Abstract
Gomisin D, a lignan compound isolated from Fructus Schisandra, is a potential antidiabetic and anti-Alzheimer’s agent. Recently, gomisin D was used as a quality marker of some traditional Chinese medicine (TCM) formulas. In this study, a rapid ultra-performance liquid chromatography/tandem mass spectrometry method (UPLC-MS/MS) was developed and validated to quantify gomisin D in rat plasma for a pharmacokinetic and bioavailability study. Acetonitrile was used to precipitate plasma proteins. Separations were performed on a BEH C18 column with a gradient mobile phase comprising of acetonitrile and water (0.1% formic acid). An electrospray ionization source was applied and operated in the positive ion mode. The multiple reaction monitoring mode (MRM) was utilized to quantify gomisin D and nomilin (internal standard, IS) using the transitions of m/z 531.2 → 383.1 and m/z 515.3 → 161.0, respectively. The calibration curve was linear over the working range from 1 to 4000 ng/mL (R2 = 0.993). The intra- and interday precision ranged from 1.9% to 12.9%. The extraction recovery of gomisin D was in the range of 79.2–86.3%. The validated UPLC-MS/MS method was then used to obtain the pharmacokinetic characteristics of gomisin D after intravenous (5 mg/kg) and intragastric (50 mg/kg) administration to rats. The bioavailability of gomisin D was 107.6%, indicating that this compound may become a promising intragastrical medication. Our results provided useful information for further preclinical studies on gomisin D.
1. Introduction
Fructus Schisandrae is a Chinese folk herb with a variety of pharmacological activities, including antihepatotoxic, antihyperlipidemic, antiasthmatic, hypoglycemic, and antigastric ulcer activities [1,2,3,4,5,6,7,8,9]. What is more, Fructus Schisandrae is frequently used in combination chemotherapy regimens with other drugs for schizophrenia patients in order to lower side effects and improve therapeutic efficacy [10,11]. Modern pharmacological studies indicate that most of the pharmacological and biological actions of Fructus Schisandrae can be attributed to lignans, which account for approximately 1% of the fruits’ composition and consist of over 100 related compounds [12,13]. Gomisin D is a lignan found in Fructus Schisandra and has been demonstrated to have an antidiabetic effect and to inhibit of UDP-Glucuronosyltransferases activity [8,14,15]. In addition, gomisin D is able to scavenge ABTS (+) radicals [16] and treat Alzheimer’s disease [17,18,19]. Recently, gomisin D is used as a quality marker of Shengmai San [18] and shenqi Jiangtang Granule [15]. However, even with the hot research on pharmacological activity, there is little information about the pharmacokinetic characteristics and bioavailability of monomer’s gomisin D. Therefore, it is necessary to develop a rapid analytical method for the determination of gomisin D in biological matrix for its further clinical application and pharmacological studies.
Some high-performance liquid chromatography (HPLC) methods had been developed to determine gomisin D in different matrices. Smejkal et al. and Shi et al. determine gomisin D from Fructus Schisandrae by HPLC with diode array detector (DAD) [13,20]. Mocan et al. reported that gomisin D was identified and determined from Schisandra chinensis (Turcz.) Baill by using LC-DAD-QTOF-MS [16]. Zhang et al. establishs an ultra-high-performance liquid chromatography coupled with mass spectrometry (UPLC-MS/MS) method to evaluate the quality of Fructus Schisandrae through simultaneous qualitative and quantitative analysis of lignans [21]. Recently, in order to investigates the pharmacokinetic profiles of seven lignans, Sun et al. developed an HPLC-MS/MS method to determine gomisin D in rat plasma after intragastrical administration of Schisandra chinensis extract [19]. The retention time is >15 min and the linear range is 1.95–78.1 ng/mL. The maximum and minimum plasma concentrations of gomisin D are <12 µg/mL and >3 µg/mL, respectively. The concentration ranges are far greater than the linear ranges of this method. Su et al. also developed an UPLC-MS/MS method for the simultaneous determination of 12 lignans in rat plasma after administration of wine-processed Schisandra Chinensis fructus (WPSCF) extract [22]. The retention time of gomisin D is >11 min and the linear range is 5.0–625.0 ng/mL. Obviously, the method with a longer retention time and narrower linear ranges was not suitable for pharmacokinetics of gomisin D. In addition, up to now, no pharmacokinetic study of a monomer of gomisin D has been performed, which hinders its clinical application. In this work, a wider linear and rapid UPLC-MS/MS method was developed to determine gomisin D in rat plasma, and was successfully applied to investigate the pharmacokinetics and bioavailability of gomisin D in rats after i.v. and i.g. administration.
