Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials
Abstract
1. Introduction
2. Epilepsy
3. Common Antiepileptic Drugs
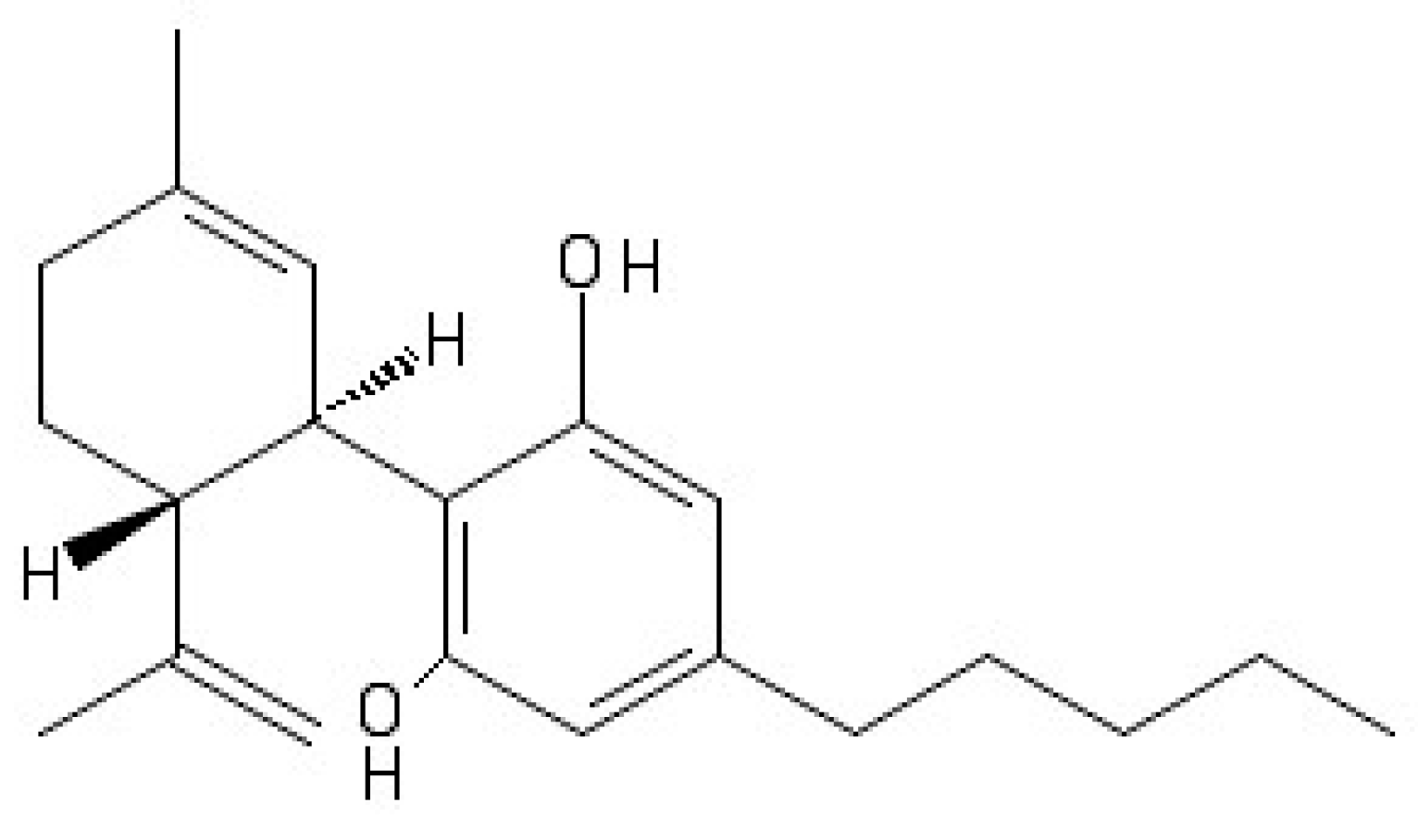
4. Cannabidiol and Molecular Targets in Epilepsy
5. Cannabidiol: Clinical Trials for Epilepsy
5.1. Completed Clinical Trials
5.2. Ongoing Clinical Trials
5.3. Clinical Trials Approved by Local Ethics Committees
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Bih, C.I.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar]
- Sanchez, A.; Garcia-Merino, A. Neuroprotective agents: cannabinoids. Clin. Immunol. 2012, 142, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Scionti, D.; Diomede, F.; Grassi, G.; Pollastro, F.; Piattelli, A.; Cocco, L.; Bramanti, P.; Mazzon, E.; Trubiani, O. Gingival stromal cells as an in vitro model: Cannabidiol modulates genes linked with amyotrophic lateral sclerosis. J. Cell. Biochem. 2017, 118, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.G.; Martins, N.M.; Sisti, F.M.; Fernandes, L.S.; Ferreira, R.S.; Queiroz, R.H.C.; Santos, A.C. The neuroprotection of cannabidiol against MPP+-induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicol. Vitr. 2015, 30, 231–240. [Google Scholar] [CrossRef]
- Chagas, M.H.N.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.; Crippa, J.A.S. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Et Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Watt, G.; Karl, T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer’s disease. Front. Pharmacol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the expression of Alzheimer’s disease-related genes in mesenchymal stem cells. Int. J. Mol. Sci. 2016, 18, 26. [Google Scholar]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.; Mestre, L.; Carrillo-Salinas, F.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hou, C.; Chen, X.; Wang, D.; Yang, P.; He, X.; Zhou, J.; Li, H. Protective effect of cannabidiol on hydrogen peroxide-induced apoptosis, inflammation and oxidative stress in nucleus pulposus cells. Mol. Med. Rep. 2016, 14, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Batista, J.; Viana, R.; Baetas, A.; Orestes, E.; Andrade, M.; Honório, K.; da Silva, A. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef]
- Soares, R.Z.; Vuolo, F.; Dall’Igna, D.M.; Michels, M.; Crippa, J.A.d.S.; Hallak, J.E.C.; Zuardi, A.W.; Dal-Pizzol, F. Evaluation of the role of the cannabidiol system in an animal model of ischemia/reperfusion kidney injury. Rev. Bras. Ter. Intensiva 2015, 27, 383–389. [Google Scholar] [CrossRef]
- González-García, C.; Torres, I.M.; García-Hernández, R.; Campos-Ruíz, L.; Esparragoza, L.R.; Coronado, M.J.; Grande, A.G.; García-Merino, A.; López, A.J.S. Mechanisms of action of cannabidiol in adoptively transferred experimental autoimmune encephalomyelitis. Exp. Neurol. 2017, 298, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Tolón, M.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic–ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, L.; Gran, B.; Constantinescu, C.S. