Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sensor Responses
2.2. Oil Chromatograms
- Octane is a straight-chain alkane composed of 8 carbon atoms. It has a role as a xenobiotic. It is a colorless liquid with an odor of gasoline, less dense than water and insoluble in water;
- 3,4-dimethyl-1,5-hexadiene is an olefin that has also been found in sugarcane bagasse oil [17];
- Methyl benzoate has a characteristic aroma that is sweet, creamy, anisic vanilla-like, slightly spicy, woody and powdery heliotropine-like. It has been found in the mushroom variety Trametes graveolens, feijoa fruit and peel (Feijoa sellowiana), white wine, cocoa, tea, guava, starfruit, Bourbon vanilla, Tahiti vanilla, mountain papaya, sapodilla fruit and Illicium verum [18].
3. Materials and Methods
3.1. Sample Preparation and Experimental Design
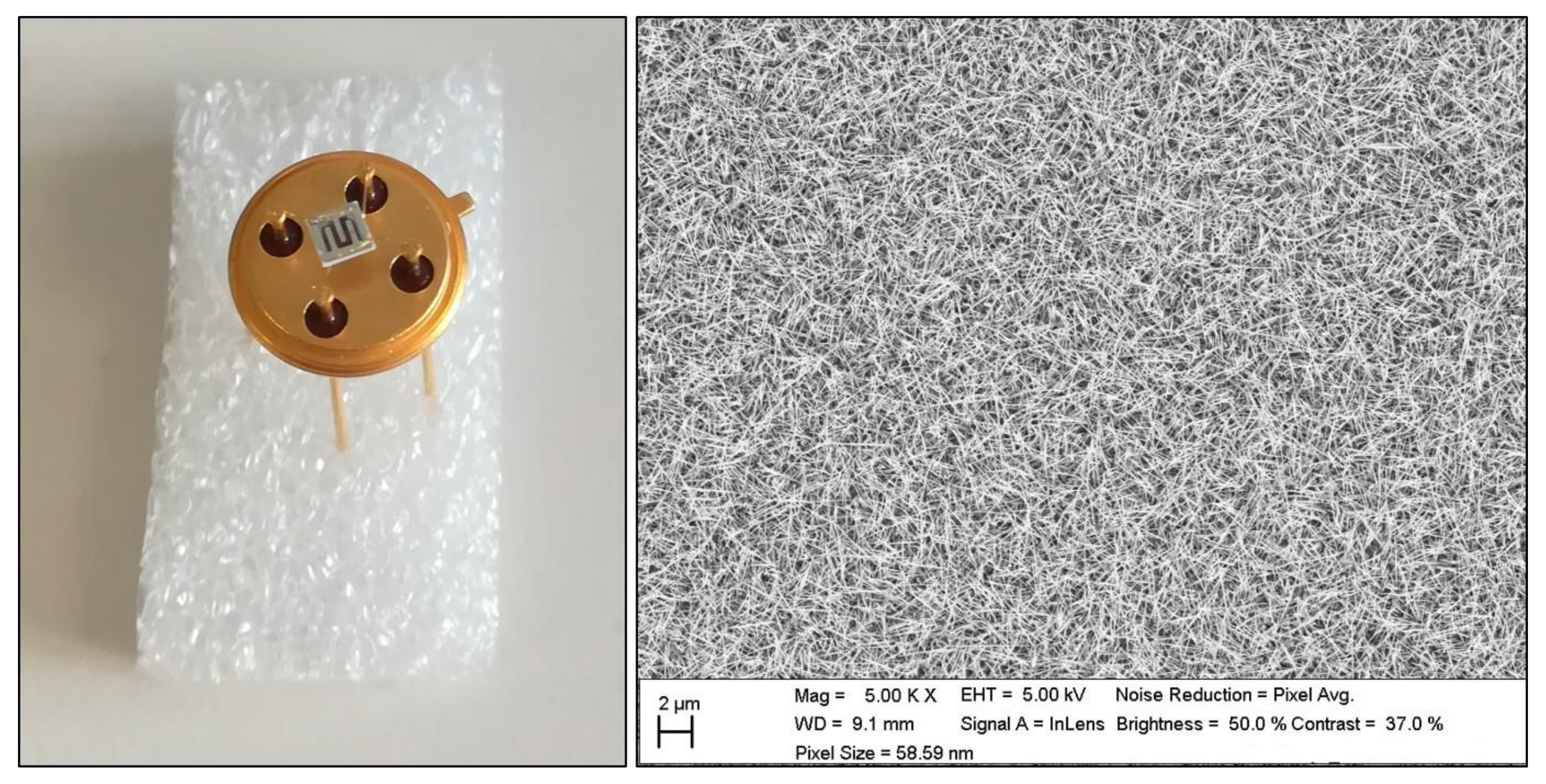
3.2. Small Sensor System (S3)
3.3. SPME-GC-MS Analysis
3.4. Data Analysis Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caporaso, N. Virgin Olive Oils: Environmental Conditions, Agronomical Factors and Processing Technology Affecting the Chemistry of Flavor Profile. J. Food Chem. Nanotechnol. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- Sacchi, R.; Mannina, L.; Fiordiponti, P.; Barone, P.; Paolillo, L.; Patumi, M.; Segre, A. Characterization of italian extra virgin olive oils using 1H-NMR spectroscopy. J. Agric. Food Chem. 1998, 46, 3947–3951. [Google Scholar] [CrossRef]
