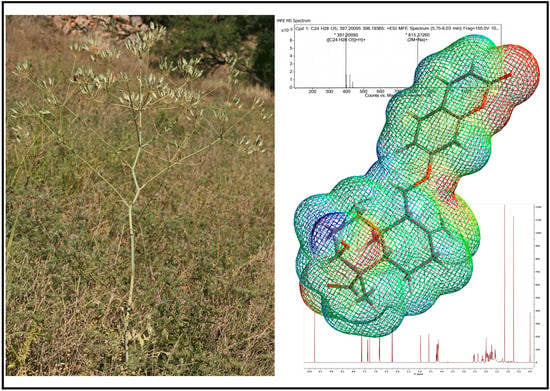
Anatolicin, a Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin from the Root Extract of Heptaptera anatolica
Abstract
:1. Introduction
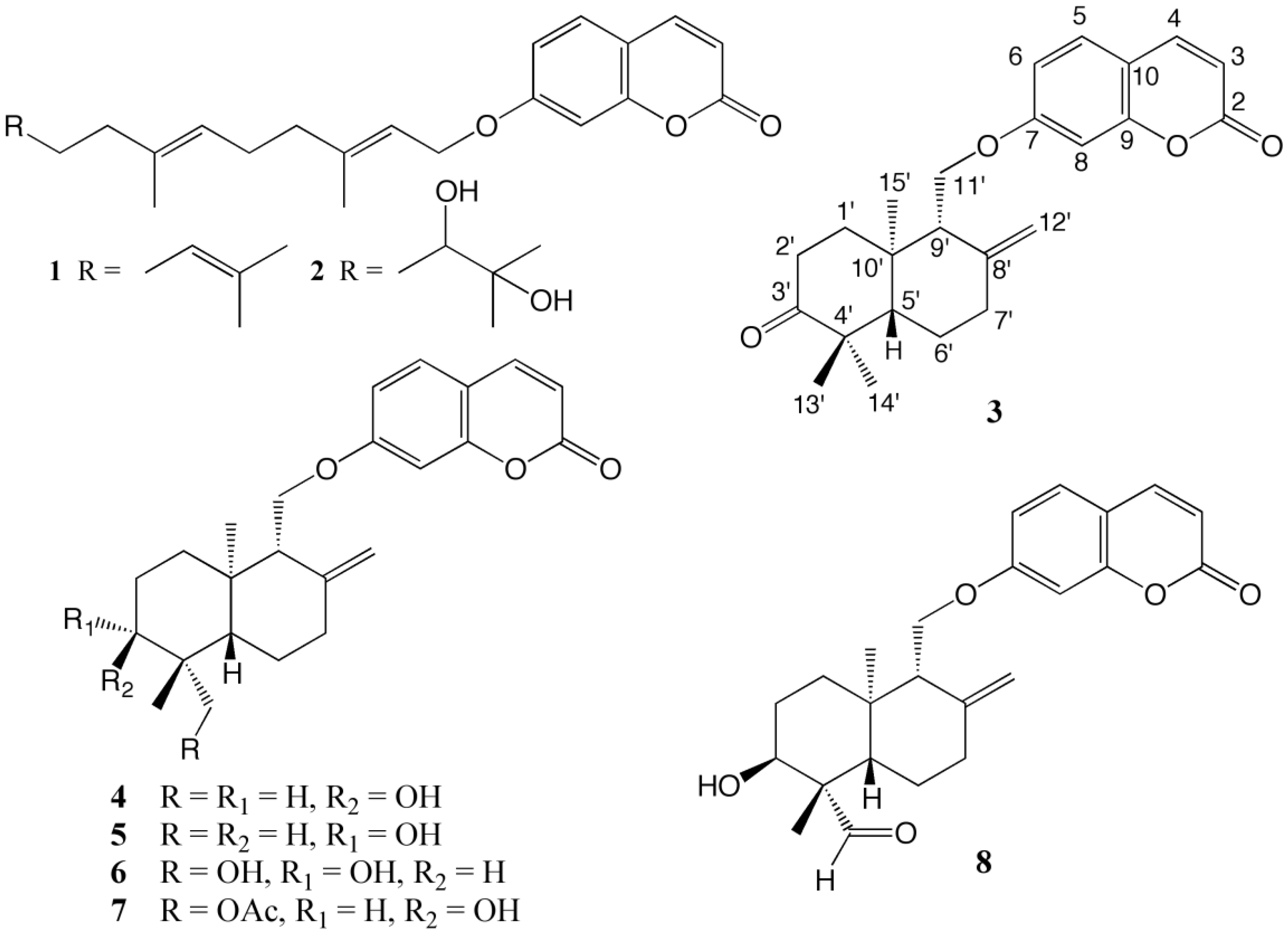
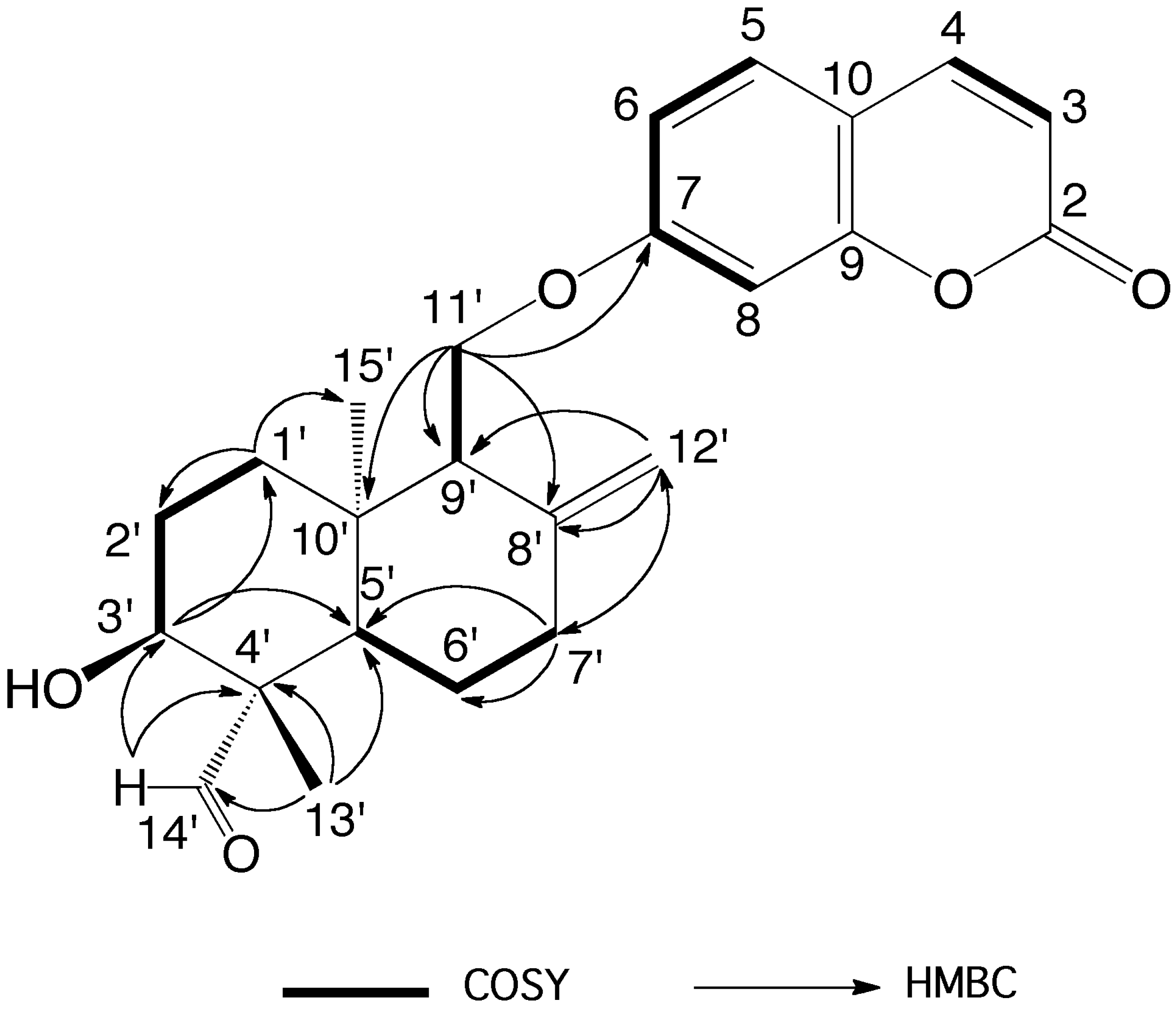
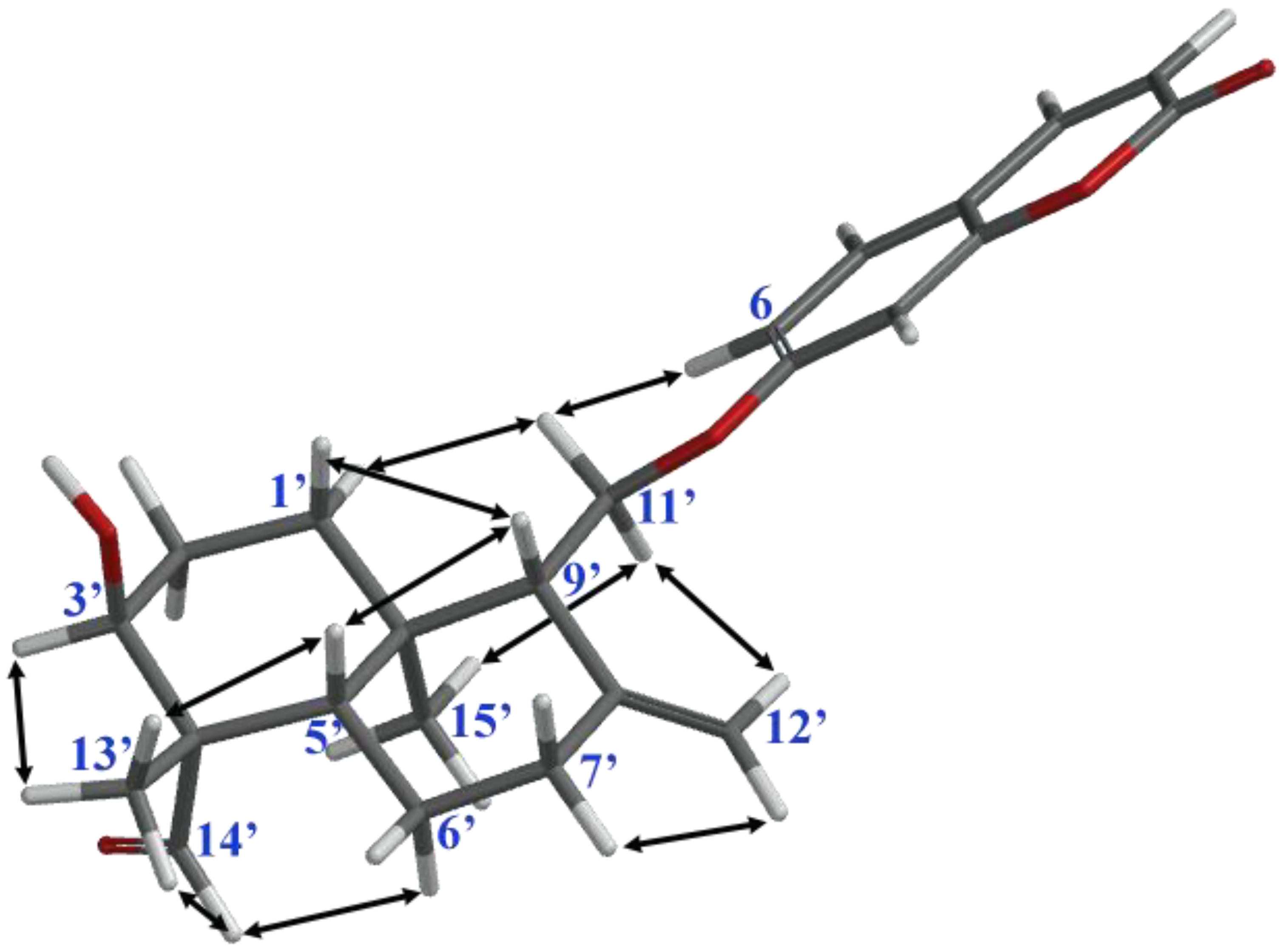
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cytotoxic Activity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Gunther, R.T. The Greek Herbal of Dioscorides; Hafner Publishing Co.: New York, NY, USA, 1959; pp. 328–331. [Google Scholar]
- Eisenman, S.W.; Zaurov, D.E.; Struwe, L. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan; Springer: New York, NY, USA; Heidelberg/Berlin, Germany; Dordrecht, The Netherlands; London, UK, 2013; p. 10. [Google Scholar]
- Nazari, Z.E.; Iranshahi, M. Biologically active sesquiterpene coumarins from Ferula species. Phytother. Res. 2011, 25, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Herrnstadt, I.; Heyn, C.C. Studies in Heptaptera (Umbelliferae) II: Taxonomic revision. Notes R. Bot. Gard. Edinb. 1971, 31, 91–107. [Google Scholar]
- Appendino, G.; Özen, H.C.; Tagliapietra, S.; Cisero, M. Coumarins from Heptaptera anisoptera. Phytochemistry 1992, 31, 3211–3213. [Google Scholar] [CrossRef]
- Appendino, G.; Özen, H.C.; Nano, G.M.; Cisero, M. Sesquiterpene coumarin ethers from the genus Heptaptera. Phytochemistry 1992, 31, 4223–4226. [Google Scholar] [CrossRef]