2. Experimental
2.1. Materials
Gomisin D (purity ≥98%) and nomilin (purity ≥98%) as an internal standard (IS) were purchased from Chengdu Munster Biotechnology Co., Ltd. (Chengdu, China) (Figure 1). Formic acid (Sigma Aldrich, St. Louis, MO, USA), methanol and acetonitrile (Merck, Darmstadt, Germany) were of HPLC grade. Ultrapure water (18.2 mΩ) was prepared from a Milli-Q system (Millipore, Bedford, MA, USA). All other reagents were HPLC or analytical grade.
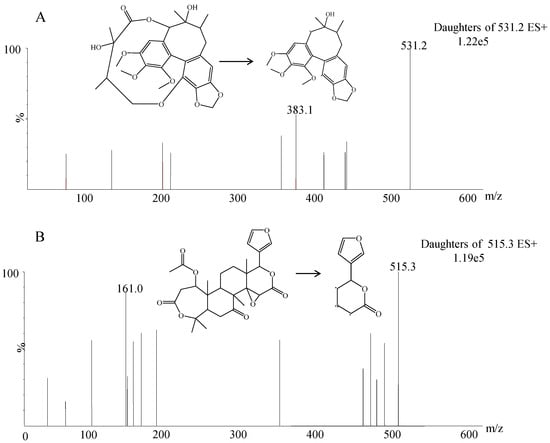
Figure 1.
Mass spectra of gomisin D (A) and nomilin (B) in scan mode with an electrospray ionization (ESI) (+) source.
2.2. Instruments and Conditions
All samples were analyzed on a Waters Acquity-UPLC (Water, Milford, MA, USA). The temperature of the autosampler was set at 4 °C. Separations was performed on an ACQUITY UPLC® BEH C18 (2.1 × 50 mm, 1.7 µm) column at temperature of 35 °C. The gradient mobile phases consisted of solvent A (0.1% formic acid) and solvent B (acetonitrile) as follows: (1) held at 10% B (0 min to 0.2 min); (2) increased from 10% to 80% B (0.2 min to 1.5 min); (3) held at 80% B (1.5 min to 2 min); (4) decreased from 80% to 10% B (2 min to 2.2 min); (5) held at 10% B (2.2 min to 4 min). The flow rate and injection volume were 0.4 mL/min and 3 µL, respectively.
The ESI source was operated in positive ion mode. The optimized mass spectrometer parameters were set as follows: capillary voltage, 1.93 kV; and desolvation temperature, 600 °C. Argon was used as a collision gas. Nitrogen was used as desolvation gas (1000 L/h) and cone gas (50 L/h). The optimal collision energies of gomisin D and IS were 18 and 32 V, respectively. The cone energy was set at 6 V for gomisin D and 35 V for IS. The ion transitions of MRM were m/z 531.2 → 383.1 and m/z 515.3 → 161.0 for gomisin D and IS, respectively.
2.3. Preparation of Standard and Quality Control Samples
Stock solutions of gomisin D and nomilin were respectively prepared at a precise concentration of 700.0 µg/mL in methanol and were then serially diluted with methanol to obtain standard working solutions of desired concentrations. Calibration standard solutions were prepared by adding the working solutions into blank rat plasma. Gomisin D was set at concentrations of 1, 2, 5, 10, 20, 50, 100, 200, 500, 1000, and 4000 ng/mL. The quality control (QC) samples with four levels (1, 2, 50, 3200 ng/mL) were prepared by spiking 10 µL of gomisin D working solution into 100 µL of blank rat plasma. All the solutions were stored at 4 °C before use.