- García-González, D.L.; Aparicio, R. Research in olive oil: Challenges for the near future. J. Agric. Food Chem. 2010, 58, 12569–12577. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Samblàs, C.; Tres, A.; Koot, A.; van Ruth, S.M.; Gonzàlez-Casado, A.; Cuadros-Rodrìguez, L. Proton transfer reaction-mass spectrometry volatile organic compound fingerprinting for monovarietal extra virgin olive oil identification. Food Chem. 2012, 134, 589–596. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Bassi, D.; Pedò, S.; Serraiocco, A. Environmental and seasonal influence on virgin olive (Olea europaea L.) oil volatiles in northern Italy. Scientia Horticulturae 2009, 122, 385–392. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Beltrán, G.; Ortega, D.; Fernández, A.; Jiménez, A.; Uceda, M. Characterisation of virgin olive oil of Italian olive cultivars: “Frantoio” and “Leccino”, grown in Andalusia. Food Chem. 2005, 89, 387–391. [Google Scholar] [CrossRef]
- Haddi, Z.; Amari, A.; Ould Ali, A.; El Bari, N.; Barhoumi, H.; Maaref, A.; Jaffrezic-Renault, N.; Bouchikhi, B. Discrimination and identification of geographical origin virgin olive oil by an e-nose based on MOS sensors and pattern recognition techniques. Procedia Eng. 2011, 25, 1137–1140. [Google Scholar] [CrossRef]
- Tena, N.; Lazzez, A.; Aparicio-Ruiz, R.; García-González, D.L. Volatile compounds characterizing Tunisian Chemlali and Chétoui virgin olive oils. J. Agric. Food Chem. 2007, 55, 7852–7858. [Google Scholar] [CrossRef]
