Cytotoxic Properties of Damiana (Turnera diffusa) Extracts and Constituents and A Validated Quantitative UHPLC-DAD Assay
Abstract
1. Introduction
2. Results
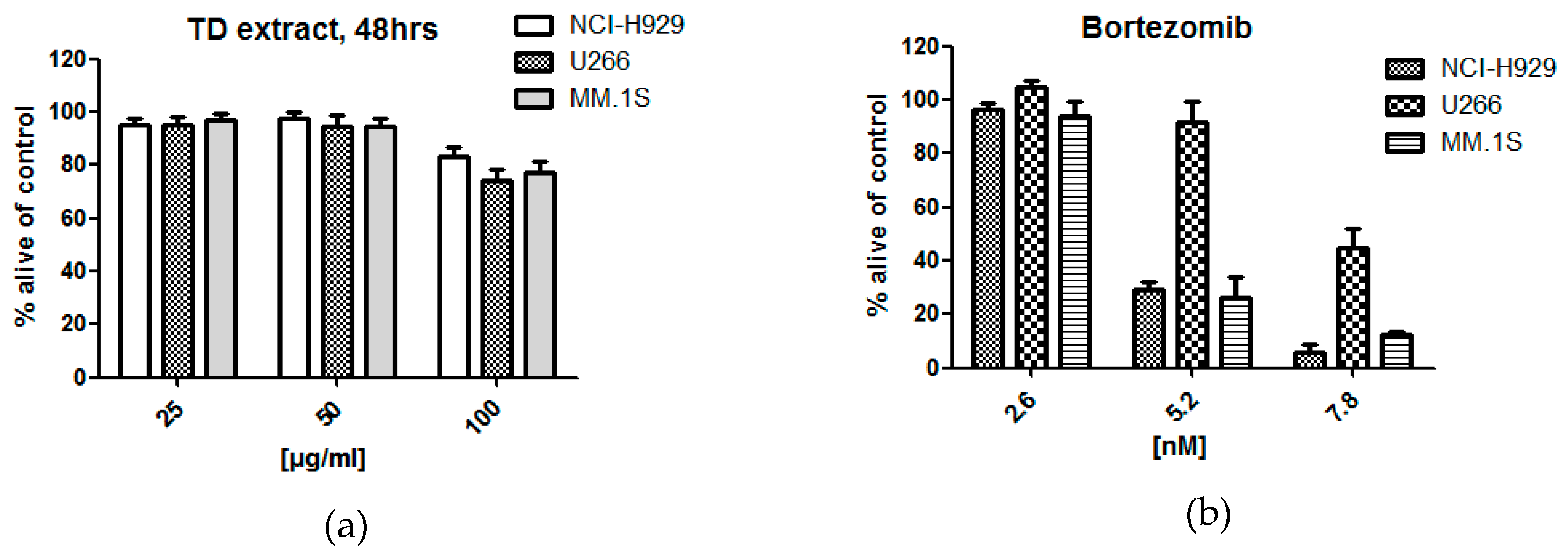
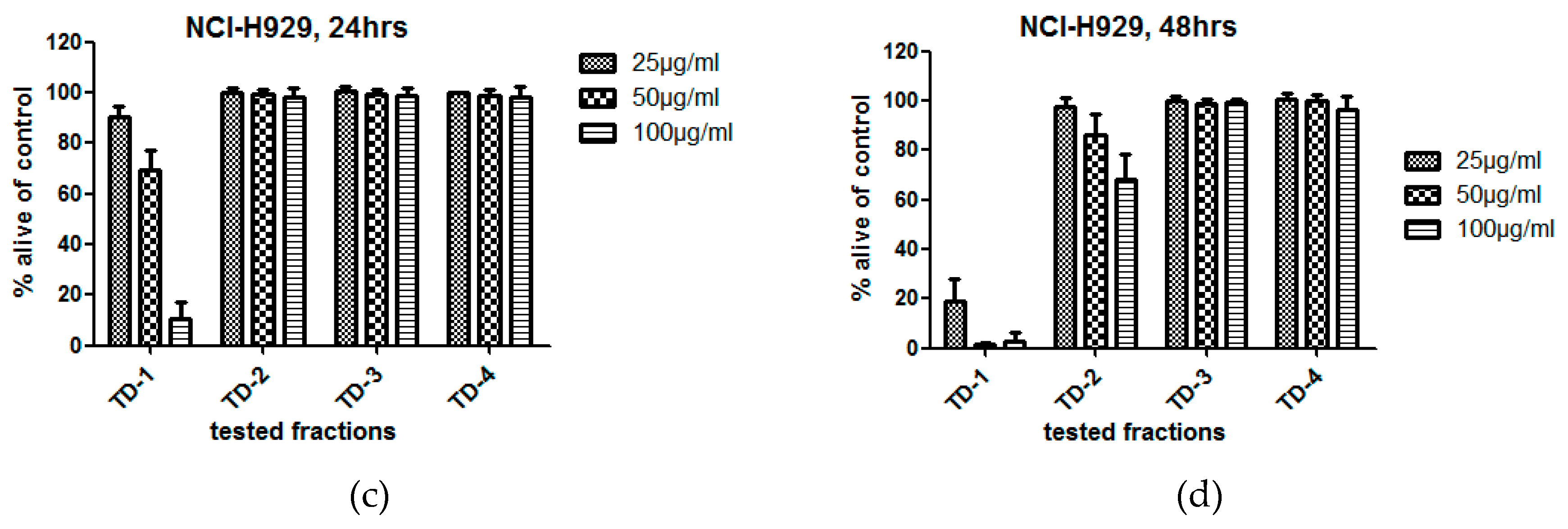