- Appendino, G.; Özen, H.C.; Jakupovic, J. A Sesquiterpene coumarin ether and a coniferyl ester from Heptaptera anisoptera. Fitoterapia 1993, 64, 505–506. [Google Scholar]
- Roselli, S.; Maggio, A.; Bellone, G.; Formisano, C.; Basile, A.; Cicala, C.; Alfieri, A.; Mascolo, N.; Bruno, M. Antibacterial and anticoagulant activities of coumarins isolated from the flowers of Magydaris tomentosa. Planta Med. 2006, 72, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A. Sesquiterpene coumarins and sesquiterpenes from Ferula sinaica. Phytochemistry 1999, 50, 109–112. [Google Scholar] [CrossRef]
- Eshbakova, K.A.; Saidkhodzhaev, A.I.; Vdovin, A.D.; Abdullaev, N.D. Terpenoid coumarins from Ferula feruloides. Chem. Nat. Comp. 2009, 45, 708–709. [Google Scholar] [CrossRef]
- The tube model of anatolicin (8) was generated using the Spartan’14 Molecular Modeling Software Package ver. 1.2.0. Wavefunction, Inc.: Irvine, CA, USA, 2014.
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.B.; Vistica, D.; Warren, J.T.; Bokesch, H.R.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Position | 3 (in CDCl3) | 4 (in CDCl3) | 5 (in CDCl3) | 6 (in CD3OD) | 7 (in CDCl3) |
|---|---|---|---|---|---|
| 3 | 6.25; d; 9.7; 1H | 6.23; d; 9.5; 1H | 6.24; d; 9.5; 1H | 6.24; d; 9.6; 1H | 6.23; d; 9.6; 1H |
