Influence of Pickling Process on Allium cepa and Citrus limon Metabolome as Determined via Mass Spectrometry-Based Metabolomics
Abstract
1. Introduction
2. Results and Discussion
2.1. Profiling of Onion and Lemon Volatiles Using SPME/GC-MS and Multivariate Data Analysis
2.2. Profiling of Onion and Lemon Secondary Metabolites via UPLC-QTOFMS and Multivariate Data Analysis
2.2.1. Phenolic Acids
2.2.2. Flavonoids
2.2.3. Limonoids/Coumarins
2.3. Onion and Lemon Primary Metabolite Profiling Using GC-MS
3. Materials and Methods
3.1. Plant Material
3.2. Pickling Protocol
3.3. Chemicals and Fibers
3.4. Headspace Volatiles Analysis of C. limon and A. cepa Bulbs
3.5. GC-MS Analysis of Silylated Primary Metabolites in Fruit Pulp and Skin
3.6. Extraction Procedure and Sample Preparation for UPLC-PDA-MS Analysis
3.7. GC-MS and UPLC-QTOFMS Data Processing for Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McFeeters, R.F. Effects of Fermentation on the Nutritional Properties of Food. In Nutritional Evaluation of Food Processing; Karmas, E., Harris, R.S., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1988; pp. 423–446. [Google Scholar]
- Montano, A.; Casado, F.J.; de Castro, A.; Sanchez, A.H.; Rejano, L. Vitamin content and amino acid composition of pickled garlic processed with and without fermentation. J. Agric. Food Chem. 2004, 52, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.-D.; Ji, B.-P.; Li, B.O.; Lu, F.E.I. Effect of fermentation on nitrate, nitrite and organic acid contents in traditional pickled chinese cabbage. J. Food Process. Preserv. 2009, 33, 175–186. [Google Scholar] [CrossRef]
- Guo, X.H.; Yang, J.S.; Zhang, J.J. The study of nitrite peakvalue change mechanism and inhibition methods during Chinese cabbage fermentation. Chin. Food Ferment. Ind. 1989, 15, 26–34. [Google Scholar]
- He, S.L.; Li, B.; Ji, B.P. Study on nitrate reductase activityduring the fermentation of pickled vegetable. Chin. Food Technol. 2005, 1, 94–97. [Google Scholar]
- Chan, T.Y.K. Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol. Lett. 2011, 200, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W. Use of starter cultures in fermentation on a household scale. Food Control 1997, 8, 241–258. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Con, A.H.; Karasu, N. Determination of Antagonistic Starter Cultures for Pickle and Olive Fermentation Processes. Czech J. Food Sci. 2009, 27, 185–193. [Google Scholar]