- Melucci, D.; Bendini, A.; Tesini, F.; Barbieri, S.; Zappi, A.; Vichi, S.; Conte, L.; Toschi, T.G. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Tokatli, F.; Buratti, S.; Alamprese, C. Discriminative capacities of infrared spectroscopy and e-nose on Turkish olive oils. Eur. Food Res. Technol. 2017, 243, 2035–2042. [Google Scholar] [CrossRef]
- Pardo, M.; Sberveglieri, G.; Taroni, A.; Masulli, F.; Valentini, G. Decompositive classification models for electronic noses. Anal. Chim. Acta 2001, 446, 221–230. [Google Scholar] [CrossRef]
- Pardo, M.; Sberveglieri, G.; Gardini, S.; Dalcanale, E. A hierarchical classification scheme for an Electronic Nose. Sens. Actuators B 2000, 69, 359–365. [Google Scholar] [CrossRef]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors affecting extra-virgin olive oil composition. In Horticultural Reviews; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 2011; Volume 38, pp. 83–147. [Google Scholar]
- Kaftan, A.; Elmaci, Y. Aroma characterization of virgin olive oil from two Turkish olive varieties by SPME/GC/MS. Int. J. Food Prop. 2011, 14, 1160–1169. [Google Scholar] [CrossRef]
- Yang, Y.; Ferro, M.D.; Cavaco, I.; Liang, Y. Detection and identification of extra virgin olive oil adulteration by GC-MS combined with chemometrics. J. Agric. Food Chem. 2013, 61, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Ahmed, N.; Zeeshan, M.; Iqbal, N.; Farooq, M.Z.; Shah, S.A. Investigation on bio-oil yield and quality with scrap tire addition in sugarcane bagasse pyrolysis. J. Clean. Prod. 2018, 196, 927–934. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 1215–1216. [Google Scholar]
