186. [Google Scholar] [CrossRef]
- Haste, N.M.; Perera, V.R.; Maloney, K.N.; Tran, D.N.; Jensen, P.; Fenical, W.; Nizet, V.; Hensler, M.E. Activity of the streptogramin antibiotic etamycin against methicillin-resistant Staphylococcus aureus. J. Antibiot. 2010, 64, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Wojts, K.P.; Riedrich, M.; Lu, J.Y.; Winter, P.; Winkler, T.; Walter, S.; Arndt, H.D. Total synthesis of nosiheptide. Angew. Chem. Int. Ed. Engl. 2016, 55, 9772–9776. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, Q.; Song, Y.; Ma, J.; Ju, J. Involvement of SgvP in carbon-sulfur bond formation during Griseoviridin biosynthesis. Chembiochem 2014, 15, 1183–1189. [Google Scholar] [CrossRef]
- Tominaga, T.; Uchida, R.; Koyama, N.; Tomoda, H. Anti-Rhizopus activity of tanzawaic acids produced by the hot spring-derived fungus Penicillium sp. BF-0005. J. Antibiot. 2018, 71, 626–632. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the 1–3 are available from the corresponding authors. |
| Test Microorganism | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | CAM | RFP | EB | |
| Mycobacterium avium JCM15430 | 0.024 | 1.56 | 0.097 | 0.19 | 0.78 | 12.5 |
| Mycobacterium intracellulare JCM6384 | 0.024 | 1.56 | 0.19 | 0.024 | 0.012 | 3.12 |
| Mycobacterium smegmatis M341 | 6.25 | >100 | 25 | 15.6 | 1.56 | 0.78 |
| Mycobacterium bovis BCG Pasteur | 0.012 | 6.25 | 0.78 | 0.12 | 0.012 | 1.56 |
| In Combination with 3 | MIC of 2 (FIC Index *) Against | |
|---|---|---|
| M. avium JCM15430 | M. intracellulare JCM6384 | |
| (µg/mL) | (µg/mL) | |
| 0 | 1.56 | 1.56 |
| +0.007 | 0.15 (0.078) | 0.078 (0.093) |
| +0.015 | 0.039 (0.046) | 0.078 (0.15) |
| +0.031 | 0.019 (0.070) | 0.039 (0.26) |
| Test Microorganism | MIC (µg/mL) | |
|---|---|---|
| 2+3 (1:1) | 2+3 (3:7) | |
| M. avium JCM15430 | 0.024 | 0.097 |
| M. intracellulare JCM6384 | 0.048 | 0.097 |
| M. smegmatis M341 | 6.25 | 6.25 |
| M. bovis BCG Pasteur | 0.024 | 0.012 |
| Test Compound | ED50 (µg/larva) 1 | MIC (µg/mL) | ED50/MIC |
|---|---|---|---|
| 1 | >50 | 6.25 | >8 |
| 2/3 | 35.4 | 6.25 | 5.6 |
| EB | 35.4 | 0.78 | 45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoda, K.; Koyama, N.; Kanamoto, A.; Tomoda, H. Discovery of Nosiheptide, Griseoviridin, and Etamycin as Potent Anti-Mycobacterial Agents against Mycobacterium avium Complex. Molecules 2019, 24, 1495. https://doi.org/10.3390/molecules24081495
Hosoda K, Koyama N, Kanamoto A, Tomoda H. Discovery of Nosiheptide, Griseoviridin, and Etamycin as Potent Anti-Mycobacterial Agents against Mycobacterium avium Complex. Molecules. 2019; 24(8):1495. https://doi.org/10.3390/molecules24081495
Chicago/Turabian StyleHosoda, Kanji, Nobuhiro Koyama, Akihiko Kanamoto, and Hiroshi Tomoda. 2019. "Discovery of Nosiheptide, Griseoviridin, and Etamycin as Potent Anti-Mycobacterial Agents against Mycobacterium avium Complex" Molecules 24, no. 8: 1495. https://doi.org/10.3390/molecules24081495
APA StyleHosoda, K., Koyama, N., Kanamoto, A., & Tomoda, H. (2019). Discovery of Nosiheptide, Griseoviridin, and Etamycin as Potent Anti-Mycobacterial Agents against Mycobacterium avium Complex. Molecules, 24(8), 1495. https://doi.org/10.3390/molecules24081495