- Giraffa, G. Studying the dynamics of microbial populations during food fermentation. FEMS Microbiol. Rev. 2004, 28, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Hansen, E.B. Commercial bacterial starter cultures for fermented foods of the future. Int. J. Food Microbiol. 2002, 78, 119–131. [Google Scholar] [CrossRef]
- Abad-García, B.; Garmón-Lobato, S.; Sánchez-Ilárduya, M.B.; Berrueta, L.A.; Gallo, B.; Vicente, F.; Alonso-Salces, R.M. Polyphenolic contents in Citrus fruit juices: Authenticity assessment. Eur. Food Res. Technol. 2014, 238, 803–818. [Google Scholar] [CrossRef]
- Spinola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MS(n) and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, M.S.; Wannes, W.A.; Ouerghemmi, I.; Jegham, S.; Ben Njima, Y.; Hamdaoui, G.; Zemni, H.; Marzouk, B. Juice components and antioxidant capacity of four Tunisian Citrus varieties. J. Sci. Food Agric. 2011, 91, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.; El-Readi, M.Z.; Tahrani, A.; Herrmann, F.; Kaufmann, D.; Farrag, N.; El-Shazly, A.; Wink, M. Secondary metabolites of ponderosa lemon (Citrus pyriformis) and their antioxidant, anti-inflammatory, and cytotoxic activities. Zeitschrift fur Naturforschung C J. Biosci. 2011, 66, 385–393. [Google Scholar] [CrossRef]
- Guyonnet, D.; Siess, M.H.; Le Bon, A.M.; Suschetet, M. Modulation of Phase II Enzymes by Organosulfur Compounds from Allium Vegetables in Rat Tissues. Toxicol. Appl. Pharmacol. 1999, 154, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Calcagno, A.M.; Hladky, S.B.; Ambudkar, S.V.; Barrand, M.A. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J. 2005, 272, 4725–4740. [Google Scholar] [CrossRef] [PubMed]
- Gülşen, A.; Makris, D.P.; Kefalas, P. Biomimetic oxidation of quercetin: Isolation of a naturally occurring quercetin heterodimer and evaluation of its in vitro antioxidant properties. Food Res. Int. 2007, 40, 7–14. [Google Scholar] [CrossRef]
- Liu, L.; Yeh, Y.Y. S-alk(en)yl cysteines of garlic inhibit cholesterol synthesis by deactivating HMG-CoA reductase in cultured rat hepatocytes. J. Nutr. 2002, 132, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.S.; Srinivasan, K. Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol. Cell. Biochem. 1997, 175, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Joshi, S.; Kapoor, H.C. Antioxidants in onion (Allium cepa L.) cultivars grown in India. J. Food Biochem. 2009, 33, 184–200. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Paganga, G.; Miller, N.; Rice-Evans, C.A. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radic. Res. 1999, 30, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Farag, M.A.; Ali, S.E.; Hodaya, R.H.; El-Seedi, H.R.; Sultani, H.N.; Laub, A.; Eissa, T.F.; Abou-Zaid, F.O.F.; Wessjohann, L.A. Phytochemical Profiles and Antimicrobial Activities of Allium cepa Red cv. and A. sativum Subjected to Different Drying Methods: A Comparative MS-Based Metabolomics. Molecules 2017, 22, 761. [Google Scholar] [CrossRef] [PubMed]
- Marsili, R. Analysis of musty microbial metabolites by stir bar sorptive extraction. In Flavor, Fragrance, and Odor Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 63–92. [Google Scholar]
- Li, Y. Confined direct analysis in real time ion source and its applications in analysis of volatile organic compounds of Citrus limon (lemon) and Allium cepa (onion). Rapid Commun. Mass Spectrom. 2012, 26, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tawfike, A.F.; Viegelmann, C.; Edrada-Ebel, R. Metabolomics and dereplication strategies in natural products. Methods Mol. Biol. 2013, 1055, 227–244. [Google Scholar] [PubMed]
- Farag, M.A. Comparative mass spectrometry & nuclear magnetic resonance metabolomic approaches for nutraceuticals quality control analysis: A brief review. Recent Pat. Biotechnol. 2014, 8, 17–24. [Google Scholar] [PubMed]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L.; Rudaz, S.; Choi, Y.H.; Kim, H.K. Plant metabolomics: From holistic data to relevant biomarkers. Curr. Med. Chem. 2013, 20, 1056–1090. [Google Scholar]
- Villas-Boas, S.G.; Mas, S.; Akesson, M.; Smedsgaard, J.; Nielsen, J. Mass spectrometry in metabolome analysis. Mass Spectrom. Rev. 2005, 24, 613–646. [Google Scholar] [CrossRef] [PubMed]
- Beato, V.M.; Sanchez, A.H.; de Castro, A.; Montano, A. Effect of processing and storage time on the contents of organosulfur compounds in pickled blanched garlic. J. Agric. Food Chem. 2012, 60, 3485–3491. [Google Scholar] [CrossRef] [PubMed]
- Song, H.H.; Kim, D.Y.; Woo, S.; Lee, H.K.; Oh, S.R. An approach for simultaneous determination for geographical origins of Korean Panax ginseng by UPLC-QTOF/MS coupled with OPLS-DA models. J. Ginseng Res. 2013, 37, 341–348. [Google Scholar] [CrossRef] [PubMed]
- McGorrin, R. Character-impact flavor and off-flavor compounds in foods. In Flavor, Fragrance, and Odor Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 207–262. [Google Scholar]