- Pohanish, R.P. Sittig’s Handbook of Pesticides and Agricultural Chemicals, 2nd ed.; Elsevier: Waltham, MA, USA, 2015; pp. 211–212. [Google Scholar]
- Serrano, R.; Nacher-Mestre, J.; Portoles, T.; Amat, F.; Hernandez, F. Non-target screening of organic contaminants in marine salts by gas chromatography coupled to high-resolution time-of-flight mass spectrometry. Talanta 2011, 85, 877–884. [Google Scholar] [CrossRef]
- Sberveglieri, V.; Bhandari, M.P.; Núñez Carmona, E.; Betto, G.; Sberveglieri, G. A novel MOS nanowire gas sensor device (S3) and GC-MS-based approach for the characterization of grated Parmigiano Reggiano cheese. Biosensors 2016, 6, 60. [Google Scholar] [CrossRef]
- Bhandari, M.P.; Carmona, E.N.; Abbatangelo, M.; Sberveglieri, V.; Duina, G.; Malla, R.; Comini, E.; Sberveglieri, G. Discrimination of quality and geographical origin of extra virgin olive oil by S3 device with metal oxides gas sensors. Proceedings 2018, 2, 1061. [Google Scholar] [CrossRef]
- Bhandari, M.P.; Núñez Carmona, E.; Galstyan, V.; Sberveglieri, V. Quality evaluation of Parmigiano Reggiano cheese by a novel nanowire device S3 and evaluation of the VOCs profile. Procedia Eng. 2016, 168, 460–464. [Google Scholar] [CrossRef]
- Sberveglieri, V.; Bhandari, M.P.; Núñez Carmona, E.; Betto, G.; Soprani, M.; Malla, R.; Sberveglieri, G. Spectrocolorimetry and nanowire gas sensor device S3 for the analysis of Parmigiano Reggiano cheese ripening. In Proceedings of the 2017 ISOCS/IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Montreal, QC, Canada, 28–31 May 2017. [Google Scholar]