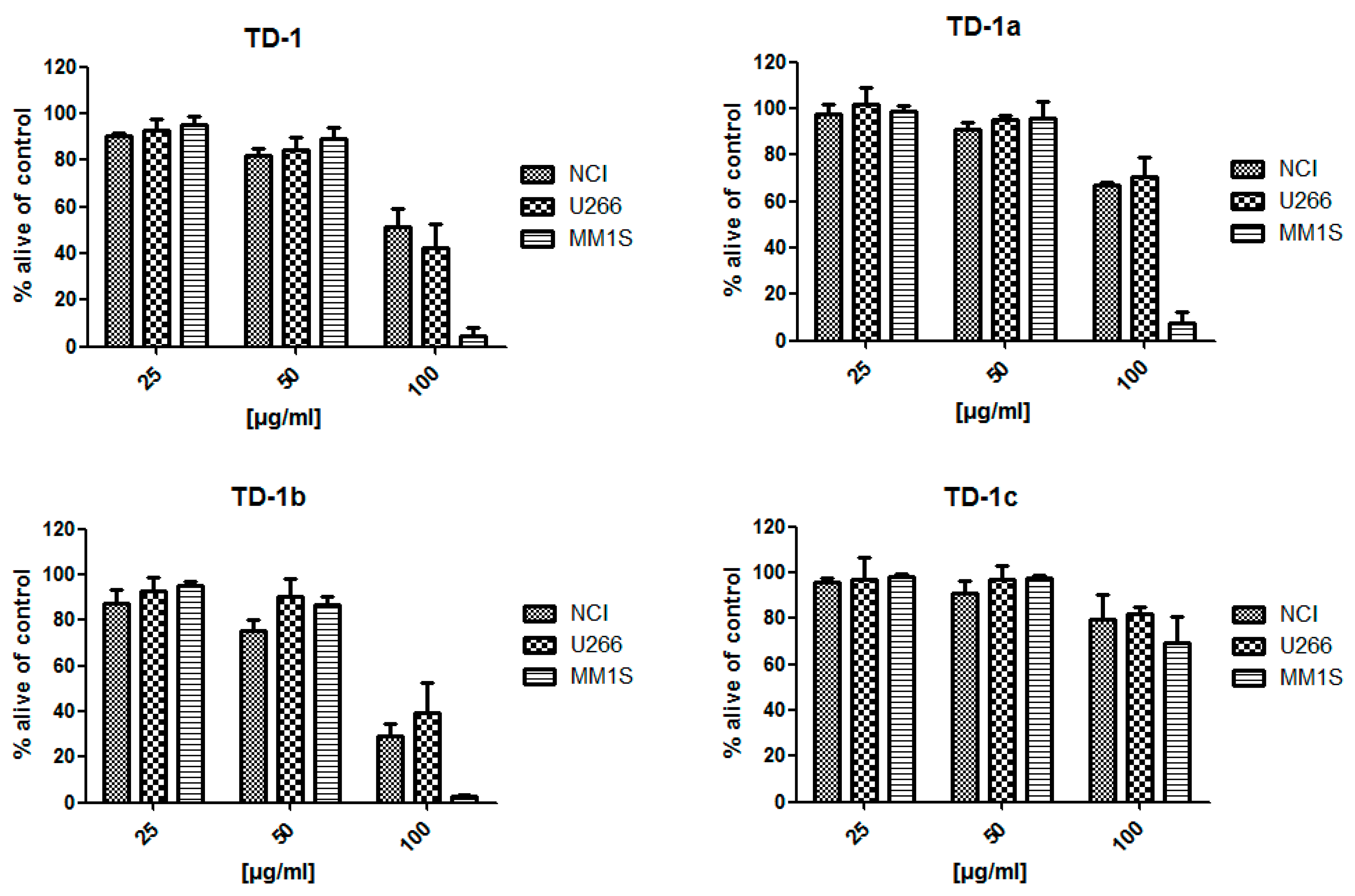
2.1. Bioactivity of Tested Fractions