- Tocmo, R.; Lin, Y.; Huang, D. Effect of processing conditions on the organosulfides of shallot (Allium cepa L. Aggregatum group). J. Agric. Food Chem. 2014, 62, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Mondy, N.; Duplat, D.; Christides, J.P.; Arnault, I.; Auger, J. Aroma analysis of fresh and preserved onions and leek by dual solid-phase microextraction-liquid extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2002, 963, 89–93. [Google Scholar] [CrossRef]
- Yu, A.-N.; Tan, Z.-W.; Wang, F.-S. Mechanism of formation of sulphur aroma compounds from l-ascorbic acid and l-cysteine during the Maillard reaction. Food Chem. 2012, 132, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.; Boccard, J.; Mehl, F.; Debrus, B.; Marcourt, L.; Merle, P.; Delort, E.; Baroux, L.; Sommer, H.; Rudaz, S.; et al. Comprehensive profiling and marker identification in non-volatile citrus oil residues by mass spectrometry and nuclear magnetic resonance. Food Chem. 2014, 150, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Yang, C.K.; Tu, H.; Zhou, J.J.; Liu, X.Q.; Cheng, Y.J.; Luo, J.; Deng, X.X.; Zhang, H.Y.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.K.; Verma, S.K.; Misra, R.; Seniya, C. Antibacterial and Phyto-chemical Analysis of Some Medicinal Plants and their Efficacy on Multidrug Resistant Bacteria. J. Pure Appl. Microbiol. 2013, 7, 2191–2204. [Google Scholar]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-F.; Kong, L.-Y.; Kurtán, T.; Liu, H.-L.; Mándi, A.; Li, J.; Gu, Y.-C.; Guo, Y.-W. Four Phragmalin Orthoesters from the Chinese Mangrove Xylocarpus granatum. Planta Med. 2014, 80, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Molina, E.; Dominguez-Perles, R.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Niedermaier, S.; Gschwander, S.; Schossig, P. Cycling stability of D-mannitol when used as phase change material for thermal storage applications. Thermochim. Acta 2018, 660, 134–143. [Google Scholar] [CrossRef]
- Farag, M.A.; Rasheed, D.M.; Kamal, I.M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME-GC-MS and chemometrics. Food Res. Int. 2015, 78, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Broeckling, C.D.; Reddy, I.R.; Duran, A.L.; Zhao, X.C.; Sumner, L.W. MET-IDEA: Data extraction tool for mass spectrometry-based metabolomics. Analyt. Chem. 2006, 78, 4334–4341. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
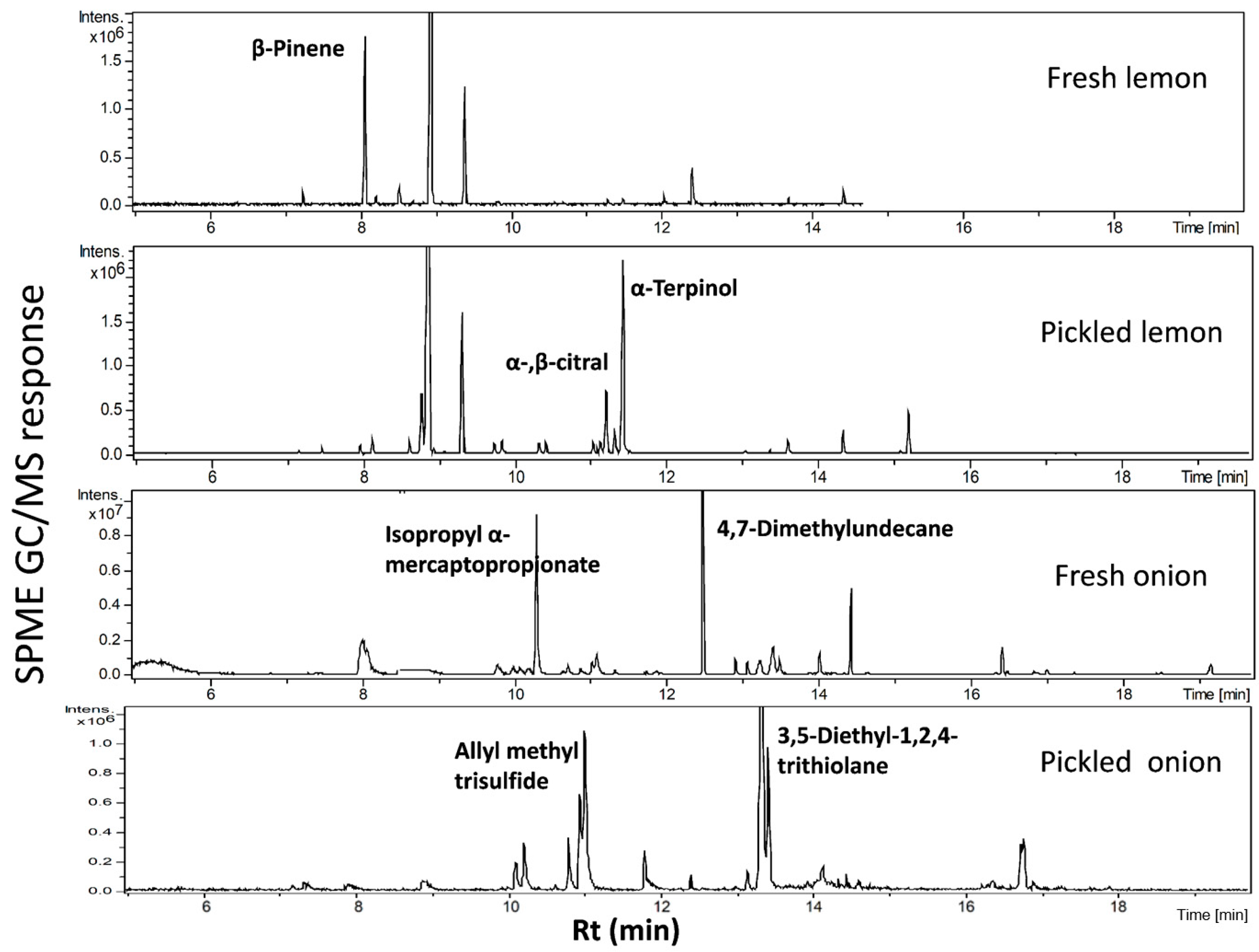
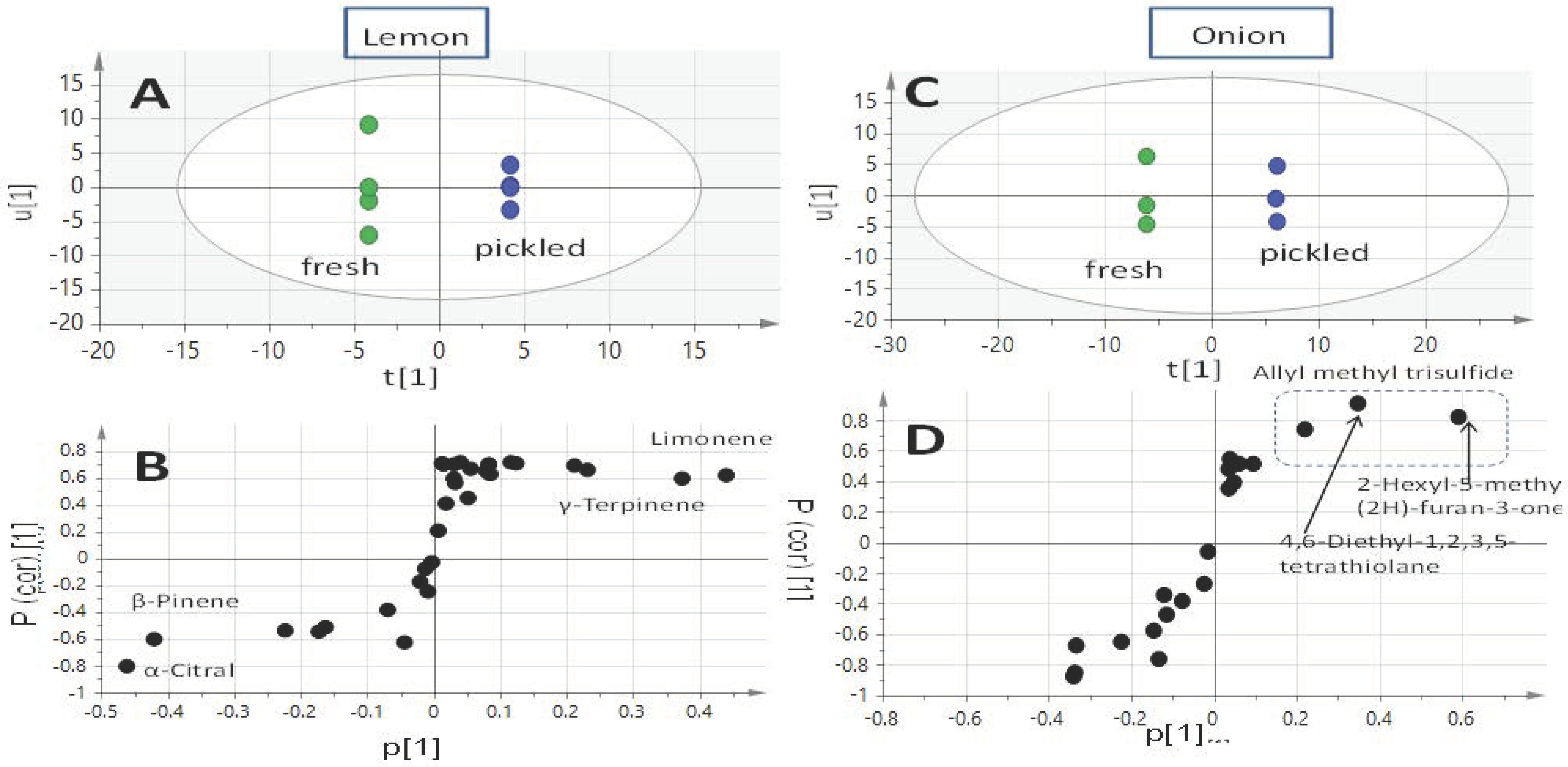
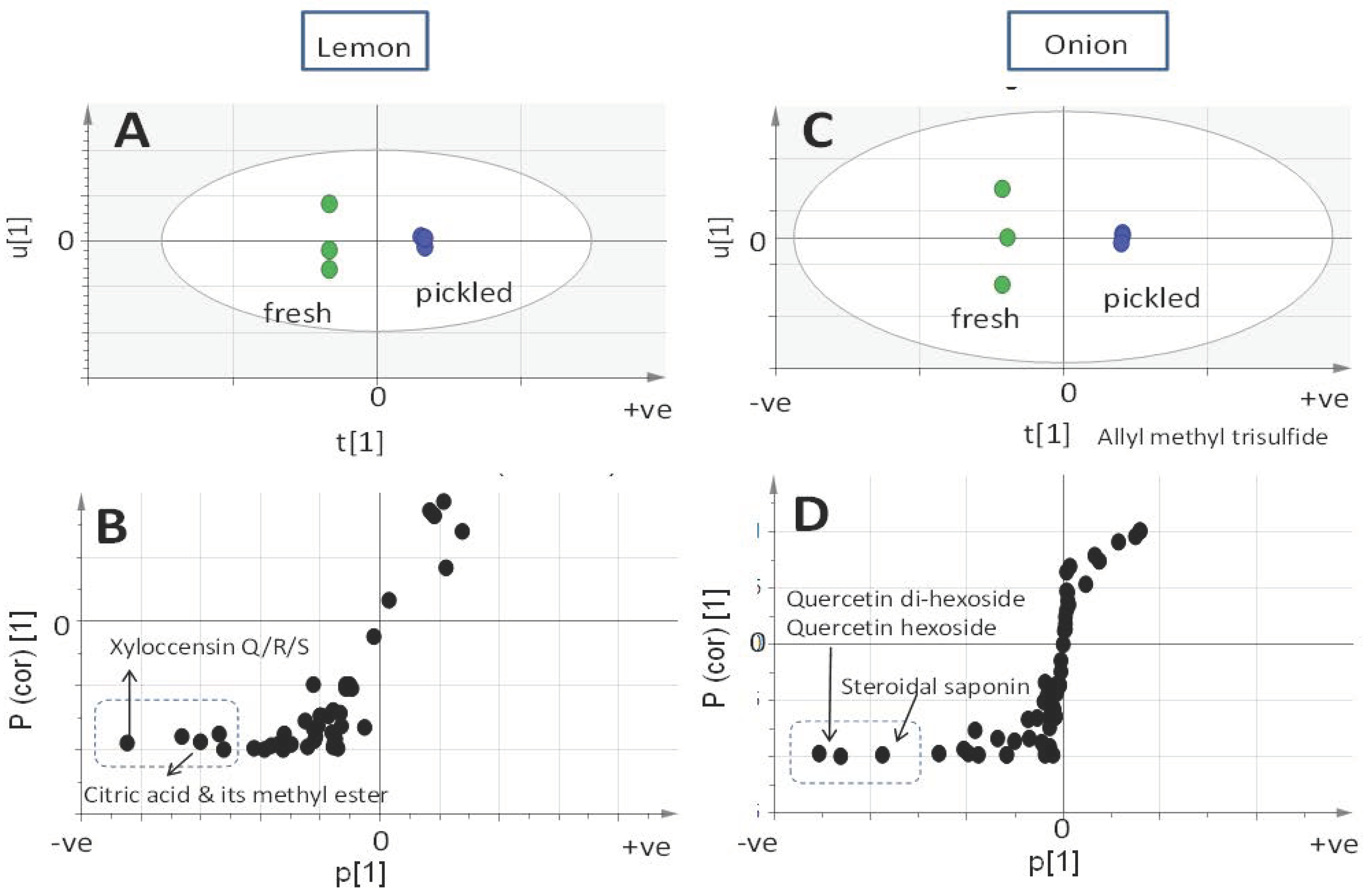