- Soprani, M.; Abbatangelo, M.; Núñez Carmona, E.; Duina, G.; Malgaretti, M.; Comini, E.; Sberveglieri, V.; Bhandari, M.P.; Bolpagni, D.; Sberveglieri, G. Array of semiconductor nanowires gas sensor for IoT in wastewater management. In Proceedings of the 2018 International Workshop on Metrology for Industry 4.0 and IoT, Brescia, Italy, 16–18 April 2018. [Google Scholar]
- Abbatangelo, M.; Núñez Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. Application of a novel S3 nanowire gas sensor device in parallel with GC-MS for the identification of rind percentage of grated Parmigiano Reggiano. Sensors 2018, 18, 1617. [Google Scholar] [CrossRef] [PubMed]
- Abbatangelo, M.; Núñez Carmona, E.; Sberveglieri, V. Application of a novel S3 nanowire gas sensor device in parallel with GC-MS for the identification of Parmigiano Reggiano from US and European competitors. J. Food Eng. 2018, 236, 36–43. [Google Scholar] [CrossRef]
Sample Availability: Garda PDO EVOO, olive oil and non-PDO EVOOs (phase1) are available from the authors. |
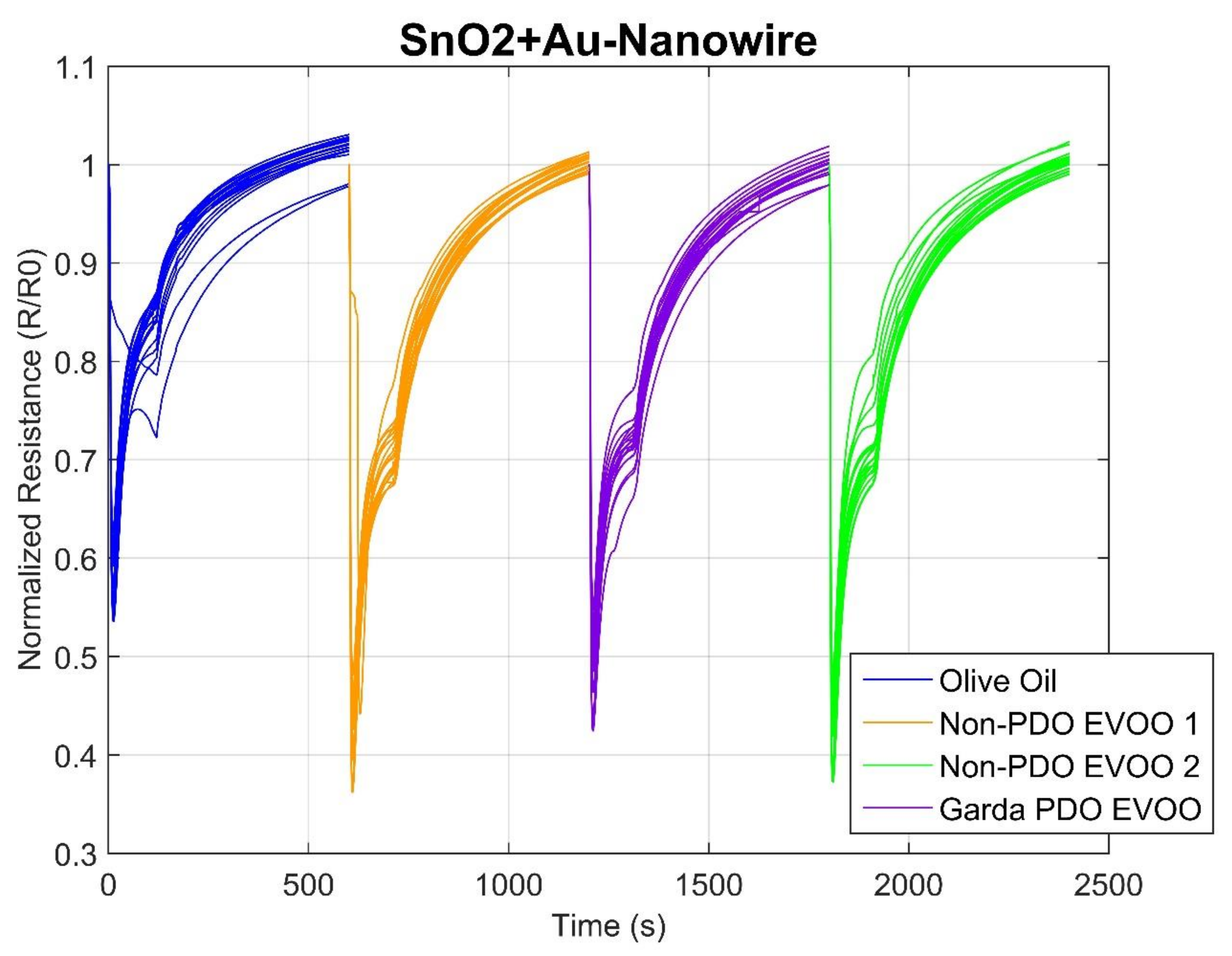
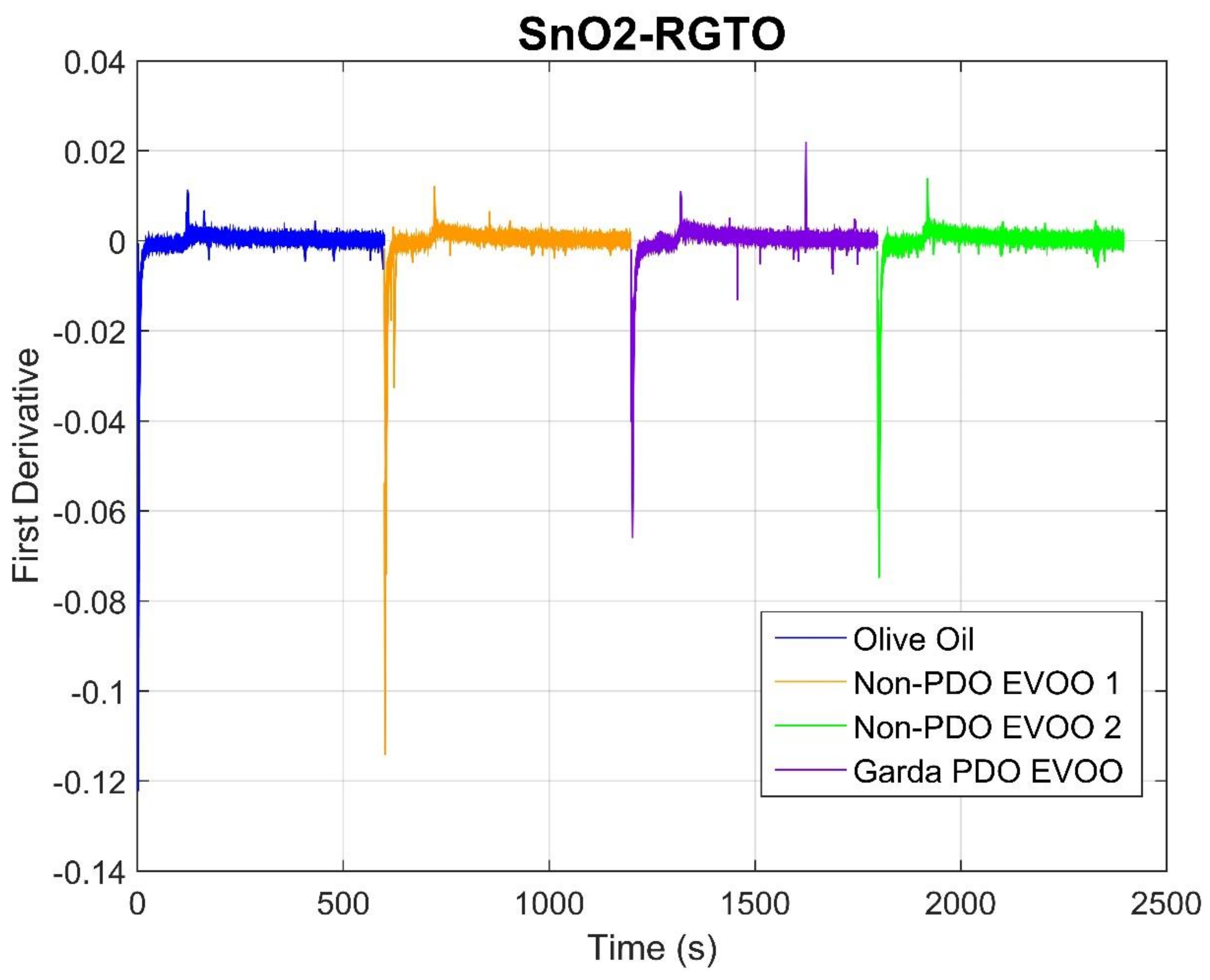
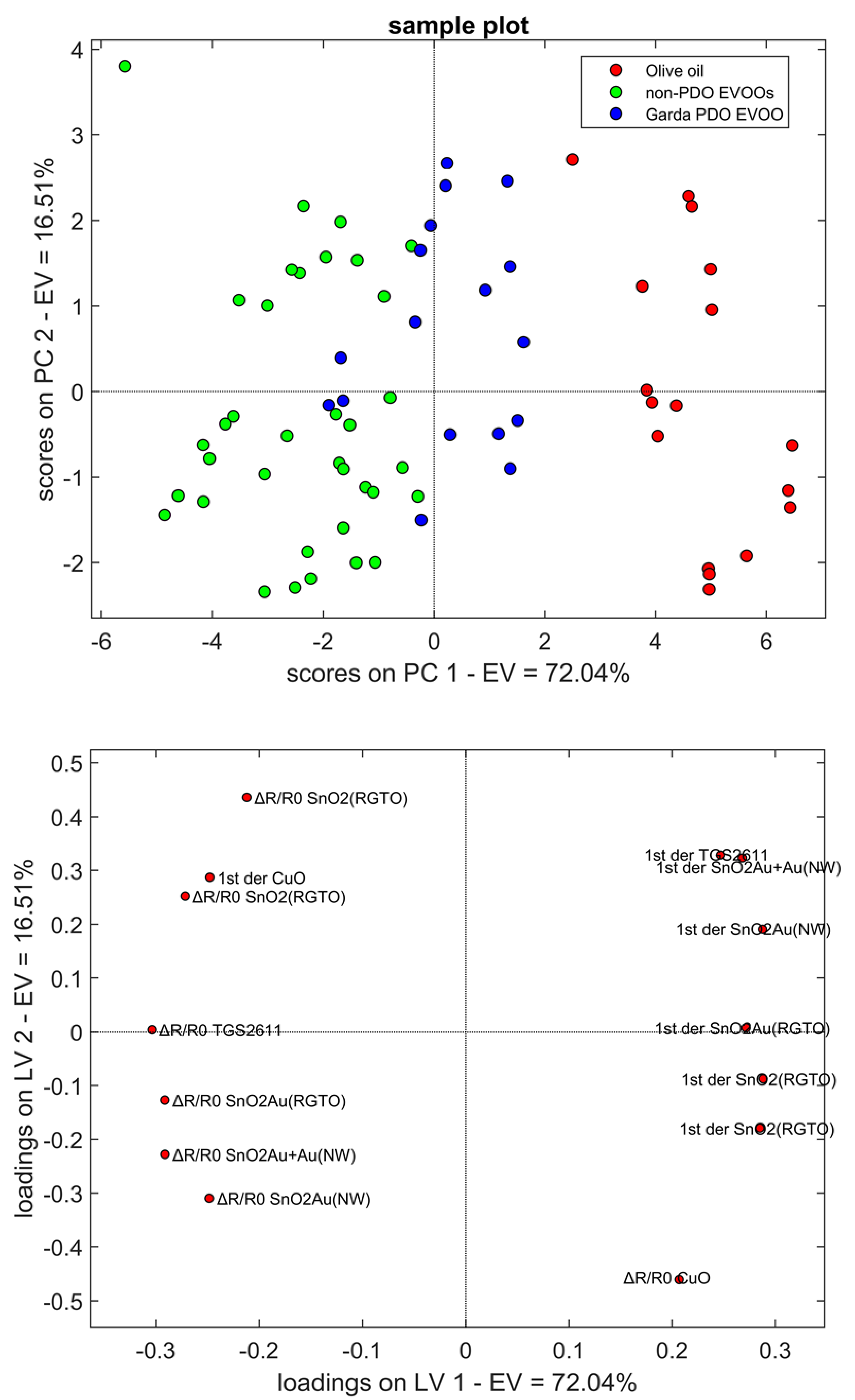
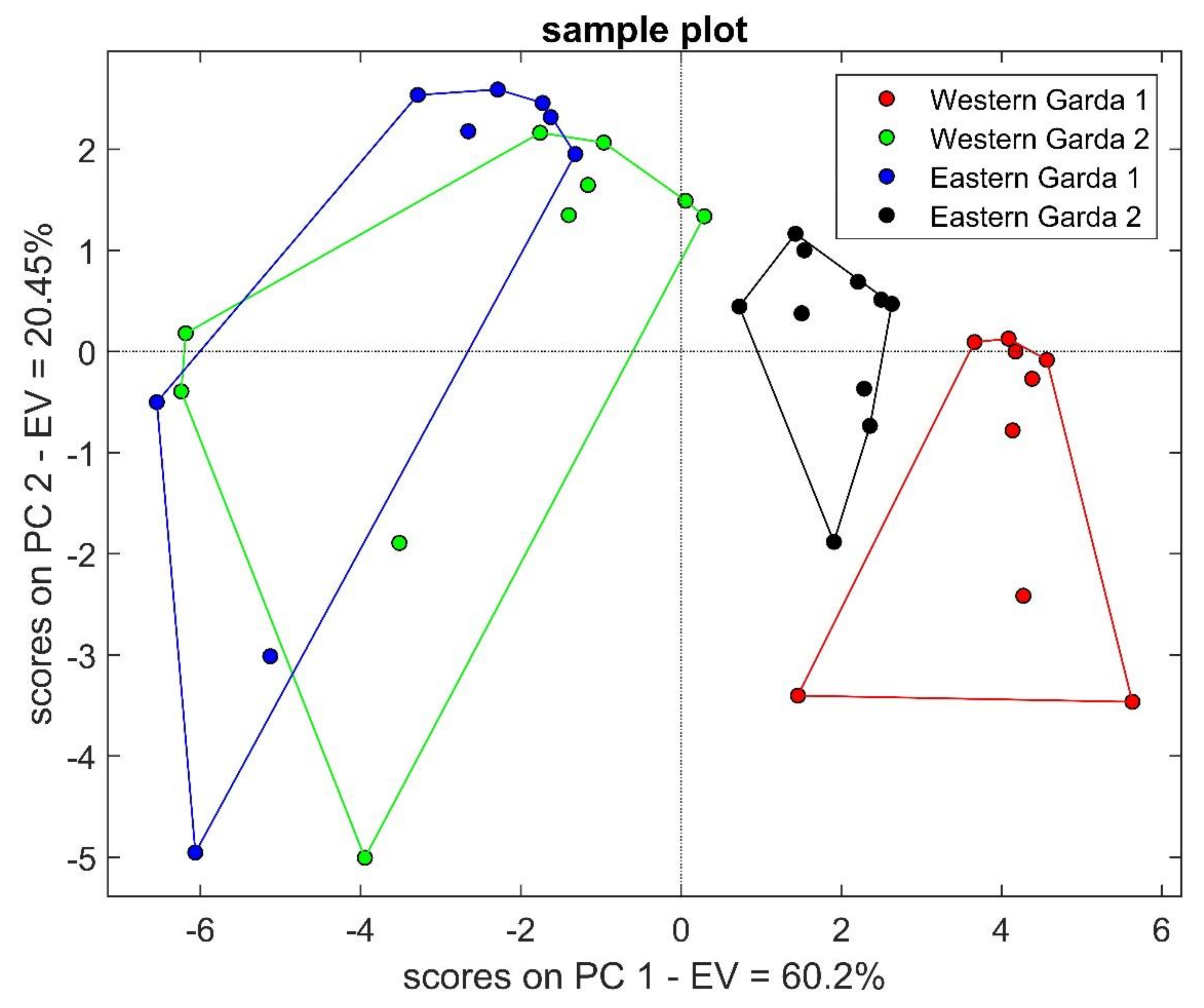
| Rt | Name | Phase 1 | Phase 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Garda PDO EVOO | Non-PDO EVOO 1 | Non-PDO EVOO 2 | Olive Oil | Western Garda EVOO 1 | Western Garda EVOO 2 | Eastern Garda EVOO 1 | Eastern Garda EVOO 2 | ||
| 11.53 | 2-Hexenal | 4.82 × 107 | 4.46 × 107 | 5.99 × 107 | 1.81 × 106 | 5.74 × 107 | 3.32 × 107 | 5.27 × 107 | 4.71 × 107 |
