Essential Oil Composition and Biological Activity of “Pompia”, a Sardinian Citrus Ecotype
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Investigation
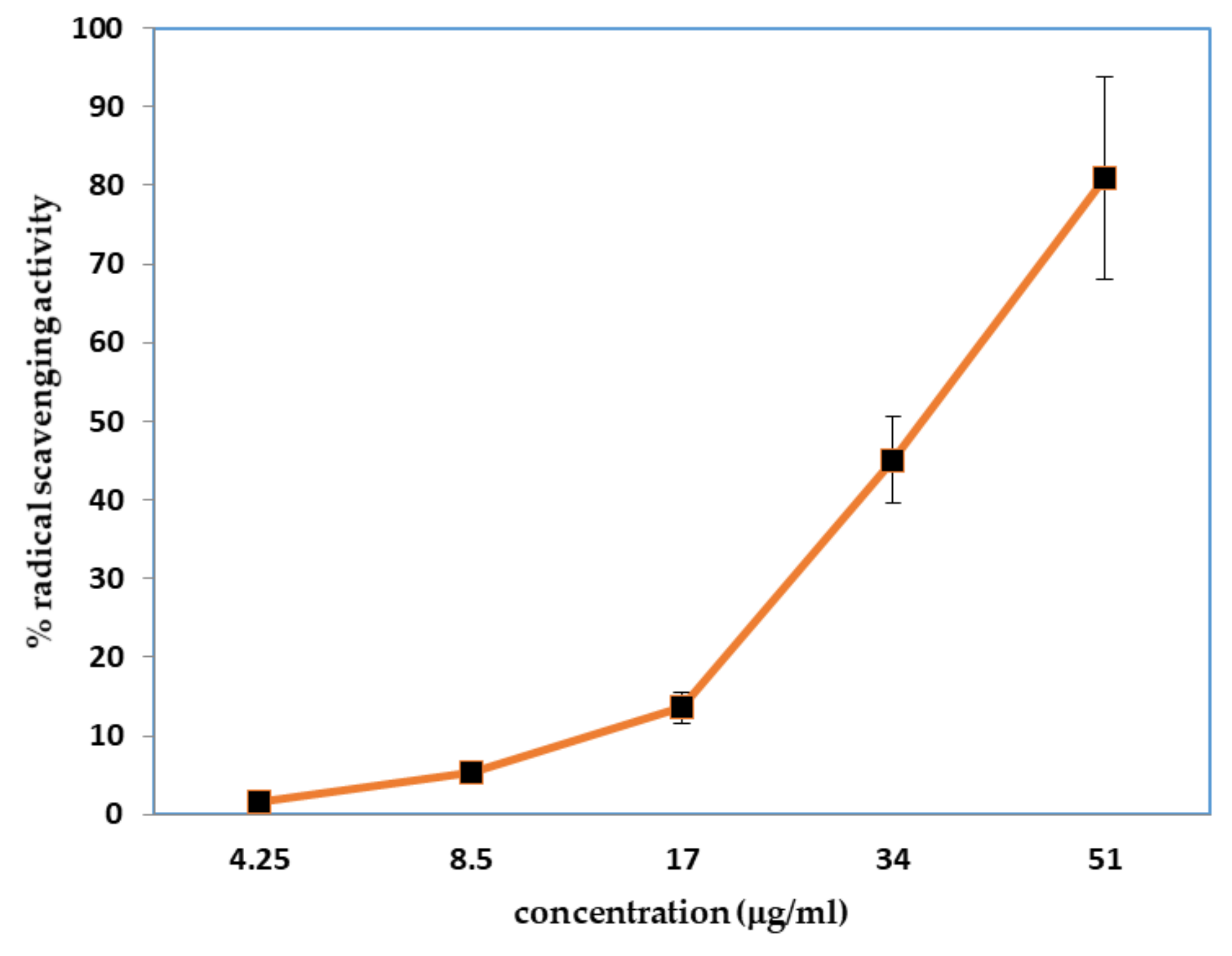
2.2. Antioxidant Activity
2.3. Antifungal Activity
2.4. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oils Extraction
4.3. Gas Chromatography–Mass Spectrometry Analyses and Peaks Identification
4.4. Antiradical Activity by Diphenyl-1-Picarylhydrazyl (DPPH) Assay
4.5. Ferric Reducing Antioxidant Power (FRAP) Assay
4.6. Antifungal Activity
4.7. Antibacterial Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camarda, I.; Mazzola, P.; Brunu, A.; Fenu, G.; Lombardo, G.; Palla, F. Un agrume nella storia della Sardegna: Citrus limon var. pompia Camarda var. nova. Quad. di Bot. Ambient. e Appl. 2013, 24, 109–118. [Google Scholar]
- D’Aquino, S.; Fronteddu, F.; Usai, M.; Palma, A. Qualitative and physiological properties of ‘Pompia’, a citron-like fruit. Plant Genet. Resour. Newsl. IPGRI 2005, 143, 40–45. [Google Scholar]
- Sa pompia: Un particolare frutto da cui si ottiene un dolce tipico di Siniscola. Available online: http://www.siniscolaonline.it/pompia.htm (accessed on 7 December 2018).