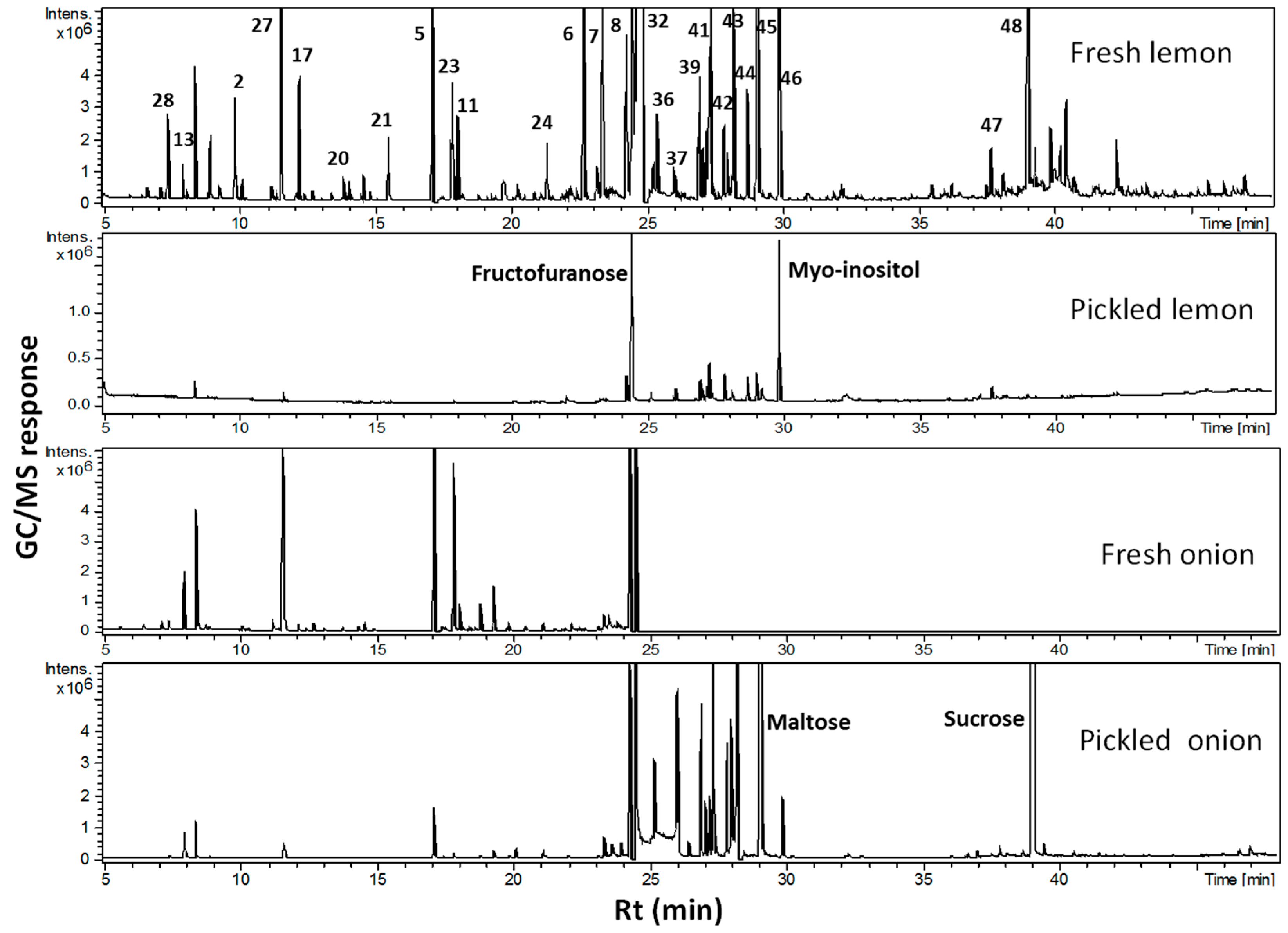
| Peak | rt (min) | KI | ID | Average ± Std. dev. Fresh | Average ± Std. dev. Pickled |
|---|---|---|---|---|---|
| 1 | 7.03 | 903 | α-Thujene | 0.15 ± 0.11 | 0.04 ± 0.03 |
| 2 | 7.18 | 912 | α-Pinene a | 0.37 ± 0.48 | 0.35 ± 0.27 |
| 3 | 7.48 | 929 | Fenchene | 0.04 ± 0.04 | 0.02 ± 0.02 |
| 4 | 8.03 | 961 | β-Pinene a | 33.24 ± 5.89 | 23.44 ± 1.26 |
| 5 | 8.16 | 967 | β-Myrcene a | 0.15 ± 0.20 | 0.36 ± 0.28 |
| 6 | 8.64 | 995 | 1,4-Cineole a | 0.02 ± 0.02 | 0.07 ± 0.06 |
| 7 | 8.66 | 996 | 2-Carene | 2.12 ± 2.69 | 0.17 ± 0.13 |
| 8 | 8.80 | 1005 | Melilotal | 0.05 ± 0.07 | 0.39 ± 0.31 |
| 9 | 8.94 | 1014 | Limonene a | 32.10 ± 10.45 | 43.49 ± 5.16 |
| 10 | 9.38 | 1042 | γ-Terpinene | 12.39 ± 8.90 | 20.97 ± 3.21 |
| 11 | 9.76 | 1066 | Terpinolene | 0.10 ± 0.13 | 0.52 ± 0.41 |
| 12 | 9.86 | 1073 | p-Cymenene | 0.02 ± 0.03 | 0.19 ± 0.15 |
| 13 | 10.43 | 1110 | α-Linalool a | 0.01 ± 0.02 | 0.37 ± 0.29 |
| 14 | 11.06 | 1154 | Bicyclo[3.2.1]oct-3-en-2-one, 4-methyl- | 0.01 ± 0.02 | 0.36 ± 0.29 |
| 15 | 11.10 | 1158 | α-Phellandren-8-ol | 0.02 ± 0.03 | 0.03 ± 0.02 |
| 16 | 11.22 | 1166 | 1-Terpinen-4-ol | 0.99 ± 0.81 | 0.89 ± 0.71 |
| 17 | 11.33 | 1173 | p-Cymen-8-ol | 0.02 ± 0.03 | 0.05 ± 0.04 |
| 18 | 11.43 | 1180 | α-Terpineol | 0.39 ± 0.49 | 3.33 ± 2.66 |
| 19 | 11.56 | 1189 | Unknown | 0.15 ± 0.18 | 2.50 ± 2.00 |
| 20 | 11.97 | 1220 | β-Citral | 3.46 ± 4.47 | 0.00 ± 0.00 |
| 21 | 12.36 | 1249 | α-Citral a | 10.86 ± 11.79 | 0.00 ± 0.00 |
| 22 | 13.16 | 1311 | δ-EIemene | 0.02 ± 0.03 | 0.08 ± 0.07 |
| 23 | 13.37 | 1328 | 6-Dimethyl-2,6-octadien-8-yl acetate | 0.70 ± 0.90 | 0.24 ± 0.19 |
| 24 | 14.28 | 1402 | (Z)-β-Farnesene | 0.33 ± 0.46 | 0.23 ± 0.18 |
| 25 | 14.34 | 1407 | (E)-α-Bergamotene | 2.29 ± 2.56 | 0.25 ± 0.20 |
| 26 | 14.70 | 1437 | Unknown sesquiterpene | 0.00 ± 0.00 | 0.01 ± 0.01 |
| 27 | 15.04 | 1464 | Unknown sesquiterpene | 0.00 ± 0.00 | 0.05 ± 0.04 |
| 28 | 15.08 | 1468 | (Z,E)-α-Farnesene | 0.02 ± 0.03 | 0.80 ± 0.63 |
| 29 | 15.12 | 1478 | β-Bisabolene | 0.01 ± 0.01 | 0.69 ± 0.54 |
| 30 | 15.80 | 1523 | epi-α-Eudesmol | - | 0.01 ± 0.01 |
| 31 | 16.06 | 1541 | Unknown sesquiterpene | - | 0.08 ± 0.07 |
| Total monoterpene hydrocarbons % | 80.66 | 89.55 | |||
| Total oxygenated monoterpenes % | 15.83 | 5.49 | |||
| Total sesquiterpene hydrocarbons % | 3.37 | 2.44 | |||
| Total oxygenated sesquiterpenes % | 0.7 | 0.25 |
| Peak | rt (min) | KI | Name | Average ± Std. dev. Fresh | Average ± Std. dev. Pickled |