| 4 | 7.63; d; 9.7; 1H | 7.62; d; 9.5; 1H | 7.62; d; 9.5; 1H | 7.88; d; 9.6; 1H | 7.62; d; 9.6; 1H |
| 5 | 7.36; d; 9.2; 1H | 7.34; d; 9.5; 1H | 7.35; d; 8.6; 1H | 7.52; d; 8.6; 1H | 7.34; d; 9.4; 1H |
| 6 | 6.82; dd; 9.2, 2.4; 1H * | 6.82; dd; 9.5, 2.4; 1H * | 6.82; dd; 8.6, 2.4; 1H * | 6.90; dd; 8.6, 2.4; 1H * | 6.82; dd; 9.4, 2.3; 1H * |
| 8 | 6.82; bd; 2.2 1H * | 6.82; bd; 2.1; 1H * | 6.81; bs, 1H * | 6.92; bd, 2.3; 1H * | 6.81; bd; 2.2 1H * |
| 1′α | 2.09; ddd; 3.6, 6.6, 13.2; 1H ** | 1.50; dt; 13.2, 3.3; 1H | 1.45; m; 1H ** | 1.73; m; 1H ** | 1.73; m; 1H ** |
| 1′β | 1.81; dt; 5.5, 13.2; 1H | 1.83; bdt; 3.2, 13.2; 1H | 1.76; m; 1H *** | 1.87; m; 1H *** | 1.84; dt; 2.8, 13.1; 1H |
| 2′α | 2.68; ddd; 6.9, 13.2, 15.1; 1H | 1.67; m; 1H ** | 1.45; m; 1H ** | 1.73; m; 1H ** | 1.73; m; 1H ** |
| 2′β | 2.41; ddd; 3.2, 5.3, 15.1; 1H | 1.67; m; 1H ** | 1.76; m; 1H *** | 1.87; m; 1H ** | 1.73; m; 1H ** |
| 3′ | - | 3.46; bt; 2.6; 1H | 3.30; dd; 4.4, 11.4; 1H | 3.43; bdd; 6.6, 10.0; 1H | 3.79; bt; 2.2; 1H |
| 5′ | 1.67; dd; 2.4, 12.1; 1H | 1.66; dd; 2.4, 12.9; 1H ** | 1.17; dd; 2.7, 12.5; 1H | 1.41; dd; 3.9, 12.6; 1H **** | 1.54; dd; 3.1, 12.3; 1H |
| 6′α | 1.57; m; 1H | 1.42; dq; 4.4, 14.0; 1H | 1.62; m; 1H | 1.41; m; 1H **** | 1.42; dq; 4.3, 13.2; 1H |
| 6′β | 1.73; m; 1H | 1.96; ddt; 2.4, 3.7, 14.0; 1H | 1.76; m; 1H *** | 1.87; m; 1H *** | 1.93; ddt; 2.2, 2.8, 13.2 1H |