- Chessa, I.; Mulas, M.; Pala, M. Gli Agrumi. In Le vecchie varietà di Sardegna. Patrimonio genetico di specie arboree da frutto; Agabbio, M., Ed.; Carlo Delfino Editore: Sassari, Italy, 1994; pp. 339–360. [Google Scholar]
- Mignani, I.; Mulas, M.; Mantegazza, R.; Lovigu, N.; Spada, A.; Nicolosi, E. Daniele Bassi Characterization by Molecular Markers of ‘Pompia’, a Natural Citrus Hybrid Cultivated in Sardinia. Acta Hortic. 2015, 1065, 165–172. [Google Scholar] [CrossRef]
- INRA Encyclopédie des ravageurs européens: Eriophyes sheldoni (Ewing). Available online: http://www7.inra.fr/hyppz/RAVAGEUR/6erishe.htm (accessed on 7 December 2018).
- Moris, G.G. Flora sardoa 1; Typographia Regia: Torino, Italy, 1837. [Google Scholar]
- Petretto, G.L.; Sarais, G.; Maldini, M.T.; Foddai, M.; Tirillini, B.; Rourke, J.P.; Chessa, M.; Pintore, G. Citrus monstruosa Discrimination among Several Citrus Species by Multivariate Analysis of Volatiles: A Metabolomic Approach. J. Food Process. Preserv. 2016, 40, 950–957. [Google Scholar] [CrossRef]
- Curk, F.; Luro, F. Classificazione ed Evoluzione Degli Agrumi. Oral communication, Workshop “Un mare di agrumi: Dalla coltivazione al prodotto finito”: Pisa, Italy, 2018. [Google Scholar]
- Deiana, M.; Montoro, P.; Jerković, I.; Atzeri, A.; Marijanović, Z.; Serreli, G.; Piacente, S.; Tuberoso, C.I.G. First characterization of Pompia intrea candied fruit: The headspace chemical profile, polar extract composition and its biological activities. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Zara, S.; Sanna, M.L.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Pompia. Available online: https://www.fondazioneslowfood.com/en/slow-food-presidia/pompia/ (accessed on 9 December 2018).