|---|---|---|---|---|---|
| 1 | 7.44 | 924 | 1-propenyl methyl disulphide | 2.91 ± 2.19 | - |
| 2 | 7.99 | 958 | Dimethyl trisulfide a | 3.24 ± 3.00 | 2.31 ± 3.18 |
| 3 | 9.79 | 1068 | 2-Acetylpyrrole | 0.26 ± 0.15 | 0.00 ± 0.00 |
| 4 | 10.12 | 1089 | Unknown sulphur | 3.45 ± 0.36 | 6.71 ± 1.27 |
| 5 | 10.31 | 1101 | Isopropyl α-mercaptopropionate | 10.42 ± 1.94 | 8.35 ± 0.70 |
| 6 | 10.89 | 1143 | Diethanol disulphide | 12.20 ± 5.82 | 10.90 ± 2.89 |
| 7 | 10.99 | 1149 | Methyl pentyl disulfide | 0.24 ± 0.10 | - |
| 8 | 11.11 | 1157 | Allyl methyl trisulfide | 11.48 ± 5.08 | 25.25 ± 7.56 |
| 9 | 11.83 | 1209 | β-Hydroxyethyl phenyl ether | 0.38 ± 0.20 | - |
| 10 | 11.88 | 1212 | Dimethyl tetrasulfide | 3.35 ± 3.67 | - |
| 11 | 12.16 | 1234 | 3-Isopropylbenzaldehyde | 0.26 ± 0.05 | - |
| 12 | 12.32 | 1246 | 4,7-Dimethylundecane | 0.20 ± 0.10 | - |
| 13 | 12.92 | 1292 | (Allylsulfanyl)acetonitrile | 2.72 ± 3.67 | - |
| 14 | 13.23 | 1316 | Dipropyl trisulfide | 4.44 ± 1.26 | 1.28 ± 2.21 |
| 15 | 13.37 | 1328 | 3,5-Diethyl-1,2,4-trithiolane | 18.95 ± 7.15 | 32.08 ± 4.53 |
| 16 | 13.42 | 1332 | Diallyl trisulfide isomer | 0.75 ± 0.42 | - |
| 17 | 14.43 | 1415 | 2-Hexyl-5-methyl-3(2H)-furanone | 0.42 ± 0.43 | 3.44 ± 1.06 |
| 18 | 15.41 | 1495 | Unknown hydrocarbon | 0.34 ± 0.29 | - |
| 19 | 16.76 | 1589 | 4,6-Diethyl-1,2,3,5-tetrathiolane | 10.03 ± 9.17 | 9.69 ± 1.13 |
| 20 | 17.00 | 1605 | 2,4-Dimethyl-5,6-dithia-2,7-Nonadienal | 13.96 ± 12.73 | - |
| Total sulphur compounds % | 98.14 | 96.57 |
| Peak | rt (min) | M + H/M − H | Error (ppm) | Formula | MSMS | Name | Class |
|---|---|---|---|---|---|---|---|
| 1 | 0.51 | 339.1088 | 2.909 | C12H21O11− | - | Methyl-O-hexosyl pentoside | Sugar |
| 2 | 0.66 | 381.078958 | −2.67 | C17H17O10+ | - | Hydroxybergaptol hexoside | Coumarin |
| 3 | 0.67 | 191.01953 | 0.9 | C6H7O7− | 111 | (Iso)citric acid | Organic acid |
| 4 | 0.96 | 205.0351 | 0.82 | C7H9O7− | 173, 111 | Methylated (iso)citric acid | Organic acid |
| 5 | 8.74 | 375.09094 | −1.25 | C15H19O11+ | 357, 339, 231, 137 | Unknown | |
| 6 | 8.95 | 357.11874 | 0.73 | C16H21O9− | 195 | O-Feruloyl-hexoside | Phenolic acid |
| 7 | 9.03 | 595.16473 | −1.02 | C27H31O15+ | 449, 287 | Luteolin O-rhamnosyl-hexoside | Flavone-O-glycoside |
| 8 | 9.31 | 625.17462 | −1.69 | C28H33O16+ | 607, 589, 487 | Diosmetin-C,C-di-hexoside | Flavone-C-glycoside |
| 9 | 9.72 | 565.15454 | −0.64 | C26H29O14+ | 433, 415, 313 | Apigenin-C-hexosyl-O-pentoside | Flavone-C-glycoside |