2.4. Animal Experiments
Twenty-four male Sprague–Dawley rats (250 ± 20 g) were obtained from Laboratory Animal Center of Wenzhou Medical University (Wenzhou, China). All experimental procedures were complied with the “Principles of Laboratory Animal Care” and approved by the Animal Ethics Committee in Wenzhou Medical University (20 July 2018, no: 2018-257). The rats were fasted for 12 h before administration but had free access to water. Twelve rats were intravenously administrated with 5 mg/kg gomisin D, and about 0.25 mL of blood samples were collected at 0.083, 0.25, 0.5, 1, 2, 4, 8, 12, 24 h after administration. Another 12 rats were intragastrically administrated with 50 mg/kg gomisin D, and about 0.25 mL of blood samples were collected at 0.167, 0.333, 0.667, 1, 2, 4, 8, 12, 24, 30 h after administration. All blood samples were taken from rat tail vein and were placed into 1.5 mL heparinized tubes. After centrifugation at 3500 rpm and 4 °C for 10 min, the supernatant was collected and frozen at −20 °C until analysis.
2.5. Sample Preparation
The frozen plasma samples were placed at room temperature and thawed before analysis. One hundred μL of plasma sample was firstly transferred to a 1.5 mL polypropylene tube together with 10 μL of IS solution. Then, 290 μL of acetonitrile was added, and vortexed for 2 min to remove protein. After centrifuging at 11,000× g for 10 min, the supernatant (80 μL) was carefully transferred to an UPLC vial for analysis.
2.6. Method Validation
The validation process of the proposed bioanalytical method complied with the latest guidelines set by the US Food and Drug Administration (FDA) guidelines [23]. The method validation items included selectivity, linearity, precision, accuracy, matrix effect, extraction recovery, and stability.
3. Results and Discussion
3.1. Method Development
ESI positive and negative modes were both compared and evaluated in method development [24,25,26]. In this work, the response of the positive ion mode showed a better signal-to-noise ratio than that of the negative ion mode. The most abundant fragment ions for MRM was m/z 531.2 → 383.1 and m/z 515.3 → 161.0 for gomisin D and IS. The ionic response intensity of m/z 531.2 → 383.1 was five times higher than that of 531.2 → 401.1 reported in literature [19,22]. In the optimization of UPLC conditions, we found that the mobile phases had an important role in obtaining good chromatographic performances. Acetonitrile and 0.1% formic acid were selected as the mobile phases because they provided sharper peak shapes and shorter retention time. The retention time of 2.29 min was much shorter than that of >11 min reported in literature [19,22]. To find a proper IS, several compounds, including eupatilin, psoralidin, alpinetin, and nomilin, were evaluated. Nomilin was selected as IS because of its similar retention time and appropriate mass response in positive ion ESI mode. Acetonitrile was selected for one-step protein precipitation because it showed a simpler and better effect than other solvents.
3.2. Method Validation
Selectivity of gomisin D was evaluated at the retention times of the typical chromatograms of blank plasma, blank plasma spiked with gomisin D and IS, and plasma samples after administration. Figure 2 shows there was no significant peak interference at the retention times of gomisin D (2.30 min) and IS (2.19 min), suggesting that no endogenous substances significantly affected the ionization of gomisin D. The calibration curves were linear from 1 to 4000 ng/mL (R2 = 0.993). The LOD and LLOQ were 0.3 ng/mL and 1 ng/mL, respectively. The extraction recovery of gomisin D was greater than 79.2%. The matrix effect of gomisin D was 80.9–86.7%. The detailed extraction recovery and matrix effect of gomisin D are shown in Table 1. To evaluate the precision (RSD) and accuracy (RE) of the method, RSD and RE for QCs at four concentrations were calculated. Table 2 shows that the inter-day RSD ranged from 1.9% to 11.9% and the intraday RSD ranged from 3.3% to 12.9%. The inter- and intraday RE ranged between 85.8% and 98.4% and between 85.4% and 96.6%, respectively. The stability RSD of gomisin D ranged between 1.5% and 10.2% at room temperature for 12 h, at 4 °C for 24 h, at −20 °C for 15 days, and three complete freeze/thaw cycles. The detailed stability RSD of gomisin D was shown in Table 3. The above results demonstrated that these values were acceptable for biological analysis method.