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology 2010, 215, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Filippis, D.; Maiuri, M.C.; De Stefano, D.; Carnuccio, R.; Iuvone, T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in β-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-κB involvement. Neurosci. Lett. 2006, 399, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’Carroll, C.M.; Seal, M.; Allen, P. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010, 35, 764. [Google Scholar] [CrossRef]
- Maione, S.; Piscitelli, F.; Gatta, L.; Vita, D.; De Petrocellis, L.; Palazzo, E.; de Novellis, V.; Di Marzo, V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br. J. Pharmacol. 2011, 162, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar]
- Shannon, S.; Opila-Lehman, J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm. J. 2016, 20, 108. [Google Scholar] [CrossRef]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J. Altern. Complementary Med. 2018. [Google Scholar] [CrossRef]
- Carlini, E.; Leite, J.; Tannhauser, M.; Berardi, A. Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J. Pharm. Pharmacol. 1973, 25, 664–665. [Google Scholar] [CrossRef]
- Consroe, P.; Benedito, M.A.; Leite, J.R.; Carlini, E.A.; Mechoulam, R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur. J. Pharmacol. 1982, 83, 293–298. [Google Scholar] [CrossRef]
- Jones, N.A.; Hill, A.J.; Smith, I.; Bevan, S.A.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Ther. 2010, 332, 569–577. [Google Scholar] [CrossRef]
- Mudigoudar, B.; Weatherspoon, S.; Wheless, J.W. Emerging antiepileptic drugs for severe pediatric epilepsies. In Seminars in pediatric neurology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 167–179. [Google Scholar]
- Hussain, S.A.; Zhou, R.; Jacobson, C.; Weng, J.; Cheng, E.; Lay, J.; Hung, P.; Lerner, J.T.; Sankar, R. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: a potential role for infantile spasms and Lennox–Gastaut syndrome. Epilepsy Behav. 2015, 47, 138–141. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Gloss, D.; Vickrey, B. Cannabinoids for epilepsy. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Fisher, R.S.; Boas, W.V.E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel Jr, J. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Sabaz, M.; Lawson, J.A.; Cairns, D.R.; Duchowny, M.S.; Resnick, T.J.; Dean, P.M.; Bye, A.M. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav. 2003, 4, 680–691. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Johnson, E.L. Seizures and Epilepsy. Med. Clin. 2019, 103, 309–324. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef]
- Dravet, C.; Bureau, M.; Oguni, H.; Fukuyama, Y.; Cokar, O. Severe myoclonic epilepsy in infancy (Dravet syndrome). Epileptic Syndr. InfancyChild. Adolesc. 2005, 4, 89–113. [Google Scholar]
- Escayg, A.; MacDonald, B.T.; Meisler, M.H.; Baulac, S.; Huberfeld, G.; An-Gourfinkel, I.; Brice, A.; LeGuern, E.; Moulard, B.; Chaigne, D. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+ 2. Nat. Genet. 2000, 24, 343. [Google Scholar] [CrossRef]