| 10 | 9.85 | 433.11185 | −1.07 | C21H21O10+ | 415, 367, 313 | Apigenin-C-hexoside | Flavone-C-glycoside |
| 11 | 10.1 | 463.12177 | −1.72 | C22H23O11+ | 445, 301 | Diosmetin-C-hexoside | Flavone-C-glycoside |
| 12 | 10.23 | 625.17487 | −1.44 | C28H33O16+ | - | Diosmetin-C,C-di-hexoside isomer | Flavone-C-glycoside |
| 13 | 10.23 | 579.16962 | −1.21 | C27H31O14+ | 433, 271 | Apigenin-O-rhamnosyl-hexoside | Flavone-O-glycoside |
| 14 | 10.33 | 609.1799 | −1.41 | C28H33O15+ | 463. 301 | Diosmetin-O-rhamnosyl-hexoside | Flavone-O-glycoside |
| 15 | 10.43 | 611.19482 | −2.23 | C28H35O15+ | 465, 449, 303 | Hesperetin-O-rhanmosyl hexoside | Flavanone-O-glycoside |
| 16 | 10.63 | 395.09641 | −0.86 | C18H19O10+ | 377, 291, 147 | Hydroxybergaptol-O-methyl ether hexoside | Coumarin |
| 17 | 10.71 | 509.1282 | −0.77 | C23H25O13+ | 347 | Limocitrin-O-hexoside | Flavonol-O-glycoside |
| 18 | 10.8 | 797.21155 | −1.93 | C35H41O21+ | 433, 347 | Limocitrin-O-glycosyl malonate | Flavonol |
| 19 | 10.82 | 767.20105 | −1.87 | C34H39O20+ | 317 | Gossypetin rhamnosyl dideoxyhexosyl hexoside | Flavonol-O-glycoside |
| 20 | 10.84 | 717.22156 | −2.1 | C31H41O19+ | 391, 255 | Xyloccensin Q/R/S | Limonoid |
| 21 | 10.97 | 687.21094 | −2.15 | C30H39O18+ | 669, 303 | Unknown hesperitin glycoside | Flavanone-O-glycoside |
| 22 | 11.06 | 653.1687 | −2.53 | C29H33O17+ | 347 | Unknown | Flavonol-O-glycoside |
| 23 | 11.13 | 479.11685 | −1.55 | C22H23O12+ | 461, 287 | Luteolin dimethyl ether-O-hexoside | Flavone-O-glycoside |
| 24 | 11.20 | 371.1485 | −2.087 | C17H23O9− | - | Citrusin E | Phenolic acid |
| 25 | 11.26 | 305.10092 | −1.04 | C16H17O6+ | 203 | Unknown | |
| 26 | 12.00 | 345.06082 | 0.33 | C17H13O8− | 330 | Limocitrin | Flavonol |
| 27 | 12.39 | 247.05951 | −0.59 | C13H11O5+ | 232 | Unknown | |
| 28 | 12.75 | 515.19141 | 0.24 | C27H31O10− | 469 | Methyllimonexic acid | Limonoid |
| 29 | 12.78 | 471.19974 | −1.6 | C26H31O8+ | 425,409, 367 | Limonine | Limonoid |
| 30 | 12.97 | 287.0907 | −0.7 | C16H15O5+ | 203 | Trichoclin | Coumarin |
| 31 | 13.00 | 327.14435 | 0.52 | C16H23O7− | 173, 111 | Unknown | |
| 32 | 13.12 | 293.17548 | 0.74 | C17H25O4− | 236, 221 | Unknown terpene | Terpene |
| 33 | 13.13 | 515.22675 | −0.81 | C28H35O9+ | 455, 411, 393, 369 | Nomilinic acid | Limonoid |
| 34 | 13.54 | 455.20485 | −1.6 | C26H31O7+ | 437, 409, 393 | Deoxylimonin/obacunone | Limonoid |