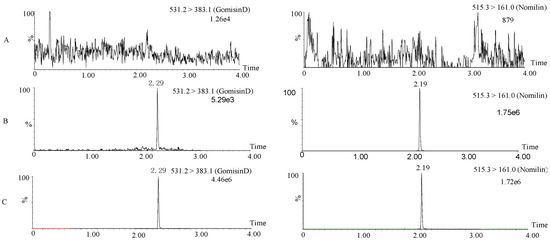
Figure 2.
Typical ultra-performance liquid chromatography/tandem mass spectrometry (UPLC-MS) chromatograms of gomisin D and internal standard (IS). Rat blank plasma (A); rat blank plasma spiked with 1 ng/mL of gomisin D (B); plasma sample collected 30 min after intravenous administration of a single 5 mg/kg dose of gomisin D (C).

Table 1.
Extraction recovery and matrix effect of gomisin D and nomilin in rat plasma (mean ± SD, n = 6).

Table 2.
Intra- and interday accuracy and precision for gomisin D in rat quality control (QC) samples.

Table 3.
Stability of gomisin D in rat plasma (mean ± SD, n = 6).
3.3. Pharmacokinetic Analysis
The developed UPLC-MS/MS method was applied to the pharmacokinetic and bioavailability study of gomisin D after i.g. and i.v. administrations of 50 and 5 mg/kg, respectively. The mean plasma concentration versus time profiles (n = 12) are illustrated in Figure 3. The main pharmacokinetic parameters from noncompartment model analysis are summarized in Table 4. For i.v. administration, Tmax was 0.083 ± 0.0 h and t1/2 was 5.0 ± 1.1 h. The V and CL of gomisin D was 11.2 ± 2.6 L/kg and 1.6 ± 0.3 L/h/kg, respectively. The AUC(0–t) and AUC(0-∞) was 3136.2 ± 548.7 µg L/h and 3261.1 ± 600.0 µg L/h, respectively. For i.g. administration, Tmax was 3.0 ± 1.2 h and t1/2 was 5.6 ± 2.0 h. The V and CL of gomisin D were 13.3 ± 6.2 L/kg and 1.5 ± 0.3 L/h/kg, respectively. The AUC(0–t) and AUC(0-∞) were 32,795.6 ± 11,104.6 µg L/h and 35,091.7 ± 8092.8 µg L/h, respectively. The result of apparent volume of distribution (V) indicated that gomisin D was distributed in extracellular fluid. The results of t1/2 showed that gomisin D experienced a slow elimination process after i.v. or i.g. administration, thus possibly obtaining a good therapeutic effect. The bioavailability could reach up to 107.6% as calculated by the formula of F = [(AUCi.g.) × (Dosei.v.)]/[(AUCi.v.) × (Dose i.g.)] × 100%, suggesting that gomisin D may become a promising intragastrical medication. In fact, most lignan compounds show a higher absorption or bioavailability, such as gomisin J [2,27,28]. Gomisin D shows better absorption than other lignans [19]. There were many factors leading to the higher bioavailability (>100%) [29,30], such as enterohepatic circulation, nonspecific binding [31], dose–vehicle effects [32], nonstationary pharmacokinetics [33], nonlinearity in pharmacokinetics [34], and in vivo isomerization [33]. Since there were no double peaks in the pharmacokinetic curve of gomisin D, thus enterohepatic circulation could be excluded. However, the specific reasons for the high bioavailability of gomisin D need to be further explored. Gomisin D is a very promising active monomer for lowering blood sugar [14,15,35]. Our research provided the information of pharmacokinetic and bioavailability of gomisin D. According to the ‘Pubmed Compound’ record, the octanol/water partition coefficient value (XLogP3-AA) of gomisin D is 3.5. In the future, water-soluble groups can be added to the molecular structure of gomisin D to increase its water-solubility.
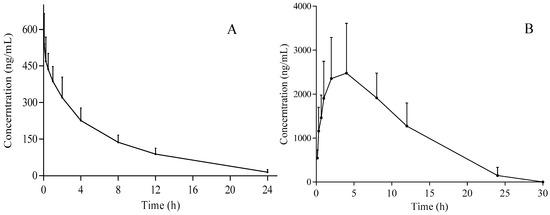
Figure 3.
