Biomimetic Mineralization of Magnetic Iron Oxide Nanoparticles Mediated by Bi-Functional Copolypeptides
Abstract
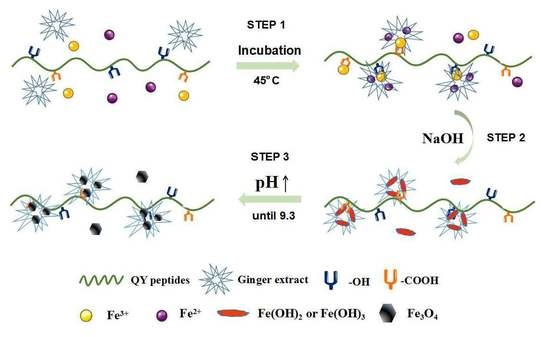
1. Introduction
2. Results
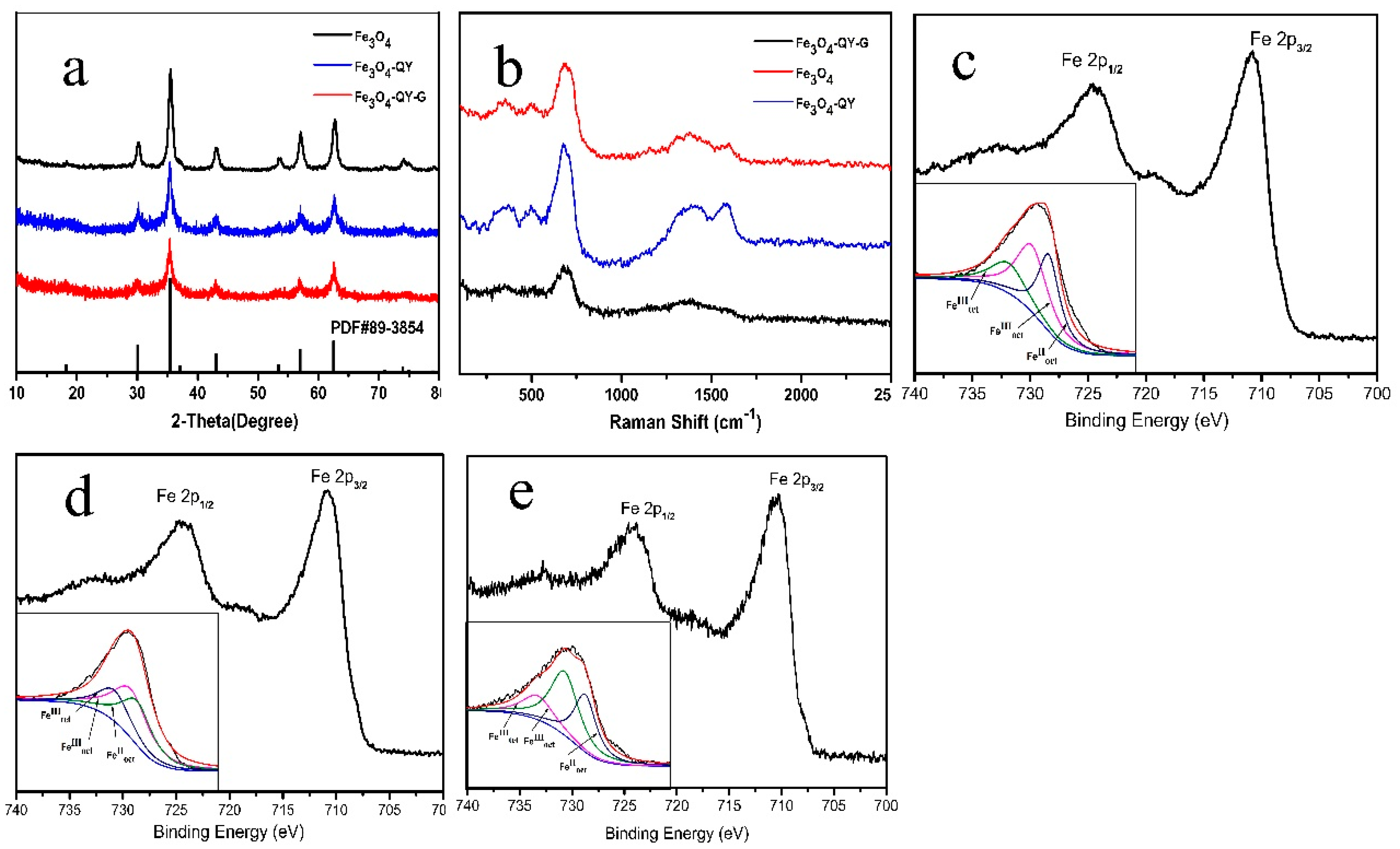
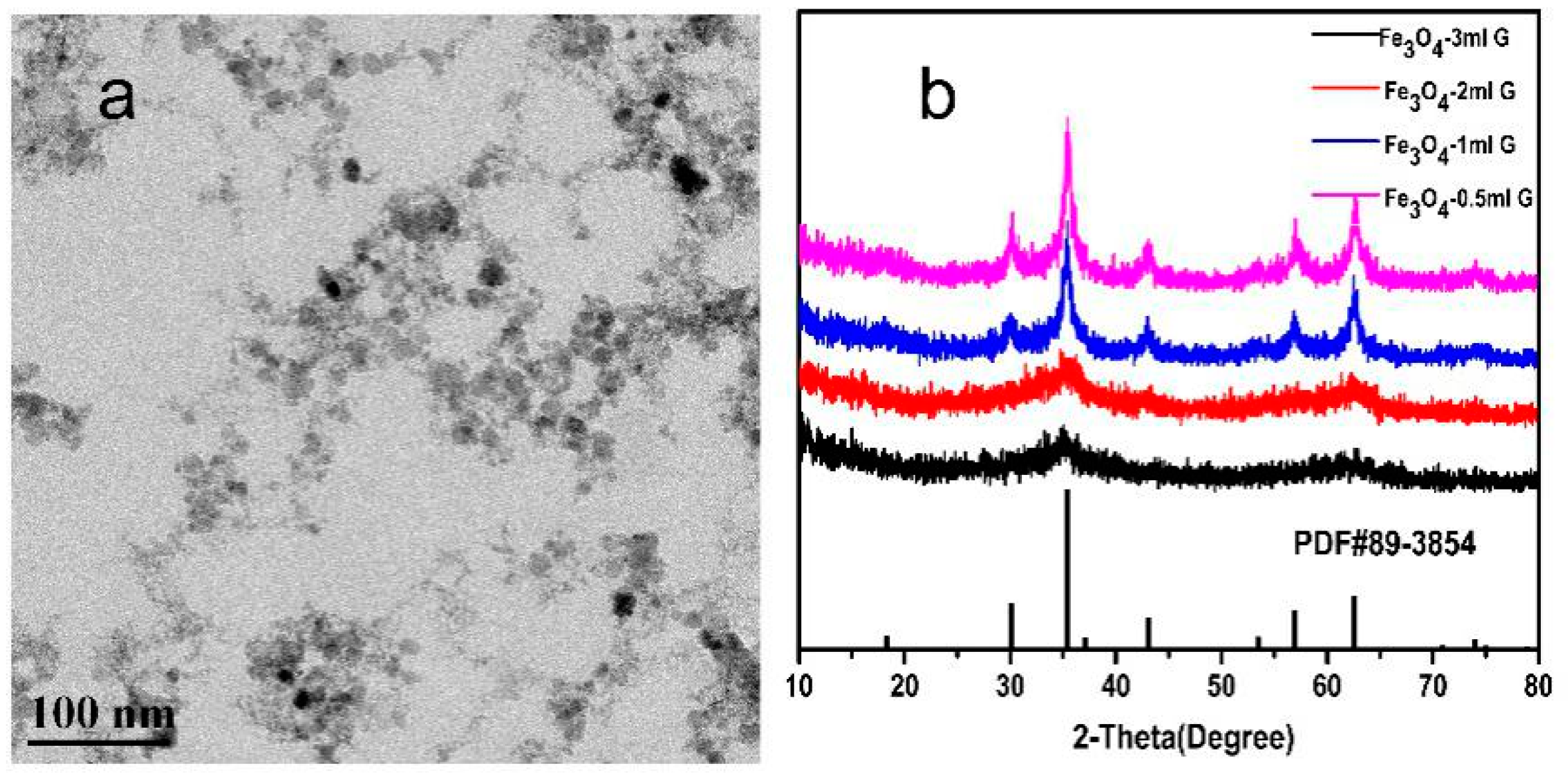
2.1. Characterization of Mineral Phases
2.2. Constituent Analysis via FT-IR
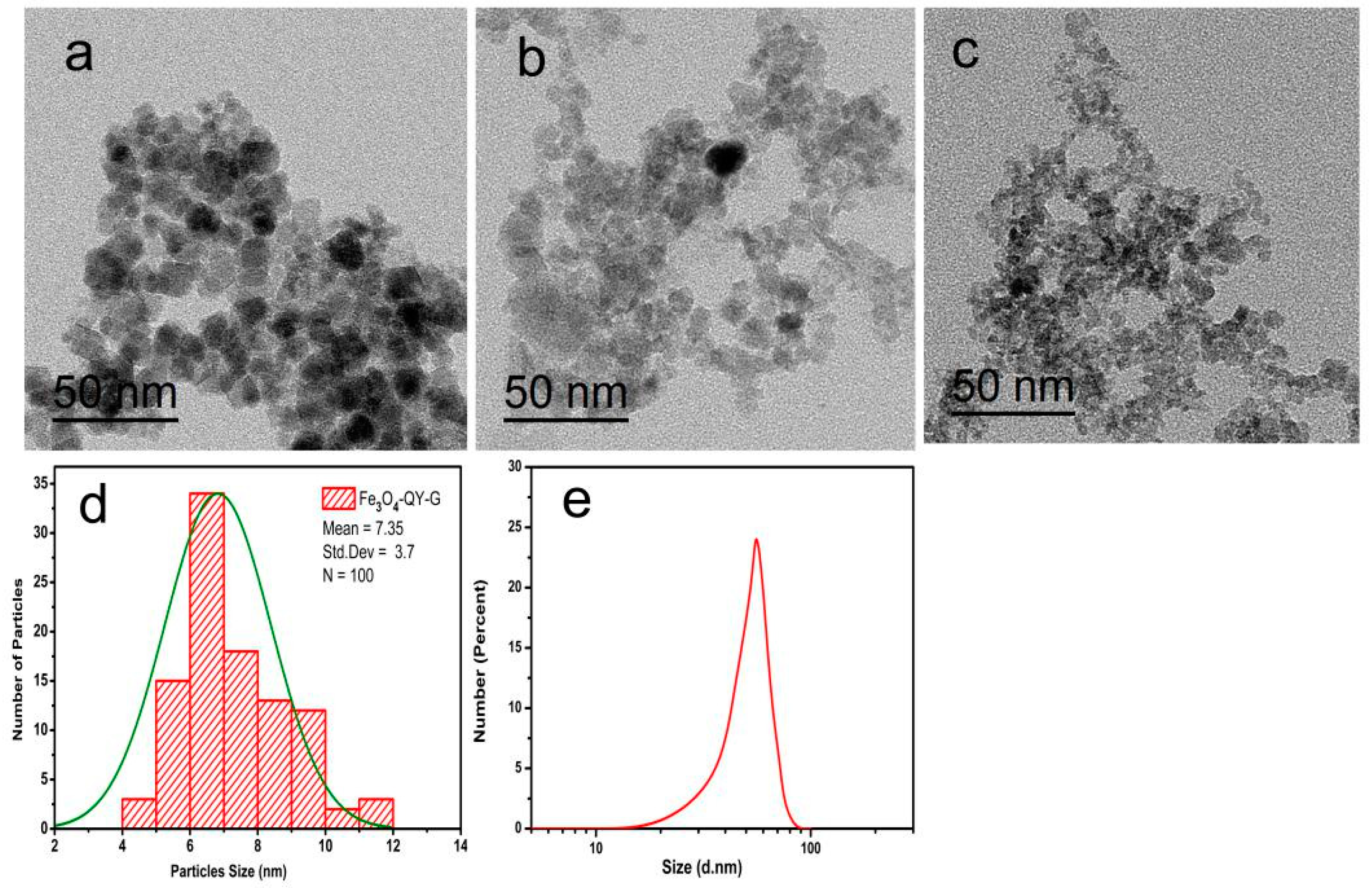
2.3. Morphology and Solvent-Dependent Stability
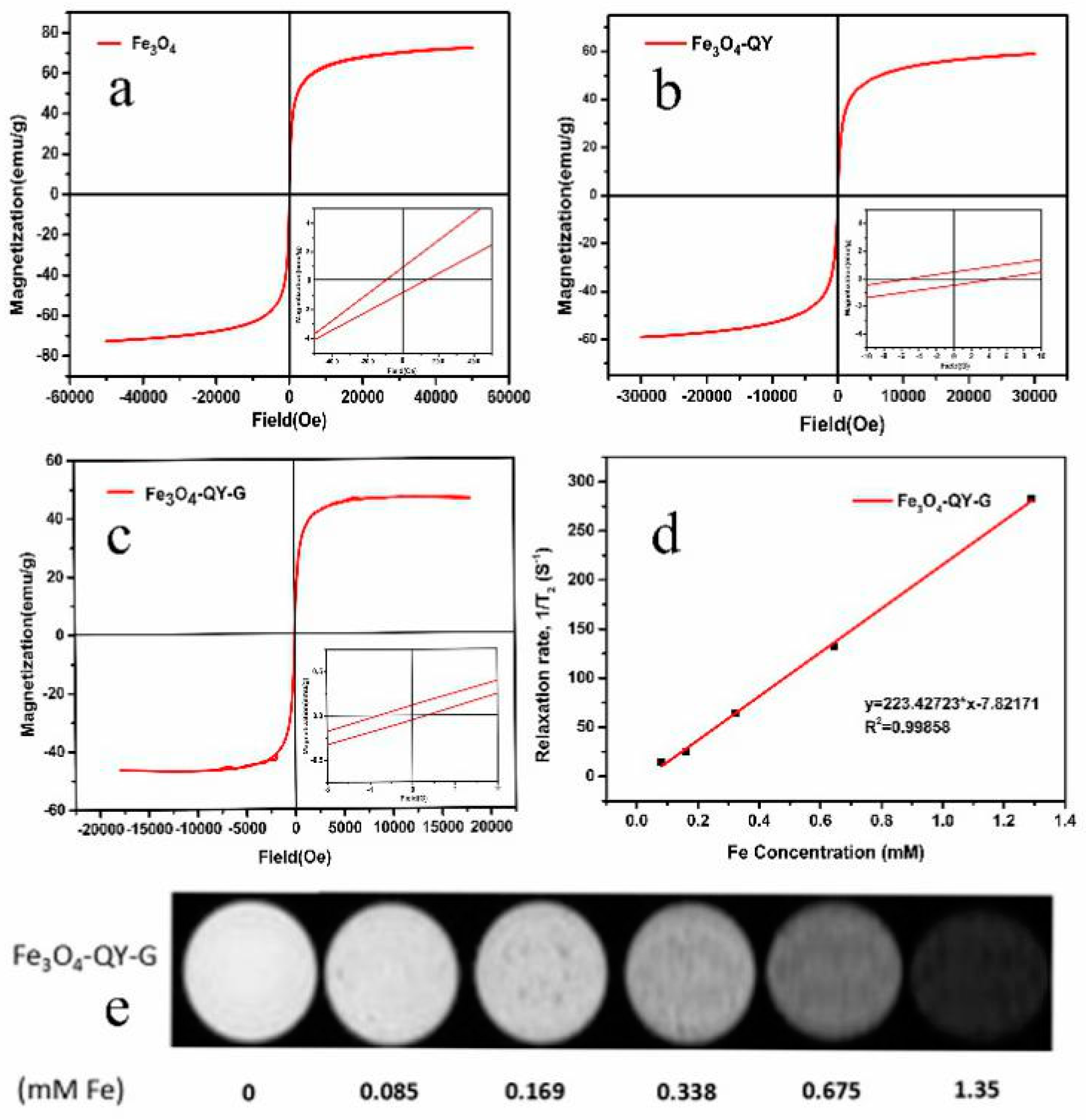
2.4. Magnetic Property and Imaging Efficiency
2.5. The Chelating Ability and Reduction Capability Results of Ginger Extract
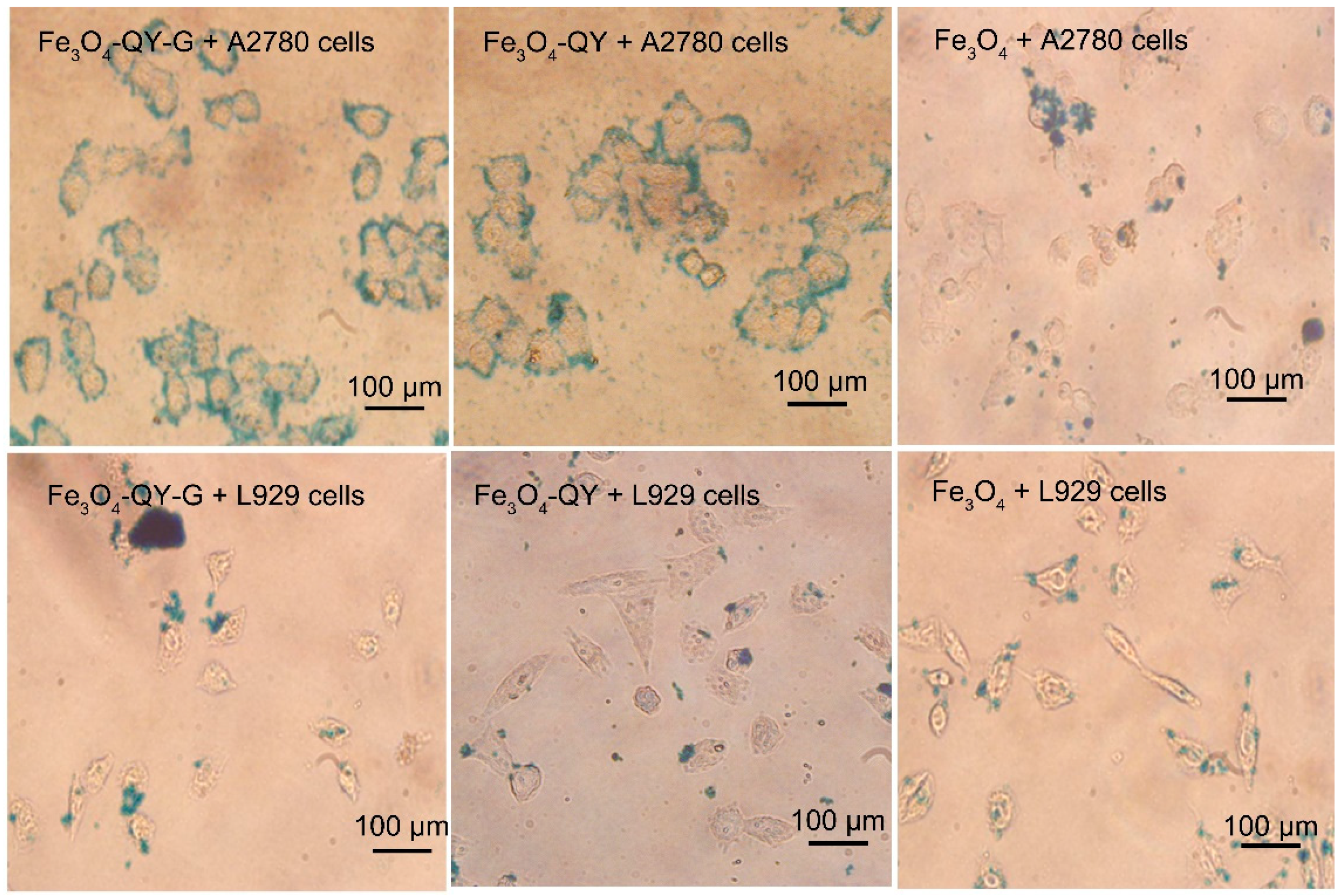
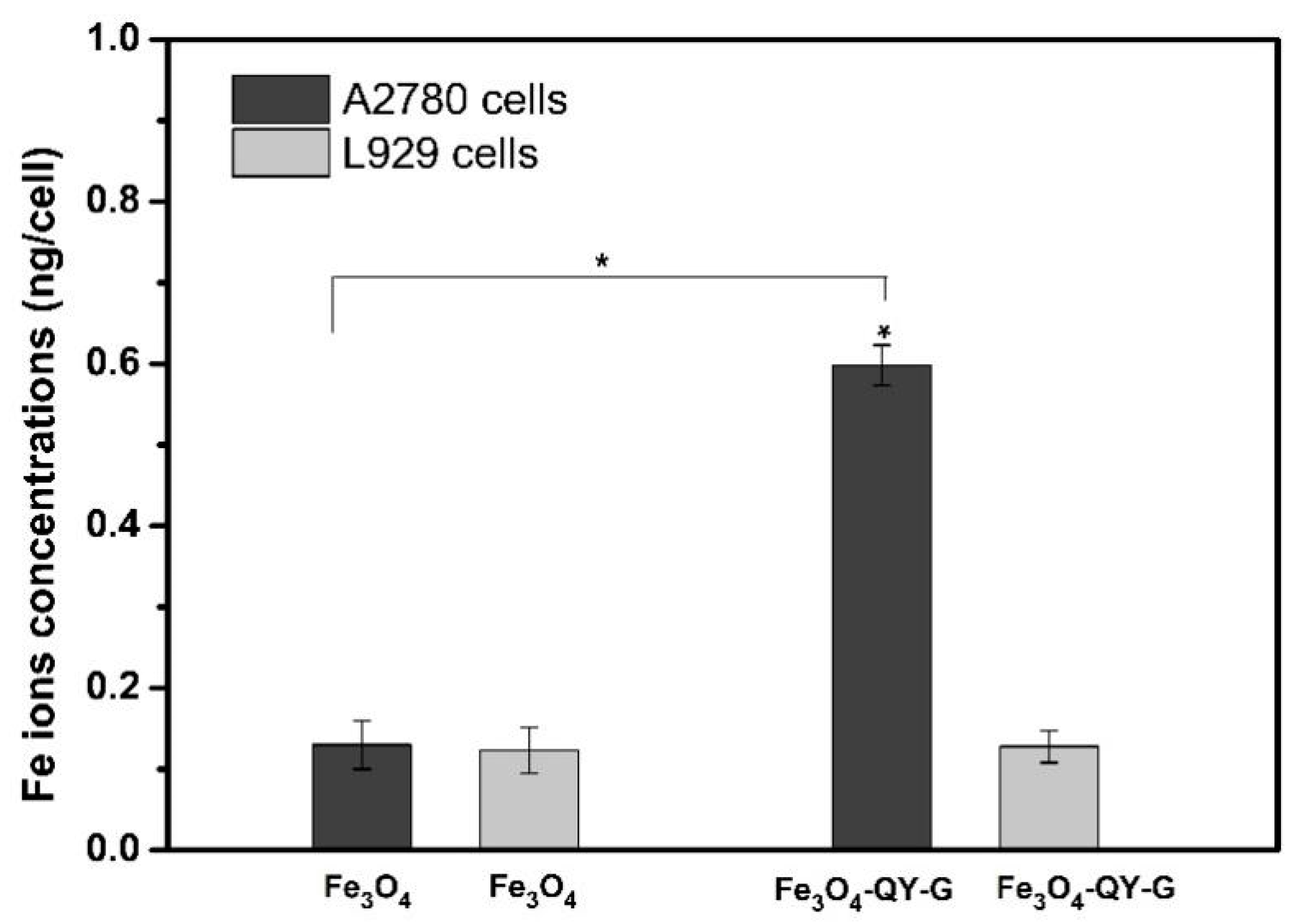
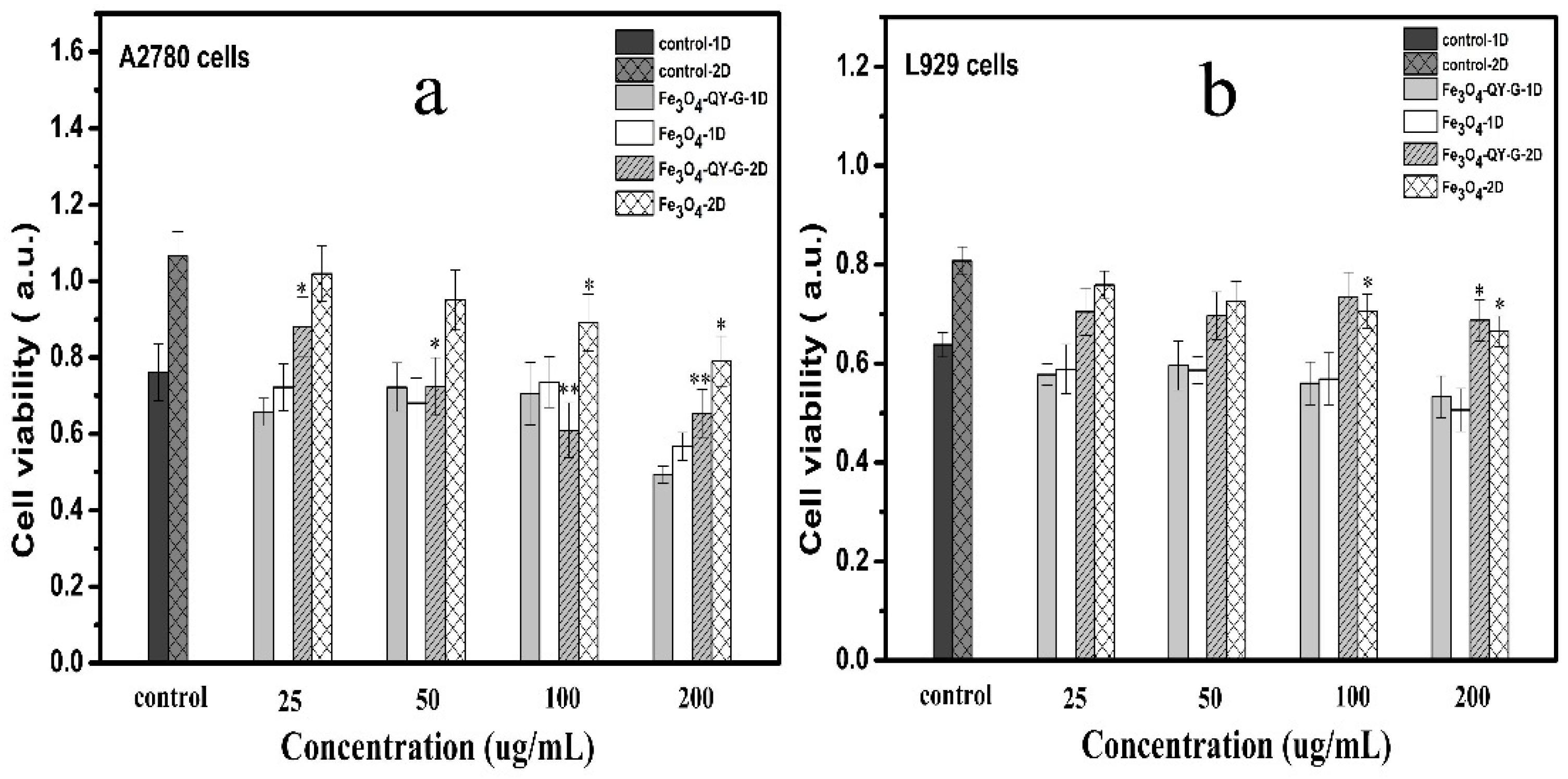
2.6. Cell Targeting and Cytotoxicity Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Fe3O4 Nanoparticles
4.3. Physicochemical Characterizations
4.4. The Chelating Ability on Fe2+ and Reduction Capability Test of Fe3+
4.5. In Vitro Cytotoxicity Study of Fe3O4-QY-G Nanoparticles
4.6. Cell Targeting Study of Fe3O4-QY-G Nanoparticles
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides—A review. Int. J. Eng. Sci. 2010, 2, 127–146. [Google Scholar] [CrossRef]
- Darja, L.; Alenka, M. Anisotropic magnetic nanoparticles: A review of their properties, syntheses and potential applications. Prog. Mater. Sci. 2018, 95, 286–328. [Google Scholar]
- Ma, M.; Wu, Y.; Zhou, J.; Sun, Y.K.; Zhang, Y.; Gu, N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 2004, 268, 33–39. [Google Scholar] [CrossRef]