| 35 | 13.55 | 269.08 | 0.95 | C16H13O4− | 214, 201 | Unknown terpene | Terpene |
| 36 | 13.59 | 309.21 | −0.05 | C18H29O4− | 291, 251 | Unknown | |
| 37 | 13.67 | 299.0924 | 1.04 | C17H15O5− | 284 | Trihydroxy-dimethylflavanone | Flavanone |
| 38 | 14.21 | 233.04378 | −0.67 | C12H9O5+ | 218, 205, 173 | Hydroxybergapten | Coumarin |
| 39 | 14.27 | 301.10632 | −0.73 | C17H17O5+ | 233 | Hydroxy-dimethoxyflavanone | Flavanone |
| 40 | 14.27 | 339.0621 | −3.09 | C22H11O4+ | - | Unknown | |
| 41 | 15.00 | 313.14386 | 0.42 | C19H21O4− | 176 | Geranyloxy hydroxy coumarin. | Fatty acyl coumarin |
| 42 | 15.45 | 481.2717 | −5.06 | C29H37O6− | - | Dihydrobergamotin octanal acetal | Coumarin |
| 43 | 15.65 | 377.11435 | −2.87 | C26H17O3+ | 241 | Unknown | |
| 44 | 16.07 | 369.16898 | −0.67 | C22H25O5+ | 233 | Hydroxybergaptol-O-dimethyl-octadienyl-Me ether | Fatty acyl coumarin |
| 45 | 16.17 | 339.15781 | −1.28 | C21H23O4+ | 203 | Bergaptin | Fatty acyl coumarin |
| 46 | 16.19 | 337.14368 | 0.24 | C21H21O4− | 267, 254 | Geranyloxypsoralen (bergaptin) | Coumarin |
| 47 | 16.22 | 329.17377 | −0.97 | C20H25O4+ | 193, 184 | Geranyloxy-methoxycoumarin | Fatty acyl coumarin |
| 48 | 16.30 | 271.2276 | 0.71 | C16H31O3− | 225 | Hydroxypalmitic acid | Fatty acid |
| 49 | 16.52 | 339.19971 | −1.63 | C15H31O8− | 183 | Unknown | Fatty acid |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Tawfike, A.F.; Donia, M.S.; Ehrlich, A.; Wessjohann, L.A. Influence of Pickling Process on Allium cepa and Citrus limon Metabolome as Determined via Mass Spectrometry-Based Metabolomics. Molecules 2019, 24, 928. https://doi.org/10.3390/molecules24050928
Farag MA, Tawfike AF, Donia MS, Ehrlich A, Wessjohann LA. Influence of Pickling Process on Allium cepa and Citrus limon Metabolome as Determined via Mass Spectrometry-Based Metabolomics. Molecules. 2019; 24(5):928. https://doi.org/10.3390/molecules24050928
Chicago/Turabian StyleFarag, Mohamed A., Ahmed F. Tawfike, Marwa S. Donia, Anja Ehrlich, and Ludger A. Wessjohann. 2019. "Influence of Pickling Process on Allium cepa and Citrus limon Metabolome as Determined via Mass Spectrometry-Based Metabolomics" Molecules 24, no. 5: 928. https://doi.org/10.3390/molecules24050928
APA StyleFarag, M. A., Tawfike, A. F., Donia, M. S., Ehrlich, A., & Wessjohann, L. A. (2019). Influence of Pickling Process on Allium cepa and Citrus limon Metabolome as Determined via Mass Spectrometry-Based Metabolomics. Molecules, 24(5), 928. https://doi.org/10.3390/molecules24050928