| 13.38 | β-Ocimene | 4.31 × 105 | 6.25 × 105 | 4.49 × 105 | 0 | 1.17 × 106 | 3.73 × 105 | 6.21 × 105 | 4.66 × 105 |
| 14.57 | Acetic acid, hexyl ester | 1.96 × 105 | 9.08 × 105 | 2.63 × 105 | 5.09 × 104 | 3.62 × 105 | 4.23 × 105 | 2.76 × 105 | 4.21 × 105 |
| 17.03 | 3-Hexen-1-ol, acetate, (Z)- | 5.66 × 105 | 4.11 × 106 | 8.24 × 105 | 3.80 × 105 | 1.18 × 106 | 1.57 × 106 | 1.08 × 106 | 8.54 × 105 |
| 19.20 | 1-Hexanol | 1.94 × 106 | 9.46 × 105 | 2.35 × 106 | 3.80 × 105 | 1.47 × 106 | 22.82 × 106 | 2.90 × 106 | 2.09 × 106 |
| 20.98 | 3-Hexen-1-ol | 5.03 × 105 | 1.17 × 106 | 1.56 × 106 | 4.15 × 105 | 1.22 × 106 | 1.29 × 106 | 1.07 × 106 | 9.95 × 105 |
| 21.29 | Nonanal | 6.26 × 105 | 2.04 × 106 | 9.31 × 105 | 1.65 × 105 | 8.68 × 105 | 5.11 × 105 | 5.77 × 105 | 3.31 × 105 |
| 22.41 | 2-Hexen-1-ol, (E)- | 5.15 × 106 | 1.13 × 106 | 6.08 × 106 | 5.72 × 105 | 2.47 × 106 | 4.44 × 106 | 5.56 × 106 | 3.00 × 106 |
| 25.54 | Ammonium acetate | 1.12 × 106 | 1.62 × 106 | 2.44 × 106 | 5.00 × 105 | 1.07 × 106 | 2.77 × 106 | 3.36 × 106 | 8.01 × 105 |
| 35.99 | Butanoic acid | 2.45 × 105 | 6.69 × 105 | 1.29 × 106 | 2.07 × 105 | 4.01 × 105 | 6.36 × 105 | 4.55 × 106 | 1.51 × 105 |
| 42.37 | α-Farnesene | 2.46 × 105 | 2.16 × 105 | 3.37 × 105 | 0 | 5.73 × 105 | 1.97 × 105 | 2.65 × 105 | 1.51 × 105 |
| 48.13 | Hexanoic acid | 7.88 × 105 | 1.50 × 106 | 2.13 × 106 | 7.18 × 105 | 6.30 × 105 | 1.85 × 106 | 5.77 × 106 | 5.79 × 105 |
| Rt | Name | Western Garda EVOO 1 | Western Garda EVOO 2 | Eastern Garda EVOO 1 | Eastern Garda EVOO 2 |