- Heba, M.Y.; Fatma, M.M.; Mariam, H.M.; Amira, B.E.M.; Asmaa, M.A.; Asmaa, A.A.; Maha, A.M.; Faten, A.M. Review of Green Methods of Iron Nanoparticles Synthesis and Applications. BioNanoScience 2018, 8, 1–13. [Google Scholar]
- Xie, S.; Yin, G.; Pu, X.; Hu, Y.; Huang, Z.; Liao, X.; Chen, X. Biomimetic Mineralization of Tumor Targeted Ferromagnetic Iron Oxide Nanoparticles Used for Media of Magnetic Hyperthermia. Curr. Drug Deliv. 2017, 14, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lenders, J. Biomimetic Macromolecule-Mediated Magnetite Mineralization; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2015. [Google Scholar]
- Yang, W.; Guo, W.; Chang, J.; Zhang, B. Protein/peptide-templated biomimetic synthesis of inorganic nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 401–417. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Yu, J.; Fang, X.; Wang, X.; Shi, D. Site-Specific Biomimetic Precision Chemistry of Bimodal Contrast Agent with Modular Peptides for Tumor-Targeted Imaging. Bioconjug. Chem. 2017, 28, 330–335. [Google Scholar] [CrossRef]
- Yang, W.; Wu, X.; Dou, Y.; Chang, J.; Xiang, C.; Yu, J.; Wang, J.; Wang, X.; Zhang, B. A human endogenous protein exerts multi-role biomimetic chemistry in synthesis of paramagnetic gold nanostructures for tumor bimodal imaging. Biomaterials 2018, 161, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shi, X.; Shi, Y.; Yao, D.; Chen, S.; Zhou, X.; Zhang, B. Beyond