Plasma concentration–time plots of gomisin D after i.v. (A) and i.g. (B) administration.

Table 4.
Pharmacokinetic parameters of gomisin D in two groups (mean ± SD, n = 12).
4. Conclusions
Compared to the previous HPLC-MS/MS methods, our UPLC-MS/MS method provided a shorter UPLC run time and wider linear range assay for the quantification of gomisin D in rat plasma. It is the first report on the pharmacokinetics and bioavailability of gomisin D. The pharmacokinetic characteristics of gomisin D will help further the understanding of its pharmacological activity and clinical application.
Author Contributions
Conceived and designed the experiments: Z.X., Performed the experiments: X.Z., F.F., X.J. Analyzed the data: J.Q., X.C. Wrote the paper: Z.X., X.Z., X.J.
Funding
This research was supported by the National Natural Science Foundation of China [grant numbers 81773691, 81703815]; Wenzhou Science and Technology Major Project, China [grant number ZS2017018].
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Jang, M.K.; Nam, J.S.; Kim, J.H.; Yun, Y.R.; Han, C.W.; Kim, B.J.; Jeong, H.S.; Ha, K.T.; Jung, M.H. Schisandra chinensis extract ameliorates nonalcoholic fatty liver via inhibition of endoplasmic reticulum stress. J. Ethnopharmacol. 2016, 185, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, H.J.; Kim, C.Y.; Han, S.Y.; Chin, Y.W.; Choi, Y.H. Simultaneous determination of nine lignans from schisandra chinensis extract using ultra-performance liquid chromatography with tandem mass spectrometry in rat plasma, urine, and gastrointestinal tract samples: Application to the pharmacokinetic study of schisandra chinensis. J. Sep. Sci. 2014, 37, 2851–2863. [Google Scholar] [PubMed]
- Hong, M.; Zhang, Y.; Li, S.; Tan, H.Y.; Wang, N.; Mu, S.; Hao, X.; Feng, Y. A network pharmacology-based study on the hepatoprotective effect of fructus schisandrae. Molecules 2017, 22, 1617. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Guo, Y.; Li, L.Y.; Hu, H.; Qu, X.L.; Sun, X.Z.; Liu, S.H.; Wang, H. A herbal composition of semen hoveniae, radix puerariae, and fructus schisandrae shows potent protective effects on acute alcoholic intoxication in rodent models. Evid.-Based Complement. Altern. Med. 2012, 2012, 638197. [Google Scholar] [CrossRef]
- Pan, S.Y.; Yu, Q.; Zhang, Y.; Wang, X.Y.; Sun, N.; Yu, Z.L.; Ko, K.M. Dietary fructus schisandrae extracts and fenofibrate regulate the serum/hepatic lipid-profile in normal and hypercholesterolemic mice, with attention to hepatotoxicity. Lipids Health Dis. 2012, 11, 120. [Google Scholar] [CrossRef]
- Pan, S.Y.; Yu, Z.L.; Dong, H.; Xiang, C.J.; Fong, W.F.; Ko, K.M. Ethanol extract of fructus schisandrae decreases hepatic triglyceride level in mice fed with a high fat/cholesterol diet, with attention to acute toxicity. Evid.-Based Complement. Altern. Med. 2011, 2011, 729412. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, D.S.; Yang, H.J.; Park, S. The lignan-rich fractions of fructus schisandrae improve insulin sensitivity via the ppar-gamma pathways in in vitro and in vivo studies. J. Ethnopharmacol. 2011, 135, 455–462. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, L.L.; Zheng, Y.N. Dibenzocyclooctadiene lignans from fructus schisandrae chinensis improve glucose uptake in vitro. Nat. Prod. Commun. 2010, 5, 231–234. [Google Scholar] [CrossRef]