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Lota, M.-L.; de Rocca Serra, D.; Tomi, F.; Bessiere, J.-M.; Casanova, J. Chemical composition of peel and leaf essential oils of Citrus medica L. and C. limonimedica Lush. Flavour Fragr. J. 1999, 14, 161–166. [Google Scholar] [CrossRef]
- Venturini, N.; Curk, F.; Desjobert, J.-M.; Karp, D.; Costa, J.; Paolini, J. Chemotaxonomic Investigations of Peel and Petitgrain Essential Oils from 17 Citron Cultivars. Chem. Biodivers. 2010, 7, 736–751. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Tundis, R.; Bonesi, M.; de Cindio, B.; Loizzo, M.R.; Conforti, F.; Statti, G.A.; Menabeni, R.; Bettini, R.; Menichini, F. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation, cold-pressing and supercritical carbon dioxide extraction. Nat. Prod. Res. 2011, 25, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.; Pistelli, L.; Zinnai, A. Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients. Molecules 2018, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, D.; Hamdane, A.M.; Said, M.B.; Abdrrabba, M. Chemical Composition and Antifungal Activity of Essential Oils from Flowers, Leaves and Peels of Tunisian Citrus aurantium Against Penicillium digitatum and Penicillium italicum. J. Essent. Oil Bear. Plants 2016, 19, 1660–1674. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, dl-limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-Scavenging Activities of Citrus Essential Oils and Their Components: Detection Using 1,1-Diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, L.; Nardoni, S.; Pinto, L.; Pistelli, L.; Leonardi, M.; Pisseri, F.; Mancianti, F. In vitro and in vivo antifungal activity of some essential oils against feline isolates of Microsporum canis. J. Mycol. Med. 2012, 22, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; et al. Chemical Composition and In Vitro Antimicrobial Efficacy of Sixteen Essential Oils against Escherichia coli and Aspergillus fumigatus Isolated from Poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, B.; Flotats, X. Citrus essential oils and their influence on the anaerobic digestion process: An overview. Waste Manag. 2014, 34, 2063–2079. [Google Scholar] [CrossRef] [PubMed]
- Chubukov, V.; Mingardon, F.; Schackwitz, W.; Baidoo, E.E.K.; Alonso-Gutierrez, J.; Hu, Q.; Lee, T.S.; Keasling, J.D.; Mukhopadhyay, A. Acute Limonene Toxicity in Escherichia coli Is Caused by Limonene Hydroperoxide and Alleviated by a Point Mutation in Alkyl Hydroperoxidase AhpC. Appl. Environ. Microbiol. 2015, 81, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Palazzolo, E.; Guarrasi, V.; Aleo, A.; Mammina, C.; Moschetti, G.; Germanà, M.A. Inhibition of foodborne pathogen bacteria by essential oils extracted from citrus fruits cultivated in Sicily. Food Control 2012, 26, 326–330. [Google Scholar] [CrossRef]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley & Sons, Inc.: New York, NY, USA, 1976; ISBN 047015019X. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1982; Volume 26. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of eSsential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 1995; ISBN 0-931710-85-5. [Google Scholar]
- Adams, R.P.; Zanoni, T.A.; Lara, A.; Barrero, A.F.; Cool, L.G. Comparisons among Cupressus arizonica Greene, C. benthamii Endl., C. lindleyi Klotz, ex Endl. and C. lusitanica Mill, using Leaf Essential Oils and DNA Fingerprinting. J. Essent. Oil Res. 1997, 9, 303–309. [Google Scholar] [CrossRef]
- Petretto, G.L.; Maldini, M.; Addis, R.; Chessa, M.; Foddai, M.; Rourke, J.P.; Pintore, G. Variability of chemical composition and antioxidant activity of essential oils between Myrtus communis var. Leucocarpa DC and var. Melanocarpa DC. Food Chem. 2016, 197, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Szőllősi, R.; Szôllôsi Varga, I. Total antioxidant power in some species of Labiatae (Adaptation of FRAP method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, approved 541 standard, 2nd ed.; CLSI document M38-A2; CLSI: Wayne, PA, USA, 2008.
- CLSI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved standard M7-A2; CLSI: Villanova, PA, USA, 1990.