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge–Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. New Engl. J. Med. 2013, 368, 1971–1979. [Google Scholar] [CrossRef]
- Comi, A.M. Presentation, diagnosis, pathophysiology and treatment of the neurologic features of Sturge-Weber Syndrome. Neurol. 2011, 17, 179. [Google Scholar] [CrossRef]
- Archer, H.L.; Evans, J.; Edwards, S.; Colley, J.; Newbury-Ecob, R.; O’Callaghan, F.; Huyton, M.; O’Regan, M.; Tolmie, J.; Sampson, J. CDKL5 mutations cause infantile spasms, early onset seizures, and severe mental retardation in female patients. J. Med. Genet. 2006, 43, 729–734. [Google Scholar] [CrossRef]
- Pellock, J.M.; Hrachovy, R.; Shinnar, S.; Baram, T.Z.; Bettis, D.; Dlugos, D.J.; Gaillard, W.D.; Gibson, P.A.; Holmes, G.L.; Nordli, D.R. Infantile spasms: a US consensus report. Epilepsia 2010, 51, 2175–2189. [Google Scholar] [CrossRef]
- Kwan, P.; Sills, G.J.; Brodie, M.J. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol. Ther. 2001, 90, 21–34. [Google Scholar] [CrossRef]
- O’Connell, B.K.; Gloss, D.; Devinsky, O. Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav. 2017, 70, 341–348. [Google Scholar] [CrossRef]
- Catterall, W.A. Forty Years of sodium channels: Structure, function, pharmacology, and epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef]
- Lucas, P.T.; Meadows, L.S.; Nicholls, J.; Ragsdale, D.S. An epilepsy mutation in the β1 subunit of the voltage-gated sodium channel results in reduced channel sensitivity to phenytoin. Epilepsy Res. 2005, 64, 77–84. [Google Scholar] [CrossRef]
- Kostyuk, P.; Molokanova, E.; Pronchuk, N.; Savchenko, A.; Verkhratsky, A. Different action of ethosuximide on low-and high-threshold calcium currents in rat sensory neurons. Neuroscience 1992, 51, 755–758. [Google Scholar] [CrossRef]
- Sitges, M.; Chiu, L.M.; Reed, R.C. Effects of levetiracetam, carbamazepine, phenytoin, valproate, lamotrigine, oxcarbazepine, topiramate, vinpocetine and sertraline on presynaptic hippocampal Na+ and Ca 2+ channels permeability. Neurochem. Res. 2016, 41, 758–769. [Google Scholar] [CrossRef]
- Holtyn, A.F.; Tiruveedhula, V.P.B.; Stephen, M.R.; Cook, J.M.; Weerts, E.M. Effects of the benzodiazepine GABAA α1-preferring antagonist 3-isopropoxy-β-carboline hydrochloride (3-ISOPBC) on alcohol seeking and self-administration in baboons. Drug Alcohol Depend. 2017, 170, 25–31. [Google Scholar] [CrossRef]
- Fisher, J.L. The anti-convulsant stiripentol acts directly on the GABAA receptor as a positive allosteric modulator. Neuropharmacology 2009, 56, 190–197. [Google Scholar] [CrossRef]
- Walters, D.C.; Arning, E.; Bottiglieri, T.; Jansen, E.E.; Salomons, G.S.; Brown, M.N.; Schmidt, M.A.; Ainslie, G.R.; Roullet, J.-B.; Gibson, K.M. Metabolomic analyses of vigabatrin (VGB)-treated mice: GABA-transaminase inhibition significantly alters amino acid profiles in murine neural and non-neural tissues. Neurochem. Int. 2019, 125, 151–162. [Google Scholar] [CrossRef]
- Curry, W.J.; Kulling, D.L. Newer antiepileptic drugs: gabapentin, lamotrigine, felbamate, topiramate and fosphenytoin. Am. Fam. Physician 1998, 57, 513–520. [Google Scholar]
