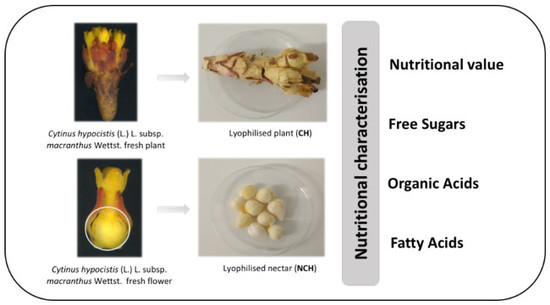
Cytinus hypocistis (L.) L. subsp. macranthus Wettst.: Nutritional Characterization
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Standards
3.2. Plant Material
3.3. Nutritional Value of Cytinus hypocistis (L.) L. subsp. macranthus Wettst
3.4. Chemical Characterization of Cytinus hypocistis (L.) L. subsp. macranthus Wettst
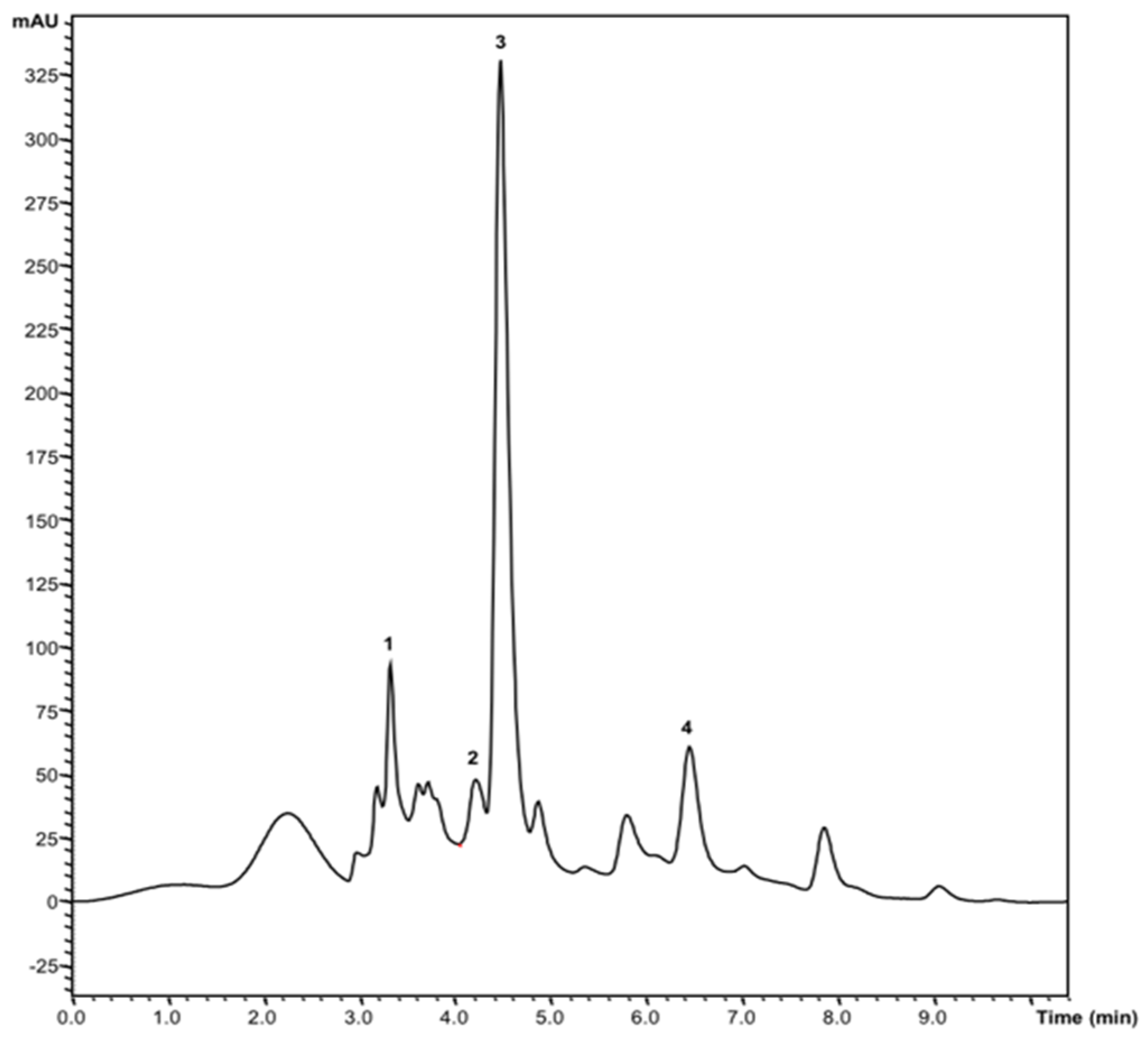
3.4.1. Soluble Sugars
3.4.2. Organic Acids
3.4.3. Fatty Acids
3.4.4. Tocopherols
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torija-Isasa, M.E.; Matallana-González, M.C. A Historical Perspective of Wild Plant Foods in the Mediterranean Area. In Mediterranean Wild Edible Plants; Springer: New York, NY, USA, 2016; pp. 3–13. [Google Scholar]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Barata, A.M. The Consumption of Wild Edible Plants. In Wild Plants, Mushrooms and Nuts; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 159–198. [Google Scholar]
- Jman Redzic, S. Wild Edible Plants and Their Traditional Use in the Human Nutrition in Bosnia-Herzegovina. Ecol. Food Nutr. 2006, 45, 189–232. [Google Scholar] [CrossRef]
- Nebel, S.; Pieroni, A.; Heinrich, M. Ta chòrta: Wild edible greens used in the Graecanic area in Calabria, Southern Italy. Appetite 2006, 47, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tarío, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Pardo-de-Santayana, M.; Tardío, J.; Blanco, E.; Carvalho, A.; Lastra, J.; San Miguel, E.; Morales, R. Traditional knowledge of wild edible plants used in the northwest of the Iberian Peninsula (Spain and Portugal): A comparative study. J. Ethnobiol. Ethnomed. 2007, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Pintus, M.; Manzo, G.; Nieddu, M.; Steri, D.; Rinaldi, A.C. Antimicrobial, antioxidant and anti-tyrosinase properties of extracts of the Mediterranean parasitic plant Cytinus hypocistis. BMC Res. Notes 2015, 8, 562. [Google Scholar] [CrossRef]
- Quivik, F.L. The Illusory Boundary: Environment and Technology in History; Berghahn Books: New York, NY, USA, 2011; Volume 16, ISBN 9780813929880. [Google Scholar]
- Rubiales, D.; Heide-Jørgensen, H.S. Parasitic Plants. Encycl. Life Sci. 2011. [Google Scholar] [CrossRef]
- Těšitel, J. Functional biology of parasitic plants: A review. Plant Ecol. Evol. 2016, 149, 5–20. [Google Scholar] [CrossRef]
- Westwood, J.H.; Yoder, J.I.; Timko, M.P.; dePamphilis, C.W. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef] [PubMed]