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Rocchigiani, G.; Pistelli, L.; Mancianti, F. Antibacterial and antifungal activity of essential oils against some pathogenic bacteria and yeasts shed from poultry. Flavour Fragr. J. 2016, 31, 302–309. [Google Scholar] [CrossRef]
Sample Availability: Samples of plant material are available at the “Azienda Scuola Agraria di Siniscola—I.P.S.A.S.R.”, Località San Narciso, Siniscola (NU, Sardinia, Italy). |
| Constituents | l.r.i. a | Relative Abundance (%) ± SD | |||
|---|---|---|---|---|---|
| HD Peel EO | CP Peel EO | HD Leaf EO | |||
| 1 | α-thujene | 931 | Tr b | - c | - |
| 2 | α-pinene | 941 | 0.43 ± 0.00 | 0.51 ± 0.04 | tr |
| 3 | camphene | 954 | tr | - | - |
| 4 | sabinene | 976 | 0.14 ± 0.00 | 0.07 ± 0.08 | 0.28 ± 0.01 |
| 5 | β-pinene | 982 | tr | - | 0.19 ± 0.00 |
| 6 | 6-methyl-5-hepten-2-one | 985 | tr | - | tr |
| 7 | myrcene | 993 | 2.12 ± 0.01 | 1.55 ± 0.04 | 0.91 ± 0.02 |
| 8 | α-phellandrene | 1005 | tr | tr | tr |
| 9 | δ-3-carene | 1011 | tr | - | 0.90 ± 0.05 |
| 10 | α-terpinene | 1018 | tr | - | - |
| 11 | p-cymene | 1027 | - | - | tr |
| 12 | limonene | 1032 | 77.44 ± 0.58 | 95.77 ± 0.30 | 28.64 ± 1.24 |
| 13 | cis-β-ocimene | 1042 | - | - | 0.46 ± 0.04 |
| 14 | trans-β-ocimene | 1052 | 0.99 ± 0.01 | 0.50 ± 0.13 | 10.50 ± 0.12 |
| 15 | isoterpinolene | 1087 | - | - | tr |
| 16 | terpinolene | 1088 | tr | - | 0.16 ± 0.02 |
| 17 | linalool | 1101 | 1.13 ± 0.06 | tr | 0.56 ± 0.02 |
| 18 | trans-p-mentha-2,8-dien-1-ol | 1121 | tr | - | - |
| 19 | trans-limonene oxide | 1141 | tr | - | tr |
| 20 | camphor | 1143 | tr | - | - |
| 21 | citronellal | 1155 | 0.33 ± 0.00 | - | 1.27 ± 0.16 |
| 22 | isoneral | 1171 | tr | - | 0.37 ± 0.14 |
| 23 | 4-terpineol | 1178 | tr | - | - |
| 24 | isogeranial | 1184 | 0.14 ± 0.01 | - | 0.44 ± 0.04 |
| 25 | α-terpineol | 1189 | 0.35 ± 0.01 | - | 0.14 ± 0.02 |
| 26 | decanal | 1204 | - | - | tr |
| 27 | trans-carveol | 1218 | tr | - | - |
| 28 | nerol | 1230 | 1.24 ± 0.04 | - | 1.49 ± 0.09 |
| 29 | neral | 1240 | 4.43 ± 0.14 | tr | 18.84 ± 0.36 |
| 30 | geraniol | 1257 | 1.46 ± 0.01 | - | 0.57 ± 0.02 |
| 31 | geranial | 1271 | 6.16 ± 0.13 | tr | 24.44 ± 1.59 |
| 32 | methyl geranate | 1325 | tr | - | - |
| 33 | citronellyl acetate | 1350 | tr | - | tr |
| 34 | neryl acetate | 1366 | 0.61 ± 0.03 | tr | 1.48 ± 0.05 |
| 35 | geranyl acetate | 1385 | 0.62 ± 0.02 | - | 3.94 ± 0.18 |
| 36 | cis-α-bergamotene | 1416 | tr | tr | - |
| 37 | β-caryophyllene | 1420 | 0.42 ± 0.01 | 0.34 ± 0.04 | 0.76 ± 0.03 |
| 38 | β-copaene | 1429 | tr | - | - |
| 39 | trans-α-bergamotene | 1438 | 0.59 ± 0.04 | 0.55 ± 0.06 | tr |
| 40 | α-humulene | 1456 | tr | tr | 0.11 ± 0.01 |