the Roles in Biomimetic Chemistry: An Insight into the Intrinsic Catalytic Activity of an Enzyme for Tumor-Selective Phototheranostics. ACS Nano 2018, 12, 12169–12180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tanya, P.; Pierre, E.; Liu, X.; David, V.; Ruslan, P.; Surya, M.; Marit, N. Self-Assembly and Biphasic Iron-Binding Characteristics of Mms6, A Bacterial Protein That Promotes the Formation of Superparamagnetic Magnetite Nanoparticles of Uniform Size and Shape. Biomacromolecules 2011, 13, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Larken, E.E.; Stephanie, G.G.; Stephen, O.B.; Timothy, J.D.; Galen, D.S.; C, B.M.; G, A.H. Cooperative Assembly of Magnetic Nanoparticles and Block Copolypeptides in Aqueous Media. Nano Lett. 2003, 3, 1489–1493. [Google Scholar]
- Wei, Y.; Yin, G.; Ma, C.; Huang, Z.; Chen, X.; Liao, X.; Yao, Y.; Yin, H. Synthesis and cellular compatibility of biomineralized Fe3O4 nanoparticles in tumor cells targeting peptides. Colloid Surf. B 2013, 107, 180–188. [Google Scholar] [CrossRef]
- Mackay, J.A.; Chen, M.; Mcdaniel, J.R.; Liu, W.; Simnick, A.J.; Chilkoti, A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat. Mater. 2009, 8, 993–999. [Google Scholar] [CrossRef]
- Nowsheen, G.; Archana, B.L.; Gary, L.B.; Dhanjay, J. An assessment of biopolymer- and synthetic polymer-based scaffolds for bone and vascular tissue engineering. Polym. Int. 2003, 62, 523–533. [Google Scholar]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 2010, 1, 517–520. [Google Scholar] [CrossRef]