- Shao, B.; Tang, J.; Ji, H.; Liu, H.; Liu, Y.; Zhu, D.; Wu, L. Enhanced oral bioavailability of wurenchun (fructus schisandrae chinensis extracts) by self-emulsifying drug delivery systems. Drug Dev. Ind. Pharm. 2010, 36, 1356–1363. [Google Scholar] [CrossRef]
- Tian, D.D.; Wang, W.; Wang, H.N.; Sze, S.C.; Zhang, Z.J. Pharmacokinetic evaluation of clozapine in concomitant use of radix rehmanniae, fructus schisandrae, radix bupleuri, or fructus gardeniae in rats. Molecules 2016, 21, 696. [Google Scholar] [CrossRef]
- Gao, J.R.; Ji, W.B.; Jiang, H.; Chen, J.F. Effects of extracts from ziziphi spinosae semen and schisandrae chinensis fructus on amino acid neurotransmitter in rats with insomnia induced by pcpa. J. Chin. Med. Mater. 2013, 36, 1635–1639. [Google Scholar]
- Chen, S.; Jia, Z.; Dong, L.; Geng, P.; Liu, Z.; Yang, S.; Wen, C.; Liu, F. Pharmacokinetic and bioavailability study of angeloylgomisin h in rat plasma by uplc-ms/ms. Int. Clin. Exp. Med. 2015, 8, 17968–17976. [Google Scholar]
- Smejkal, K.; Slapetova, T.; Krmencik, P.; Babula, P.; Dall’Acqua, S.; Innocenti, G.; Vanco, J.; Casarin, E.; Carrara, M.; Kalvarova, K.; et al. Evaluation of cytotoxic activity of schisandra chinensis lignans. Planta Med. 2010, 76, 1672–1677. [Google Scholar] [CrossRef]
- Song, J.H.; Cui, L.; An, L.B.; Li, W.T.; Fang, Z.Z.; Zhang, Y.Y.; Dong, P.P.; Wu, X.; Wang, L.X.; Gonzalez, F.J.; et al. Inhibition of udp-glucuronosyltransferases (ugts) activity by constituents of schisandra chinensis. Phytother. Res. 2015, 29, 1658–1664. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.J.; Jiang, H.J.; Xu, C.; Tong, S.Q.; Yan, J.Z. Screening and identification of alpha-glucosidase inhibitors from shenqi jiangtang granule by ultrafiltration liquid chromatography and mass spectrometry. J. Sep. Sci. 2018, 41, 797–805. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of schisandra chinensis (turcz.) baill. Using hplc-esi-tof-ms and hplc-online teac: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.; Min, B.S.; Ngoc, T.M.; Lee, I.; Zhang, X.; Bae, K. Acetylcholinesterase inhibitory effect of lignans isolated from schizandra chinensis. Arch. Pharmacal Res. 2007, 30, 685–690. [Google Scholar] [CrossRef]
- Zhang, A.H.; Yu, J.B.; Sun, H.; Kong, L.; Wang, X.Q.; Zhang, Q.Y.; Wang, X.J. Identifying quality-markers from shengmai san protects against transgenic mouse model of alzheimer’s disease using chinmedomics approach. Phytomedicine 2018, 45, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xiao-tong, W.; Fan, H.; Xiao-ling, S. Pharmacokinetics study of seven lignans in alzheimer’s rats. Int. J. Pharmacol. 2018, 14, 68–75. [Google Scholar]
- Shi, P.; He, Q.; Zhang, Y.; Qu, H.; Cheng, Y. Characterisation and identification of isomeric dibenzocyclooctadiene lignans from schisandra chinensis by high-performance liquid chromatography combined with electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2009, 20, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Wang, Q.; Wang, Y.; Wang, X.J.; Pu, J.X.; Gu, Y.; Wang, R. Application of ultrahigh-performance liquid chromatography coupled with mass spectrometry for analysis of lignans and quality control of fructus schisandrae chinensis. J. Sep. Sci. 2012, 35, 2203–2209. [Google Scholar] [CrossRef]