- De Vega, C.; Ortiz, P.L.; Arista, M.; Talavera, S. The endophytic system of mediterranean Cytinus (cytinaceae) developing on five host Cistaceae species. Ann. Bot. 2007, 100, 1209–1217. [Google Scholar] [CrossRef]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea; Cambridge University Press: Cambridge, UK, 1993; ISBN 9780521410076. [Google Scholar]
- Thorogood, C.J.; Hiscock, S.J. Host Specificity in the Parasitic Plant Cytinus hypocistis. Res. Lett. Ecol. 2007, 2007, 84234. [Google Scholar]
- Carvalho, A.M.; Morales, R. Persistence of Wild Food and Wild Medicinal Plant Knowledge in a Northeastern Region of Portugal. In Ethnobotany in the New Era: People, Health and Wild Plant Resources; Pardo de Santayana, M., Pieroni, A., Puri, R., Eds.; Berghahn Books: Oxford, UK, 2010; pp. 147–171. ISBN 9781845458140. [Google Scholar]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Nepi, M.; Pacini, E. Nectaries and Nectar; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9781402059377. [Google Scholar]
- Schildknecht, H.; Herb, R.; Kunzelmann, P. Die Chemie der Schmarotzerblumen, II. Isoterchebin: Struktur des gelben Ellagitannin-Farbstoffes ausCytinus hypocistis (Rafflesiaceae). Liebigs Ann. der Chemie 1985, 1985, 1448–1456. [Google Scholar] [CrossRef]
- Magiatis, P.; Pratsinis, H.; Kalpoutzakis, E.; Konstantinidou, A.; Davaris, P.; Skaltsounis, A.-L. Hydrolyzable Tannins, the Active Constituents of Three Greek Cytinus Taxa against Several Tumor Cell Lines. Biol. Pharm. Bull. 2001, 24, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Hanover, L.M.; White, J.S. Manufacturing, composition, and applications of fructose. Am. J. Clin Nutr. 1993, 58, 724S–732S. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. The Unexplored Potential of Edible Flowers Lipids. Agriculture 2018, 8, 146. [Google Scholar] [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a Low-Carbohydrate Diet on Appetite, Blood Glucose Levels, and Insulin Resistance in Obese Patients with Type 2 Diabetes. Ann. Intern. Med. 2005, 142, 403. [Google Scholar] [CrossRef]
- Peyron-Caso, E.; Taverna, M.; Guerre-Millo, M.; Véronèse, A.; Pacher, N.; Slama, G.; Rizkalla, S.W. Dietary (n-3) Polyunsaturated Fatty Acids Up-Regulate Plasma Leptin in Insulin-Resistant Rats. J. Nutr. 2002, 132, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560s–569s. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Geelen, A.; Brouwer, I.A.; Geleijnse, J.M.; Zock, P.L.; Katan, M.B. Effect of Fish Oil on Heart Rate in Humans. Circulation 2005, 112, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Ness, A. Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series 916. Report of a Joint WHO/FSA Expert Consultation. Int. J. Epidemiol. 2004, 33, 914–915. [Google Scholar] [CrossRef]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.S.; Teixeira, M.C.; Brodelius, M. Fatty acids profile of selected Artemisia spp. plants: Health promotion. LWT - Food Sci. Technol. 2011, 44, 293–298. [Google Scholar] [CrossRef]