- Ghodke-Puranik, Y.; Thorn, C.F.; Lamba, J.K.; Leeder, J.S.; Song, W.; Birnbaum, A.K.; Altman, R.B.; Klein, T.E. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharm. Genom. 2013, 23, 236. [Google Scholar] [CrossRef]
- Madeja, M.; Margineanu, D.G.; Gorji, A.; Siep, E.; Boerrigter, P.; Klitgaard, H.; Speckmann, E.-J. Reduction of voltage-operated potassium currents by levetiracetam: a novel antiepileptic mechanism of action? Neuropharmacology 2003, 45, 661–671. [Google Scholar] [CrossRef]
- Srinivasan, J.; Richens, A.; Davies, J.A. Effects of felbamate on veratridine-and K+-stimulated release of glutamate from mouse cortex. Eur. J. Pharmacol. 1996, 315, 285–288. [Google Scholar] [CrossRef]
- Kanda, T.; Kurokawa, M.; Tamura, S.; Nakamura, J.; Ishii, A.; Kuwana, Y.; Serikawa, T.; Yamada, J.; Ishihara, K.; Sasa, M. Topiramate reduces abnormally high extracellular levels of glutamate and aspartate in the hippocampus of spontaneously epileptic rats (SER). Life Sci. 1996, 59, 1607–1616. [Google Scholar] [CrossRef]
- Theodore, W.H.; Wiggs, E.A.; Martinez, A.R.; Dustin, I.H.; Khan, O.I.; Appel, S.; Reeves-Tyer, P.; Sato, S. Serotonin 1A receptors, depression, and memory in temporal lobe epilepsy. Epilepsia 2012, 53, 129–133. [Google Scholar] [CrossRef]
- Naziroglu, M. TRPV1 Channel: A Potential Drug Target for Treating Epilepsy. Curr. Neuropharmacol. 2015, 13, 239–247. [Google Scholar] [CrossRef]
- Vilela, L.R.; Lima, I.V.; Kunsch, E.B.; Pinto, H.P.P.; de Miranda, A.S.; Vieira, E.L.M.; de Oliveira, A.C.P.; Moraes, M.F.D.; Teixeira, A.L.; Moreira, F.A. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: Pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. EB 2017, 75, 29–35. [Google Scholar] [CrossRef]
- Gharedaghi, M.H.; Seyedabadi, M.; Ghia, J.-E.; Dehpour, A.R.; Rahimian, R. The role of different serotonin receptor subtypes in seizure susceptibility. Exp. Brain Res. 2014, 232, 347–367. [Google Scholar] [CrossRef]
- Theodore, W.H. Does serotonin play a role in epilepsy? Epilepsy Curr. 2003, 3, 173–177. [Google Scholar] [CrossRef]
- Guiard, B.P.; Di Giovanni, G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front. Pharmacol. 2015, 6, 46. [Google Scholar] [CrossRef]
- Theodore, W.H.; Hasler, G.; Giovacchini, G.; Kelley, K.; Reeves-Tyer, P.; Herscovitch, P.; Drevets, W. Reduced hippocampal 5HT1A PET receptor binding and depression in temporal lobe epilepsy. Epilepsia 2007, 48, 1526–1530. [Google Scholar] [CrossRef]
- Chung, P.C.S.; Kieffer, B.L. Delta opioid receptors in brain function and diseases. Pharmacol. Ther. 2013, 140, 112–120. [Google Scholar] [CrossRef]
- Snead, O.C., III. Opiate-induced seizures: a study of μ and δ specific mechanisms. Exp. Neurol. 1986, 93, 348–358. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. 2017, 114, 11229–11234. [Google Scholar] [CrossRef]