- Zayed, M.F.; Eisa, W.H.; Shabaka, A.A. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim. Acta A 2012, 98, 423–428. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim. Acta A 2013, 101, 184–190. [Google Scholar] [CrossRef]
- Kumar, K.P.; Paul, W.; Sharma, C.P. Green synthesis of gold nanoparticles with Zingiber officinale extract: Characterization and blood compatibility. Process. Biochem. 2011, 46, 2007–2013. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crop. Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef]
- Olena, I.; Jacek, G.; Barbara, P.; Łucja, P.; Tomasz, Z.; Grzegorz, N.; Marcin, J.; Anna, M.G.; Stefan, J. Self-organizing silver and ultrasmall iron oxide nanoparticles prepared with ginger rhizome extract: Characterization, biomedical potential and microstructure analysis of hydrocolloids. Mater. Des. 2017, 133, 307–324. [Google Scholar]
- You, F.; Yin, G.; Pu, X.; Li, Y.; Hu, Y.; Huang, Z.; Liao, X.; Yao, Y.; Chen, X. Biopanning and characterization of peptides with Fe3O4 nanoparticles-binding capability via phage display random peptide library technique. Colloid Surf. B 2016, 141, 537–545. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochim. Acta 2009, 495, 57–62. [Google Scholar]
- Soumya, D.; Hendry, M.J. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem. Geol. 2011, 290, 101–108. [Google Scholar]