- French, M.A.; Sundram, K.; Clandinin, M.T. Cholesterolaemic effect of palmitic acid in relation to other dietary fatty acids. Asia Pac. J. Clin. Nutr. 2002, 11, S401–S407. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P.; et al. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escribá, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. U S A 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) AOAC Official Methods of Analysis of AOAC INTERNATIONAL, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 49, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Bioactivity and phytochemical characterization of Arenaria montana L. Food Funct. 2014, 5, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the plant species are available from the authors. |

| Moisture (%) | CH | NCH | Student’s t-Test p-Value |
|---|---|---|---|
| 78 ± 1 | 25 ± 1 | <0.001 | |
| Nutritional value | g/100 g dw | ||
| Fat | 0.67 ± 0.03 | 1.4 ± 0.1 | <0.001 |
| Proteins | 4.90 ± 0.07 | 9.4 ± 0.3 | <0.001 |
| Ash | 2.87 ± 0.02 | 3.05 ± 0.05 | 0.005 |
| Fiber | 4.8 ± 0.1 | 1.03 ± 0.05 | <0.001 |
| Carbohydrates | 86.8 ± 0.2 | 85.1 ± 0.4 | 0.002 |
| Energy contribution (kcal/100 g dw) | 382.4 ± 0.1 | 392.9 ± 0.1 | <0.001 |
| Soluble sugars | g/100 g dw | ||
| Fructose | 6.3 ± 0.1 | 0.71 ± 0.01 | <0.001 |
| Glucose | 1.92 ± 0.05 | 0.22 ± 0.02 | <0.001 |
| Sucrose | 1.37 ± 0.05 | 0.85 ± 0.01 | <0.001 |
| Trehalose | 0.95 ± 0.02 | 0.80 ± 0.04 | 0.001 |
| Total | 10.5 ± 0.2 | 2.58 ± 0.07 | <0.001 |
| Organic acids | g/100 g dw | ||
| Oxalic acid | 0.030 ± 0.001 | tr. | - |
| Malic acid | 0.40 ± 0.01 | 0.45 ± 0.02 | 0.007 |
| Shikimic acid | tr. | nd. | - |
| Ascorbic acid | nd. | 0.180 ± 0.002 | - |
| Citric acid | 0.41 ± 0.01 | 1.48 ± 0.01 | <0.001 |
| Total | 0.85 ± 0.02 | 2.11 ± 0.03 | <0.001 |
| Fatty Acids (Relative Percentage, %) | CH | NCH | Student’s t-Test p-Value |
|---|---|---|---|
| Caproic acid (C6:0) | nd. | 0.100 ± 0.001 | - |
| Caprilic acid (C8:0) | 0.030 ± 0.003 | 0.033 ± 0.001 | 0.178 |
| Capric acid (C10:0) | 0.037 ± 0.003 | 0.036 ± 0.001 | 0.011 |
| Undecylic acid (C11:0) | 0.016 ± 0.001 | 0.042 ± 0.001 | <0.001 |
| Lauric acid (C12:0) | 0.315 ± 0.002 | 0.268± 0.001 | <0.001 |
| Myristic acid (C14:0) | 0.425 ± 0.001 | 0.384 ± 0.001 | <0.001 |