| 7′α | 2.53; ddd; 2.3, 4.0, 13.3; 1H | 2.45; ddd; 2.5, 4.2, 13.2; 1H | 2.46; ddd; 2.3, 4.0, 13.2; 1H | 2.46; ddd; 2.3, 3.9, 13.0; 1H | 2.46; ddd; 2.3, 4.1, 13.3; 1H |
| 7′β | 2.14; bdt; 4.8, 13.3; 1H ** | 2.13; bdt; 5.0, 13.2; 1H | 2.09; bdt; 5.1, 13.2; 1H | 2.07; bdt; 5.5, 13.2; 1H | 2.15; bdt; 5.0, 13.3; 1H |
| 9′ | 2.30; bt; 5.4; 1H | 2.33; bdd; 4.2, 7.3; 1H | 2.20; bdd; 4.3, 7.6; 1H | 2.22; bdd; 3.6, 6.9; 1H | 2.34; bdd; 4.5, 6.9; 1H |
| 11′a | 4.24; dd; 6.8, 9.8; 1H *** | 4.23; dd; 4.3, 10.0; 1H | 4.21; dd; 4.3, 9.7; 1H | 4.28; dd; 3.9, 10.3; 1H | 4.22; dd; 4.3, 9.9; 1H |
| 11′b | 4.17; dd; 5.2, 9.8; 1H *** | 4.16; dd; 7.6, 10.0; 1H | 4.17; dd; 7.6, 9.7; 1H | 4.22; dd; 7.7, 10.3; 1H | 4.17; dd; 7.4, 9.9; 1H ** |
| 12′a | 4.97; bs; 1H | 4.90; bd; 1.3; 1H | 4.91; bs; 1H | 4.89; bs; 1H | 4.90; bs; 1H |
| 12′b | 4.60; bs; 1H | 4.53; bd; 1.3; 1H | 4.53; bs; 1H | 4.56; bs; 1H | 4.54; bs; 1H |
| 13′ | 1.12; s; 3H | 0.98; s; 3H | 0.81; s; 3H | 1.23; s; 3H | 1.09; s; 3H |
| 14′a | 1.06; s; 3H | 0.86; s; 3H | 1.02; s; 3H | 4.13; d; 11.1; 1H | 4.18; d; 11.3; 1H ** |
| 14′b | 3.39; d; 11.1; 1H | 3.96; d; 11.3; 1H | |||
| 15′ | 1.04; s; 3H | 0.85; s; 3H | 0.84; s; 3H | 0.85; s; 3H | 0.84; s; 3H |
| OAc | - | - | - | - | 2.04; s; 3H |
| Position | δH (in ppm, m, J in Hz) | δC (in ppm) | HMBC (H -> C) Correlations |
|---|---|---|---|
| 2 | - | 161.41 | |
| 3 | 6.25; d; 9.7; 1H | 113.22 | C-2; C-10 |