- Yamaori, S.; Ebisawa, J.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011, 88, 730–736. [Google Scholar] [CrossRef]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K.; Group, G.P.A.S. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. New Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. New Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P.; Program, U.C. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Bebin, E.M.; Cutter, G.; DeWolfe, J.; Dure, L.S.; Gaston, T.E.; Kankirawatana, P.; Liu, Y.; Singh, R.; Standaert, D.G. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018, 87, 131–136. [Google Scholar] [CrossRef]
- Porcari, G.S.; Fu, C.; Doll, E.D.; Carter, E.G.; Carson, R.P. Efficacy of artisanal preparations of cannabidiol for the treatment of epilepsy: Practical experiences in a tertiary medical center. Epilepsy Behav. 2018, 80, 240–246. [Google Scholar] [CrossRef]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019. [Google Scholar] [CrossRef]
- Hess, E.J.; Moody, K.A.; Geffrey, A.L.; Pollack, S.F.; Skirvin, L.A.; Bruno, P.L.; Paolini, J.L.; Thiele, E.A. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 2016, 57, 1617–1624. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Sands, T.T.; Rahdari, S.; Oldham, M.S.; Nunes, E.C.; Tilton, N.; Cilio, M.R. Long-term safety, tolerability, and efficacy of cannabidiol in children with refractory epilepsy: results from an expanded access program in the US. Cns Drugs 2019, 33, 47–60. [Google Scholar] [CrossRef]
- Kaplan, E.H.; Offermann, E.A.; Sievers, J.W.; Comi, A.M. Cannabidiol treatment for refractory seizures in Sturge-Weber syndrome. Pediatric Neurol. 2017, 71, 18–23.e12. [Google Scholar] [CrossRef]
- Rosenberg, E.C.; Louik, J.; Conway, E.; Devinsky, O.; Friedman, D. Quality of Life in Childhood Epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia 2017, 58, e96–e100. [Google Scholar] [CrossRef]
- Devinsky, O.; Verducci, C.; Thiele, E.A.; Laux, L.C.; Patel, A.D.; Filloux, F.; Szaflarski, J.P.; Wilfong, A.; Clark, G.D.; Park, Y.D. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018, 86, 131–137. [Google Scholar] [CrossRef]
- Chen, K.A.; Farrar, M.; Cardamone, M.; Gill, D.; Smith, R.; Cowell, C.T.; Truong, L.; Lawson, J.A. Cannabidiol for treating drug-resistant epilepsy in children: the New South Wales experience. Med. J. Aust. 2018, 209, 217–221. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Bebin, E.M.; Comi, A.M.; Patel, A.D.; Joshi, C.; Checketts, D.; Beal, J.C.; Laux, L.C.; De Boer, L.M.; Wong, M.H. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia 2018, 59, 1540–1548. [Google Scholar] [CrossRef]
| Identifier | Study Title | Subjects | Conditions | CBD Dose | Concomitant AEDs | Efficacy | Security | Ref |
|---|---|---|---|---|---|---|---|---|
| NCT02987114 | A Study to Evaluate the Safety, Tolerability and Efficacy of Oral Administration of PTL101 (Cannabidiol) as an Adjunctive Treatment for Pediatric Intractable Epilepsy | 16 children (2 to 15 years) | Pediatric Intractable Epilepsy | 25–450 mg/kg/day | - | - | - | - |
| NCT02324673 | Cannabidiol Oral Solution in Pediatric Participants With Treatment-resistant Seizure Disorders | 61 children (1 to 17 years) | Resistant Seizure Disorders | 10, 20, 40 mg/kg/day | - | Improvement of illness | SAEs in 5% of patients with medium-dose and in 9.5% with high-dose | - |