- Li, Y.; Church, J.S.; Woodhead, A.L. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, C.; Vandenberghe, R.E.; Markova-Deneva, I. Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. J. Magn. Magn. Mater. 2010, 322, 1904–1911. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Qian, H.; Ning, J.; Xu, S. Magnetite (Fe3O4) tetrakaidecahedral microcrystals: Synthesis, characterization, and micro-Raman study. Mater. Charact. 2011, 62, 148–151. [Google Scholar] [CrossRef]
- Aliahmad, M.; Nasiri, M.N. Synthesis of maghemite (γ-Fe2O3) nanoparticles by thermal-decomposition of magnetite (Fe3O4) nanoparticles. Mater. Sci. 2013, 31, 264–268. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Chuang, C.H.; Chen, H.C.; Wan, C.J.; Chen, T.L.; Lin, L.Y. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT-Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Aicha, J.; Ângela, F.; Lillian, B.; Hassiba, C.; Lotfi, A.; Isabel, F.; Hassen, B.C. Chemical and antioxidant parameters of dried forms of ginger rhizomes. Ind. Crop. Prod. 2015, 77, 30–35. [Google Scholar]
- Payne, K.J.; Veis, A. Fourier Transform IR Spectroscopy of Collagen and Gelatin Solutions: Deconvolution of the Amide I Band for Conformational Studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Kang, H.J.; Fasséli, C.; Fiona, C.; Thomas, P.; Edward, N.B. Stabilizing Isopeptide Bonds Revealed in Gram-Positive Bacterial Pilus Structure. Science 2007, 318, 1625–1628. [Google Scholar] [CrossRef]