| 4 | 7.63; d; 9.7; 1H | 143.32 | C-2; C-5; C-9; C-10 |
| 5 | 7.35; d; 8.3; 1H | 128.90 | C-4; C-7; C-9 |
| 6 | 6.82; dd; 8.3, 2.2; 1H * | 113.19 | C-7; C-8; C-10 |
| 7 | - | 162.21 | |
| 8 | 6.81; bs, 1H * | 101.46 | C-7; C-9; C-10 |
| 9 | - | 156.00 | |
| 10 | - | 112.64 | |
| 1′α | 1.57; dt; 13.2, 3.3; 1H | 31.85 | C-2′; C-15′ |
| 1′β | 1.83; ddd; 3.3, 13.2, 13.2; 1H | ||
| 2′α | 1.73; m; 1H | 26.65 | C-1′ |
| 2′β | 1.92; m; 1H | ||
| 3′ | 4.16; bt; 3.1; 1H ** | 69.10 | C-1′; C-5′ |
| 4′ | - | 52.42 | |
| 5′ | 2.00; dd; 2.8, 13.3; 1H | 48.60 | C-4′; C-6′; C-13′; C-14′; C-15′ |
| 6′α | 1.76; m; 1H | 23.12 | C-5′; C-7′ |
| 6′β | 1.96; m; 1H | ||
| 7′α | 2.53; ddd; 2.6, 4.5, 13.2; 1H | 37.78 | C-5′; C-6′; C-8′; C-9′; C-12′ |
| 7′β | 2.15; ddd; 4.5, 13.2, 13.2; 1H | ||
| 8′ | - | 145.69 | |
| 9′ | 2.36; bdd; 3.5, 7; 1H | 53.58 | C-8′; C-10′; C-11′; C-12′ |
| 10′ | 39.30 | ||
| 11′a | 4.23; dd; 4.0, 9.8; 1H | 65.67 | C-7; C-8′; C-9′; C-10′ |
| 11′b | 4.17; dd; 7.8, 9.8; 1H ** | ||
| 12′a | 4.96; bs; 1H | 108.68 | C-7′; C-8′; C-9′ |
| 12′b | 4.58; bs; 1H | ||
| 13′ | 1.15; s; 3H | 20.13 | C-3′; C-4′; C-5′; C-14′ |
| 14′ | 9.76; s; 1H | 204.49 | C-3′; C-4′ |
| 15′ | 0.74; s; 3H | 14.34 | C-1′; C-5′; C-9′; C-10′ |
| Compounds | Cytotoxic Activity (IC50 values in μM) | |||||
|---|---|---|---|---|---|---|
| COLO205 | KM12 | A498 | UO31 | A673 | TC32 | |