| NCT02551731 | Cannabidiol Oral Solution for Treatment of Refractory Infantile Spasms | 9 infants (6 to 36 Months) | Refractory Infantile Spasms | 20–40 mg/kg/day | - | Complete resolution of spasm in 14.3% of children after 14 days of treatment | No SAEs were recorded | - |
| NCT02318602 | Cannabidiol Oral Solution as an Adjunctive Treatment for Treatment-resistant Seizure Disorder | 52 children and young adults (1 to 18 years) | Treatment-resistant Seizure Disorder | 10, 20, 40 mg/kg/day | - | - | SAEs in 77.78% of infants, in 38.46% of children and in 0% of adults | - |
| NCT02224703 | GWPCARE2 A Study to Investigate the Efficacy and Safety of Cannabidiol (GWP42003-P) in Children and Young Adults With Dravet Syndrome | 150 children and young adults (2 to 18 years) | Dravet Syndrome | 10, 20 mg/kg/day | - | - | - | - |
| NCT02695537 | Safety, and Tolerability of Epidiolex In Patients (Ages 1–19 Years) With Intractable Epilepsy | 100 children and young adults (1 to 18 years) | Intractable Epilepsy | 5–50 mg/kg/day | CLB, Valproate, Levetiracetam, Phenobarbital, Clonazepam, Phenytoin, Carbamazepine, Lamotrigine, Oxcarbazepine, Ethosuximide, Topiramate, Vigabatrin, Zonisamide, Eslicarbazepine, Ezogabine, Pregabalin, Perampanel, Rufinamide, Lacosamide | - | 4 children with concomitant valproate showed elevate damage of liver function | [78] |
| Reduction of seizures of 63.6% after 12 weeks of treatment | Improvement of AE Profile | [79] | ||||||
| NCT02700412 | University of Alabama at Birmingham (UAB) Adult CBD Program | 100 children and adults (15 to 99 years) | EpilepsySeizures | 5–50 mg/kg/day | - | 4 children with concomitant valproate showed elevate damage of liver function | [78] | |
| Reduction of seizures of 63.6% after 12 weeks of treatment | Improvement of AE Profile | [79] | ||||||
| NCT02224560 | Efficacy and Safety of GWP42003-P for Seizures Associated With Lennox-Gastaut Syndrome in Children and Adults (GWPCARE3) | 225 children and adults (2 to 55 years) | Epilepsy Lennox Gastaut Syndrome | 10, 20 mg/kg/day | CLB Valproate Lamotringine Levetiracetam Rufinamide | The median percent reduction in seizures frequency from baseline was 37.2% in the 10 mg/kg/day CBD group; 41.9% in the 20 mg/kg/day CBD group | SAEs were reported in 19.40% of patients at the dose of 10 mg/kg/day of the CBD and in 15.85% at the 20 mg/kg/day | [75] |
| NCT02091206 | A Dose-ranging Pharmacokinetics and Safety Study of GWP42003-P in Children With Dravet Syndrome (GWPCARE1) | 34 children (4 to 10 years) | Dravet Syndrome | 5, 10, 20 mg/kg/day | CLB Valproate Stiripentol Levetiracetam Topiramate | - | TEAEs in 5 patients; SAE in 10% of patients at the dose of 5 mg/kg/day, in 25% at the 10 mg/kg/day and in 11.11% at the 20 mg/kg/day dose. 6 patients with concomitant valproate had elevated ALT or AST | [73] |
| NCT02091375 | Antiepileptic Efficacy Study of GWP42003-P in Children and Young Adults With Dravet Syndrome (GWPCARE1) | 120 children, young adults (2 to 18 years) | Dravet Syndrome | 20 mg/kg/day | CLB Valproate Stiripentol Levetiracetam Topiramate | The median frequency of seizures decreased from 12.4 to 5.9, compared to the placebo-treated group | SAEs in 16.39% of patients | [74] |