| 41 | trans-β-farnesene | 1460 | 0.16 ± 0.01 | - | - |
| 42 | 9-epi-trans-caryophyllene | 1467 | - | tr | - |
| 43 | germacrene D | 1478 | tr | - | - |
| 44 | γ-curcumene | 1480 | tr | - | - |
| 45 | valencene | 1492 | 0.13 ± 0.01 | - | tr |
| 46 | bicyclogermacrene | 1495 | 0.27 ± 0.02 | 0.26 ± 0.01 | 0.45 ± 0.01 |
| 47 | cis-α-bisabolene | 1504 | tr | tr | - |
| 48 | β-bisabolene | 1509 | 0.89 ± 0.06 | 0.48 ± 0.11 | 0.13 ± 0.01 |
| 49 | δ-cadinene | 1524 | tr | - | tr |
| 50 | trans-α-bisabolene | 1531 | tr | - | - |
| 51 | trans-nerolidol | 1565 | tr | - | 0.40 ± 0.02 |
| 52 | germacrene D-4-ol | 1575 | tr | - | tr |
| 53 | spathulenol | 1576 | - | - | 1.22 ± 0.11 |
| 54 | caryophyllene oxide | 1581 | - | - | 0.73 ± 0.02 |
| 55 | β-oplopenone | 1606 | - | - | tr |
| 56 | epoxy-alloaromadendrene | 1639 | - | - | tr |
| 57 | epi-α-cadinol | 1640 | - | - | 0.21 ± 0.06 |
| 58 | α-cadinol | 1654 | - | - | 0.39 ± 0.01 |
| 59 | valerianol | 1656 | tr | - | - |
| 60 | β-bisabolol | 1672 | - | - | 0.10 ± 0.14 |
| 61 | epi-α-bisabolol | 1686 | tr | - | tr |
| Monoterpene hydrocarbons | 81.12 ± 0.59 | 98.38 ± 0.00 | 42.01 ± 1.49 | ||
| Oxygenated monoterpenes | 16.44 ± 0.44 | tr | 53.50 ± 1.45 | ||
| Sesquiterpene hydrocarbons | 2.45 ± 0.15 | 1.62 ± 0.00 | 1.44 ± 0.04 | ||
| Oxygenated sesquiterpenes | tr | - | 3.04 ± 0.07 | ||
| Non-terpene derivatives | tr | - | tr | ||
| Total identified (%) | 100.00 ± 0.00 | 100.00 ± 0.00 | 99.98 ± 0.01 | ||
| Extraction yield (% w/w) | 0.3 | n.a. | <0.1% | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flamini, G.; Pistelli, L.; Nardoni, S.; Ebani, V.V.; Zinnai, A.; Mancianti, F.; Ascrizzi, R.; Pistelli, L. Essential Oil Composition and Biological Activity of “Pompia”, a Sardinian Citrus Ecotype. Molecules 2019, 24, 908. https://doi.org/10.3390/molecules24050908
Flamini G, Pistelli L, Nardoni S, Ebani VV, Zinnai A, Mancianti F, Ascrizzi R, Pistelli L. Essential Oil Composition and Biological Activity of “Pompia”, a Sardinian Citrus Ecotype. Molecules. 2019; 24(5):908. https://doi.org/10.3390/molecules24050908
Chicago/Turabian StyleFlamini, Guido, Laura Pistelli, Simona Nardoni, Valentina Virginia Ebani, Angela Zinnai, Francesca Mancianti, Roberta Ascrizzi, and Luisa Pistelli. 2019. "Essential Oil Composition and Biological Activity of “Pompia”, a Sardinian Citrus Ecotype" Molecules 24, no. 5: 908. https://doi.org/10.3390/molecules24050908
APA StyleFlamini, G., Pistelli, L., Nardoni, S., Ebani, V. V., Zinnai, A., Mancianti, F., Ascrizzi, R., & Pistelli, L. (2019). Essential Oil Composition and Biological Activity of “Pompia”, a Sardinian Citrus Ecotype. Molecules, 24(5), 908. https://doi.org/10.3390/molecules24050908