- Bijan, Z.; Mark, H. Spontaneous Intermolecular Amide Bond Formation between Side Chains for Irreversible Peptide Targeting. J. Am. Chem. Soc. 2010, 132, 4526–4527. [Google Scholar]
- Jonathan, M.B.; Luciano, A.M.; Puneet, S.; Julian, P.W.; Kym, F.F.; Olaf, S. Amide bonds assemble pili on the surface of bacilli. Proc. Natl. Acad. Sci. USA 2008, 105, 10215–10220. [Google Scholar]
- Barick, K.C.; Aslam, M.; Lin, Y.P.; Bahadur, D.; Prasad, P.V.; Dravid, V.P. Novel and efficient MR active aqueous colloidal Fe3O4 nanoassemblies. J. Mater. Chem. 2009, 19, 7023–7029. [Google Scholar] [CrossRef]
- Barick, K.C.; Hassan, P.A. Glycine passivated Fe3O4 nanoparticles for thermal therapy. Colloid Surf. A 2012, 369, 96–102. [Google Scholar] [CrossRef]
- Chen, X.Y.; Ahn, D.U. Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+ or ultraviolet light. J. Am. Oil Chem. Soc. 1998, 75, 1717–1721. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Song, R.Q.; Cölfen, H. Additive controlled crystallization. CrystEngComm 2011, 13, 1249–1276. [Google Scholar] [CrossRef]
- Evans, J.S. “Tuning in” to Mollusk Shell Nacre- and Prismatic-Associated Protein Terminal Sequences. Implications for Biomineralization and the Construction of High Performance Inorganic? Organic Composites. Chem. Rev. 2008, 108, 4455–4462. [Google Scholar] [CrossRef]
- Graeme, K.H.; Jason, O.Y.; Bernd, G.; Mikko, K.; Harvey, A.G. The Flexible Polyelectrolyte Hypothesis of Protein−Biomineral Interaction. Langmuir 2010, 26, 18639–18646. [Google Scholar]