| NCT02224690 | A Study to Investigate the Efficacy and Safety of Cannabidiol (GWP42003-P; CBD) as Adjunctive Treatment for Seizures Associated With Lennox-Gastaut Syndrome in Children and Adults (GWPCARE4) | 171 children and adults (2 to 55 years) | Lennox-Gastaut Syndrome | 20 mg/kg/day | CLB Valproate Lamotrigine Levetiracetam Rufinamide | The monthly frequency of seizures decreased by a median of 43,·9% from baseline in the CBD group | Serious TEAEs occurred in 4 patients;SAEs in 23.26% of patients. 16 of the 36 patients on valproate had transaminase elevations | [76] |
| NCT02224573 | GWPCARE5 - An Open Label Extension Study of Cannabidiol (GWP42003-P) in Children and Young Adults With Dravet or Lennox-Gastaut Syndromes | 264 children, and adults (2 years and older) | Dravet Syndrome Lennox-Gastaut Syndrome | - | CLB Valproate Stiripentol Levetiracetam Topiramate | The monthly frequency of seizures decreased by a median ranged from 38% to 44% | SAEs in 29.2% of patients | [77] |
| NCT02565108 | A Randomized Controlled Trial to Investigate Possible Drug-drug Interactions Between Clobazam and Cannabidiol | 20 adults (18 to 65 years) | Epilepsy | 20 mg/kg/day | CLB | All participants reduced the maintenance dose of CBD from 10% for the day | 2 patients withdrew from the study due to SAEs (seizure cluster) | - |
| NCT02564952 | An Open-label Extension Study to Investigate Possible Drug-drug Interactions Between Clobazam and Cannabidiol | 18 adults (18 to 65 years) | Epilepsy | Initial 20 mg/kg/d titrated to maximum dose of 30 mg/kg/day | CLB | - | SAEs in 11% of patients | - |
| Study Design | Subjects | Conditions | CBD Dose | Concomitant AEDs | Efficacy | Safety | Ref |
|---|---|---|---|---|---|---|---|
| A prospective, open-label, expanded access study | 214 children and adults (1 to 30 years) | Drug Resistant Epilepsy | Initial 2–5 mg/kg/day titrated to maximum dose of 50 mg/kg/day | CLB Valproate | The median reduction in monthly motor seizure was of 36.5% | Treatment-related SAEs were recorded in 20 patients; SAEs were reported in 30% of patients. Thrombocytopenia and elevated liver function test in patients with concomitant valproate | [83] |
| A prospective, open-label study | Children and adults (1 to 30 years) | Drug Resistant Epilepsy | Initial 2–5 mg/kg/day, titrated to maximum dose of 50 mg/kg/day | - | Overall quality of life significantly improved in 48 patients, The median monthly seizures frequency was 13.9 | - | [86] |
| A prospective, multicentre, open-label study | 55 children and adults (1 to 30 years) | Epilepsy Dravet Syndrome CDKL5 deficiency disorder Aicardi Doose syndromes Dup15q syndromes | Initial 5 mg/kg/day titrated to maximum dose of 50 mg/kg/day | CLB Valproic acid Levetiracetam Rufinamide Felbamate Topiramate | Median monthly convulsive seizure frequency decreased from baseline by 51.4% at week 12 and by 59.1% at week 48 | A serious treatment-emergent AEs such as status epilepticus (9%) and respiratory infection (5%) | [87] |