- Liu, K.; Song, Y.; Liu, Y.; Peng, M.; Li, H.; Li, X.; Feng, B.; Xu, P.; Su, D. An integrated strategy using uplc-qtof-ms(e) and uplc-qtof-mrm (enhanced target) for pharmacokinetics study of wine processed schisandra chinensis fructus in rats. J. Pharm. Biomed. Anal. 2017, 139, 165–178. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Wang, L.Z.; Phan, D.D.K.; Syn, N.; Xiang, X.Q.; Song, H.Y.; Thuya, W.L.; Yang, S.L.; Wong, A.L.A.; Kumar, A.P.; Yong, W.P.; et al. A sensitive liquid chromatography-tandem mass spectrometry method for the determination of nimbolide in mouse serum: Application to a preclinical pharmacokinetics study. Pharmaceutics 2018, 10, 123. [Google Scholar] [CrossRef]
- Sun, L.L.; Ding, F.F.; You, G.J.; Liu, H.; Wang, M.; Ren, X.L.; Deng, Y.R. Development and validation of an uplc-ms/ms method for pharmacokinetic comparison of five alkaloids from jinqi jiangtang tablets and its monarch drug coptidis rhizoma. Pharmaceutics 2018, 10, 4. [Google Scholar] [CrossRef]
- Balla, A.; Cho, K.H.; Kim, Y.C.; Maeng, H.J. Simultaneous determination of procainamide and n-acetylprocainamide in rat plasma by ultra-high-pressure liquid chromatography coupled with a diode array detector and its application to a pharmacokinetic study in rats. Pharmaceutics 2018, 10, 41. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, H.; Cheon, S.M.; Ko, S.M.; Ham, S.H.; Kwon, Y.D.; Lee, Y.B.; Cho, H.Y. A sensitive uhplc-ms/ms method for the simultaneous quantification of three lignans in human plasma and its application to a pharmacokinetic study. J. Sep. Sci. 2017, 40, 3430–3439. [Google Scholar] [CrossRef]
- Wei, B.; Li, Q.; Su, D.; Fan, R.; Zhao, L.; Geng, L.; He, B.; Chen, X.; Jia, Y.; Bi, K. Development of a uflc-ms/ms method for simultaneous determination of six lignans of schisandra chinensis (turcz.) baill. In rat plasma and its application to a comparative pharmacokinetic study in normal and insomnic rats. J. Pharm. Biomed. Anal. 2013, 77, 120–127. [Google Scholar] [CrossRef]
- Ward, K.W.; Hardy, L.B.; Kehler, J.R.; Azzarano, L.M.; Smith, B.R. Apparent absolute oral bioavailability in excess of 100% for a vitronectin receptor antagonist (sb-265123) in rat. II. Studies implicating transporter-mediated intestinal secretion. Xenobiotica 2004, 34, 367–377. [Google Scholar] [CrossRef]
- Ward, K.W.; Azzarano, L.M.; Evans, C.A.; Smith, B.R. Apparent absolute oral bioavailability in excess of 100% for a vitronectin receptor antagonist (sb-265123) in rat. I. Investigation of potential experimental and mechanistic explanations. Xenobiotica 2004, 34, 353–366. [Google Scholar] [CrossRef]
- Darbar, D.; Dell’Orto, S.; Wilkinson, G.R.; Roden, D.M. Loss of quinidine gluconate injection in a polyvinyl chloride infusion system. Am. J. Health-Syst. Pharm. 1996, 53, 655–658. [Google Scholar] [CrossRef]
- Curry, S.H.; Lorenz, A.; Chu, P.I.; Limacher, M.; Stacpoole, P.W. Disposition and pharmacodynamics of dichloroacetate (dca) and oxalate following oral dca doses. Biopharm. Drug Dispos. 1991, 12, 375–390. [Google Scholar] [CrossRef]
- Ludden, T.M. Nonlinear pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 1991, 20, 429–446. [Google Scholar] [CrossRef]
- Ward, K.W.; Proksch, J.W.; Azzarano, L.M.; Salyers, K.L.; McSurdy-Freed, J.E.; Molnar, T.M.; Levy, M.A.; Smith, B.R. Sb-239063, a potent and selective inhibitor of p38 map kinase: Preclinical pharmacokinetics and species-specific reversible isomerization. Pharm. Res. 2001, 18, 1336–1344. [Google Scholar] [CrossRef]
- Hui, Z.; Yuanyuan, W.; Chenye, H.; Xiaojing, Z.; Jizhong, Y. Interaction between gomizine d and α-glucosidase. China J. Chin. Mater. Med. 2017, 42, 4631–4635. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).