- Lajos, K.; Daniel, H.; Gabor, V.; Peter, T. Structural disorder in proteins brings order to crystal growth in biomineralization. Bone 2012, 51, 528–534. [Google Scholar]
- Beverly, D.B.; Marc, R.K. Nanotechnology Meets Biology: Peptide-based Methods for the Fabrication of Functional Materials. J. Phys. Chem. Lett. 2012, 3, 405–418. [Google Scholar]
- Qiang, L.; Yang, T.; Li, Z.; Wang, H.; Chen, X.; Cui, X. Molecular dynamics simulations of the interaction between Fe3O4 and biocompatible polymer. Colloid Surf. A 2014, 456, 62–66. [Google Scholar] [CrossRef]
- Jens, B.; Archan, D.; Paul, H.B.; Cécile, L.C.; Peter, F.; Nico, A.S.; Damien, F. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar]
- Jens, B.; Maria, A.C.; Kevin, M.E.; Peter, W.; Damien, F. Biomimetic Magnetite Formation: From Biocombinatorial Approaches to Mineralization Effects. Langmuir 2014, 30, 2129–2136. [Google Scholar]
- Lionel, V.; Corinne, C.; Elisabeth, T.; Jean, P.J. Size Tailoring of Magnetite Particles Formed by Aqueous Precipitation: An Example of Thermodynamic Stability of Nanometric Oxide Particles. J. Colloid Interface Sci. 1998, 205, 205–212. [Google Scholar]
- Lenders, J.J.M.; Zope, H.R.; Yamagishi, A.; Bomans, P.H.H.; Arakaki, A.; Kros, A.; Sommerdijk, N.A.S. Bioinspired magnetite crystallization directed by random copolypeptides. Adv. Funct. Mater. 2015, 25, 711–719. [Google Scholar] [CrossRef]