|---|---|---|---|---|---|
| 2.40 | 4,4-Dimethyloxazolidine | 0 | 0 | 0 | 2.12 × 104 |
| 2.85 | 1-Fluorooctane | 0 | 0 | 0 | 5.53 × 105 |
| 4.17 | 4-Allyl-5-furan-2-yl-2,4-dihydro-[1,2,4]triazole-3-thione | 0 | 0 | 0 | 1.43 × 104 |
| 4.37 | 3,5-Dimethyloctane | 0 | 0 | 0 | 2.56 × 105 |
| 5.33 | Heptylbenzene | 0 | 0 | 0 | 3.39 × 104 |
| 7.89 | Cyclobut-1-enylmethanol | 0 | 8.99 × 104 | 0 | 0 |
| 10.52 | trans-1,2-bis-(1-methylethenyl)cyclobutane | 2.84 × 105 | 0 | 0 | 0 |
| 14.08 | 1-(3-cyclohexen-1-yl)-ethanone | 1.21 × 105 | 0 | 0 | 0 |
| 18.12 | Methyl heptenone | 1.83 × 105 | 0 | 0 | 0 |
| 20.04 | 4-Butoxy-1-butene | 1.47 × 105 | 0 | 0 | 0 |
| 24.52 | 2,6-Dimethyl-1,3,5,7-octatetraene, E,E- | 1.45 × 105 | 0 | 0 | 0 |
| 25.15 | 1-Octen-3-ol | 0 | 5.46 × 104 | 0 | 0 |
| 28.77 | 3,5-Octadien-2-one, (E,E)- | 1.95 × 105 | 0 | 0 | 0 |
| 30.80 | 1-Deoxy-d-arabitol | 0 | 1.64 × 105 | 0 | 0 |
| 64.37 | n-Hexadecanoic acid | 0 | 0 | 1.36 × 105 | 0 |
| 69.21 | Silver decanoate | 0 | 0 | 1.03 × 105 | 0 |
| 76.49 | Cholest-7-en-3β,5α-diol-6α-benzoate | 0 | 0 | 2.60 × 105 | 0 |
| 81.33 | tert-Butyldimethylsilyl 2-(2-(2-butoxyethoxy)ethoxy)acetate | 1.20 × 105 | 0 | 0 | 0 |
| 84.29 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 0 | 0 | 5.54 × 106 | 0 |
| 86.46 | Bis(1-chloro-2-propyl) (3-chloro-1-propyl)phosphate | 1.84 × 105 | 0 | 0 | 0 |
| 90.55 | Octadecanoic acid | 0 | 0 | 1.17 × 107 | 0 |
| Materials (Type) | Composition | Morphology | Operating Temperature (°C) |
|---|---|---|---|
| Phase 1 | |||