| A prospective, open-label study | 40 children (1 to 17 years) | Drug Resistant Epilepsy | Initial 5 mg/kg/day titrated to maximum dose of 25 mg/kg/day | - | 12 patients reported substantial improvement of the condition | 4 patients withdrew from the study because of AEs; SAEs were reported in 15 patients | [88] |
| A prospective, multiple center, open-label study | 607 children (average age 13 years) | Drug Resistant Epilepsy | Initial 2–10 mg/kg/day to maximum dose of 50 mg/kg/day | A median monthly seizure frequency of 51% was recorded after 12 months of treatment and maintained at weeks 96 | SAEs were reported in 33% of patients; | [89] | |
| Expanded access program | 5 infants (1 to 45 months) | Sturge-Weber Syndrome | 2–25 mg/kg/day | Levetiracetam Valproic acid Felbamate CLB Rufinamide Perampanel Clorazepate Oxcarbazepine Lacosamide Topiramate | 50% of seizures reductions in all patients; Improvements in quality of life in all patients | AEs were recorded during the study | [85] |
| Retrospective study | 210 children (≤ 19 years) | Epilepsy | 2.9, 5.8 mg/kg/day | CLB | 50% in seizures reduction in 33% of patients in the CBD group; in 44% of CBD + CLB and in 38% of CLB group | AEs in 36% of patients in the CLB group and in 7% of patients in CBD + CLB group | [80] |
| Expand access investigational new drug (IND) trial | 13 children and young (4 to 19 years) | Refractory Epilepsy | 5–25 mg/kg/day | CLB | 50% of reduction in seizures in 69.23% of patients | No serious AEs in 77% of patients | [72] |
| Open-label, fixed-sequence trial | 78 healthy subjects | - | 750 mg twice daily | CLB Stiripentol Valproate | - | Moderate AEs in 8 patients; mild AEs in most of patients | [81] |
| Expanded access study | 18 children and adults (2 to 31 years) | Tuberous Sclerosis Complex | 5–50 mg/kg/day | CLB Lacosamide Levetiracetam Lamotrigine Valproic acid Rufinamide | 4 patients recorded a reduction in seizure rate greater than 80%; 1 patient became seizures-free | AEs in 66.7% of patients | [82] |
| Expanded access program | 26 children (1 to 17 years) | Refractory epilepsy | 5–25 mg/kg/day | CLB | A 50% reduction in seizures | SAEs in 23.1% of patients | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, S.; Mammana, S.; Cavalli, E.; Bramanti, P.; Mazzon, E. Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules 2019, 24, 1459. https://doi.org/10.3390/molecules24081459
Silvestro S, Mammana S, Cavalli E, Bramanti P, Mazzon E. Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules. 2019; 24(8):1459. https://doi.org/10.3390/molecules24081459
Chicago/Turabian StyleSilvestro, Serena, Santa Mammana, Eugenio Cavalli, Placido Bramanti, and Emanuela Mazzon. 2019. "Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials" Molecules 24, no. 8: 1459. https://doi.org/10.3390/molecules24081459
APA StyleSilvestro, S., Mammana, S., Cavalli, E., Bramanti, P., & Mazzon, E. (2019). Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules, 24(8), 1459. https://doi.org/10.3390/molecules24081459