| Pentadecylic acid (C15:0) | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.001 |
| Palmitic acid (C16:0) | 24.12 ± 0.07 | 24.76 ± 0.02 | <0.001 |
| Palmitoleic acid (C16:1) | 0.662 ± 0.001 | 0.628± 0.001 | <0.001 |
| Margaric acid (C17:0) | 0.311 ± 0.004 | 0.305 ± 0.001 | <0.001 |
| Stearic acid (C18:0) | 5.19 ± 0.04 | 4.79 ± 0.01 | <0.001 |
| Elaidic acid (C18:1n9t) | 1.10 ± 0.02 | 0.86 ± 0.01 | <0.001 |
| Oleic acid (C18:1n9c) | 15.4 ± 0.1 | 13.7 ± 0.1 | <0.001 |
| Linolelaidic acid (C18:2n6t) | 2.16 ± 0.01 | 1.88 ± 0.01 | 0.001 |
| Linoleic acid (C18:2n6c) | 40.08 ± 0.02 | 39.90 ± 0.03 | <0.001 |
| γ-Linolenic acid (C18:3n6) | 1.088 ± 0.001 | 0.940 ± 0.005 | <0.001 |
| α-Linolenic acid (C18:3n3) | 2.07 ± 0.06 | 3.72 ± 0.02 | <0.001 |
| Arachidic acid (C20:0) | 1.87 ± 0.01 | 1.45 ± 0.01 | <0.001 |
| Eicosanoic acid (C20:1) | 0.366 ± 0.004 | 0.121 ± 0.004 | <0.001 |
| cis-11,14-Eicosadienoic acid (C20:2) | 1.471 ± 0.005 | 1.273 ± 0.001 | 0.001 |
| Heneicosanoic acid (C21:0) | 0.22 ± 0.01 | 0.25 ± 0.01 | 0.001 |
| Arachidonic acid (C20:4n6) | 0.028 ± 0.001 | 0.034 ± 0.002 | <0.001 |
| Behenic acid (C22:0) | 1.86 ± 0.06 | 1.57 ± 0.01 | 0.001 |
| cis-13,16-Docosadienoic acid (C22:2) | 0.058 ± 0.001 | 0.037 ± 0.001 | <0.001 |
| Tricosanoic acid (C23:0) | 0.182 ± 0.003 | 0.191 ± 0.004 | 0.003 |
| Lignoceric acid (C24:0) | 0.83 ± 0.03 | 2.60 ± 0.02 | <0.001 |
| SFA | 35.56 ± 0.09 | 35.36 ± 0.02 | 0.006 |
| MUFA | 17.5 ± 0.1 | 15.3 ± 0.1 | <0.001 |
| PUFA | 46.95 ± 0.04 | 49 ± 1 | 0.022 |
| UFA | 64.4 ± 0.1 | 63.8 ± 0.8 | 0.282 |
| PUFA/SFA | 1.32 ± 0.01 | 1.37 ± 0.02 | 0.015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.R.; Fernandes, Â.; García, P.A.; Barros, L.; Ferreira, I.C.F.R. Cytinus hypocistis (L.) L. subsp. macranthus Wettst.: Nutritional Characterization. Molecules 2019, 24, 1111. https://doi.org/10.3390/molecules24061111
Silva AR, Fernandes Â, García PA, Barros L, Ferreira ICFR. Cytinus hypocistis (L.) L. subsp. macranthus Wettst.: Nutritional Characterization. Molecules. 2019; 24(6):1111. https://doi.org/10.3390/molecules24061111
Chicago/Turabian StyleSilva, Ana Rita, Ângela Fernandes, Pablo A. García, Lillian Barros, and Isabel C.F.R. Ferreira. 2019. "Cytinus hypocistis (L.) L. subsp. macranthus Wettst.: Nutritional Characterization" Molecules 24, no. 6: 1111. https://doi.org/10.3390/molecules24061111
APA StyleSilva, A. R., Fernandes, Â., García, P. A., Barros, L., & Ferreira, I. C. F. R. (2019). Cytinus hypocistis (L.) L. subsp. macranthus Wettst.: Nutritional Characterization. Molecules, 24(6), 1111. https://doi.org/10.3390/molecules24061111