| SnO2Au (n) | SnO2 functionalized with Au clusters | RGTO | 400 °C |
| SnO2 (n) | SnO2 | RGTO | 300 °C |
| SnO2 (n) | SnO2 | RGTO | 400 °C |
| SnO2Au + Au (n) | SnO2 grown with Au and functionalized with gold clusters | Nanowire | 350 °C |
| SnO2Au (n) | SnO2 grown with Au | Nanowire | 350 °C |
| CuO (p) | CuO | Nanowire | 400 °C |
| Phase 2 | |||
| SnO2Au + Au (n) | SnO2 grown with Au and functionalized with gold clusters | Nanowire | 350 °C |
| SnO2Au + Au (n) | SnO2 grown with Au and functionalized with gold clusters | Nanowire | 400 °C |
| CuO (p) | CuO | Nanowire | 350 °C |
| SnO2 (n) | SnO2 | RGTO | 450 °C |
| SnO2Au (n) | SnO2 with gold clusters | RGTO | 400 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbatangelo, M.; Núñez-Carmona, E.; Duina, G.; Sberveglieri, V. Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO. Molecules 2019, 24, 1457. https://doi.org/10.3390/molecules24081457
Abbatangelo M, Núñez-Carmona E, Duina G, Sberveglieri V. Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO. Molecules. 2019; 24(8):1457. https://doi.org/10.3390/molecules24081457
Chicago/Turabian StyleAbbatangelo, Marco, Estefanía Núñez-Carmona, Giorgio Duina, and Veronica Sberveglieri. 2019. "Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO" Molecules 24, no. 8: 1457. https://doi.org/10.3390/molecules24081457
APA StyleAbbatangelo, M., Núñez-Carmona, E., Duina, G., & Sberveglieri, V. (2019). Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO. Molecules, 24(8), 1457. https://doi.org/10.3390/molecules24081457