| 1 | >50 | >50 | >50 | 1.8 | >50 | >50 |
| 2 | >50 | >50 | >50 | 7.6 | >50 | >50 |
| 3 | >50 | >50 | >50 | 11 | >50 | >50 |
| 4 | >50 | 9.1 | 20 | 0.38 | >50 | >50 |
| 5 | 19 | 2.5 | 21 | 0.75 | >50 | 45 |
| 6 | >50 | >50 | >50 | >50 | >50 | >50 |
| 7 | >50 | >50 | >50 | 0.017 | >50 | >50 |
| 8 | >50 | >50 | >50 | 0.024 | >50 | >50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosun, F.; Beutler, J.A.; Ransom, T.T.; Miski, M. Anatolicin, a Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin from the Root Extract of Heptaptera anatolica. Molecules 2019, 24, 1153. https://doi.org/10.3390/molecules24061153
Tosun F, Beutler JA, Ransom TT, Miski M. Anatolicin, a Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin from the Root Extract of Heptaptera anatolica. Molecules. 2019; 24(6):1153. https://doi.org/10.3390/molecules24061153
Chicago/Turabian StyleTosun, Fatma, John A. Beutler, Tanya T. Ransom, and Mahmut Miski. 2019. "Anatolicin, a Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin from the Root Extract of Heptaptera anatolica" Molecules 24, no. 6: 1153. https://doi.org/10.3390/molecules24061153
APA StyleTosun, F., Beutler, J. A., Ransom, T. T., & Miski, M. (2019). Anatolicin, a Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin from the Root Extract of Heptaptera anatolica. Molecules, 24(6), 1153. https://doi.org/10.3390/molecules24061153