- Christer, B.A.; Kenneth, R.S. The hydrogen bond and crystal engineering. Chem. Soc. Rev. 1993, 22, 397–407. [Google Scholar]
- Borisenko, V.; Sansom, M.S.P.; Woolley, G. Protonation of Lysine Residues Inverts Cation/Anion Selectivity in a Model Channel. Biophys. J. 2000, 78, 1335–1348. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E. Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chem. 2005, 92, 193–202. [Google Scholar] [CrossRef]
- Iris, H.; Damien, D.H.J.; Raimo, H. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Pu, X.; Yin, G.; Chen, X.; Yin, J.; Wu, Y. Biomimetic Mineralization of Magnetic Iron Oxide Nanoparticles Mediated by Bi-Functional Copolypeptides. Molecules 2019, 24, 1401. https://doi.org/10.3390/molecules24071401
Liu L, Pu X, Yin G, Chen X, Yin J, Wu Y. Biomimetic Mineralization of Magnetic Iron Oxide Nanoparticles Mediated by Bi-Functional Copolypeptides. Molecules. 2019; 24(7):1401. https://doi.org/10.3390/molecules24071401
Chicago/Turabian StyleLiu, Liu, Ximing Pu, Guangfu Yin, Xianchun Chen, Jie Yin, and Yuhao Wu. 2019. "Biomimetic Mineralization of Magnetic Iron Oxide Nanoparticles Mediated by Bi-Functional Copolypeptides" Molecules 24, no. 7: 1401. https://doi.org/10.3390/molecules24071401
APA StyleLiu, L., Pu, X., Yin, G., Chen, X., Yin, J., & Wu, Y. (2019). Biomimetic Mineralization of Magnetic Iron Oxide Nanoparticles Mediated by Bi-Functional Copolypeptides. Molecules, 24(7), 1401. https://doi.org/10.3390/molecules24071401