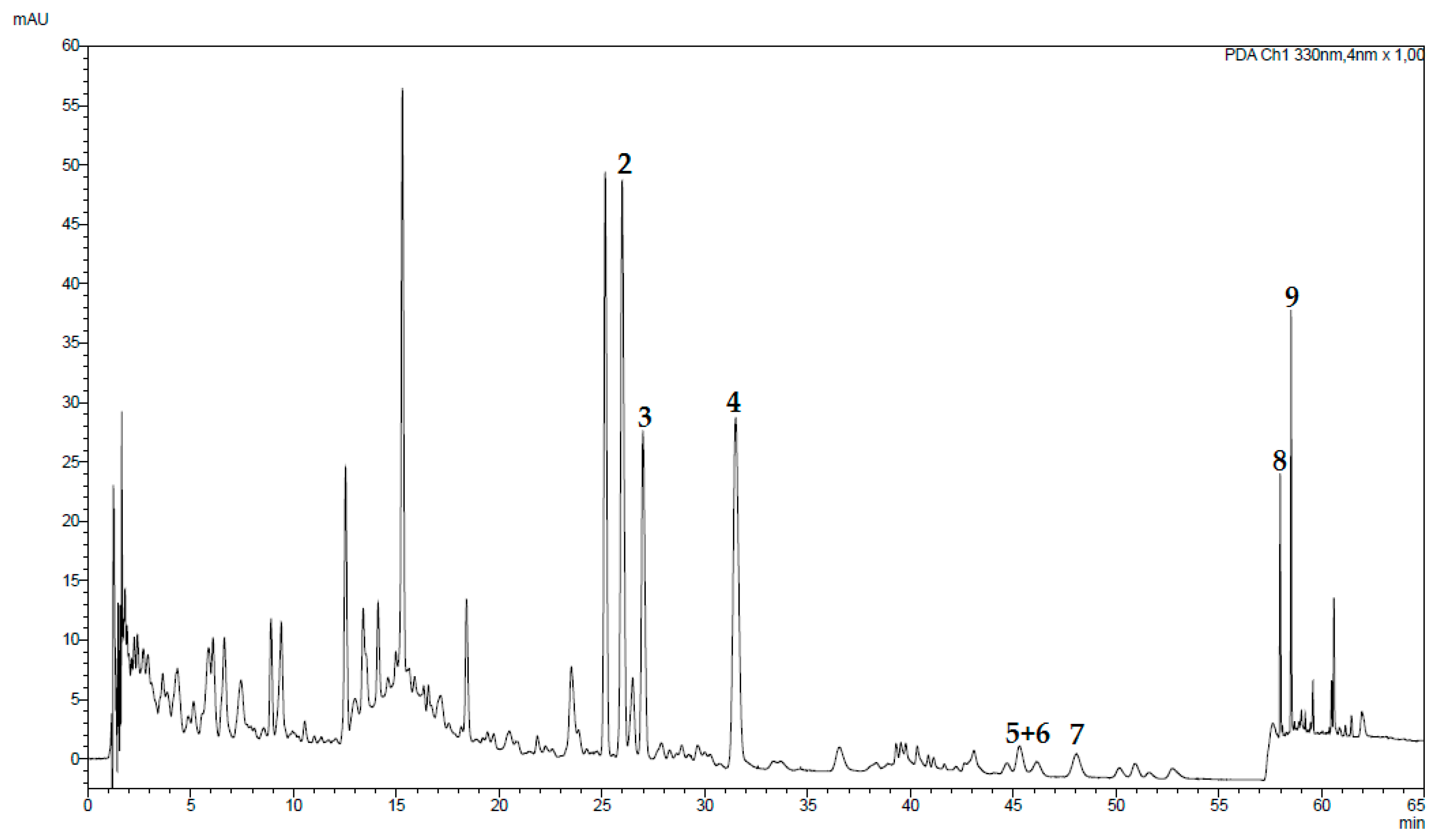
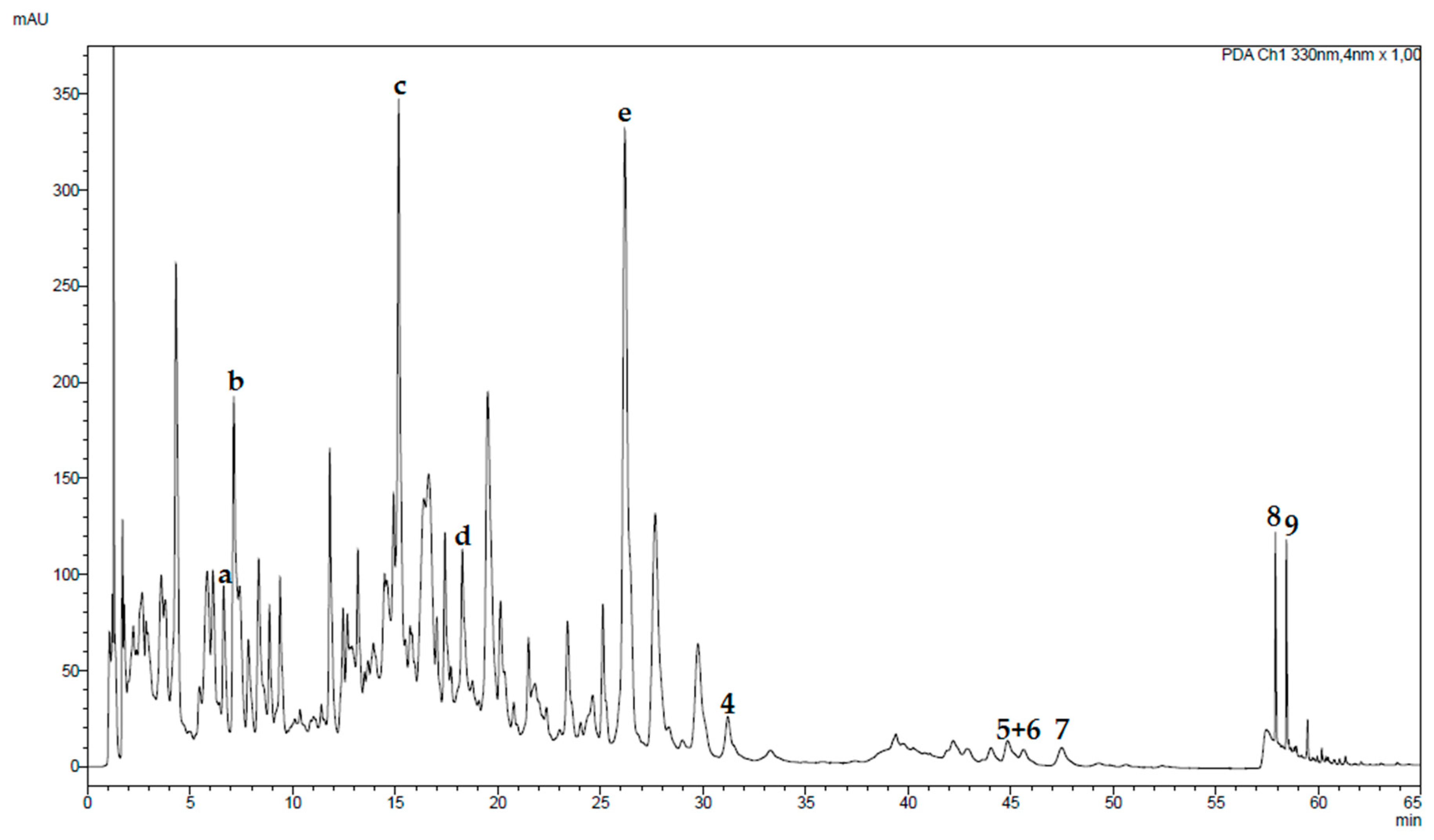
2.2. Chromatographic Analyses of Tested Fractions
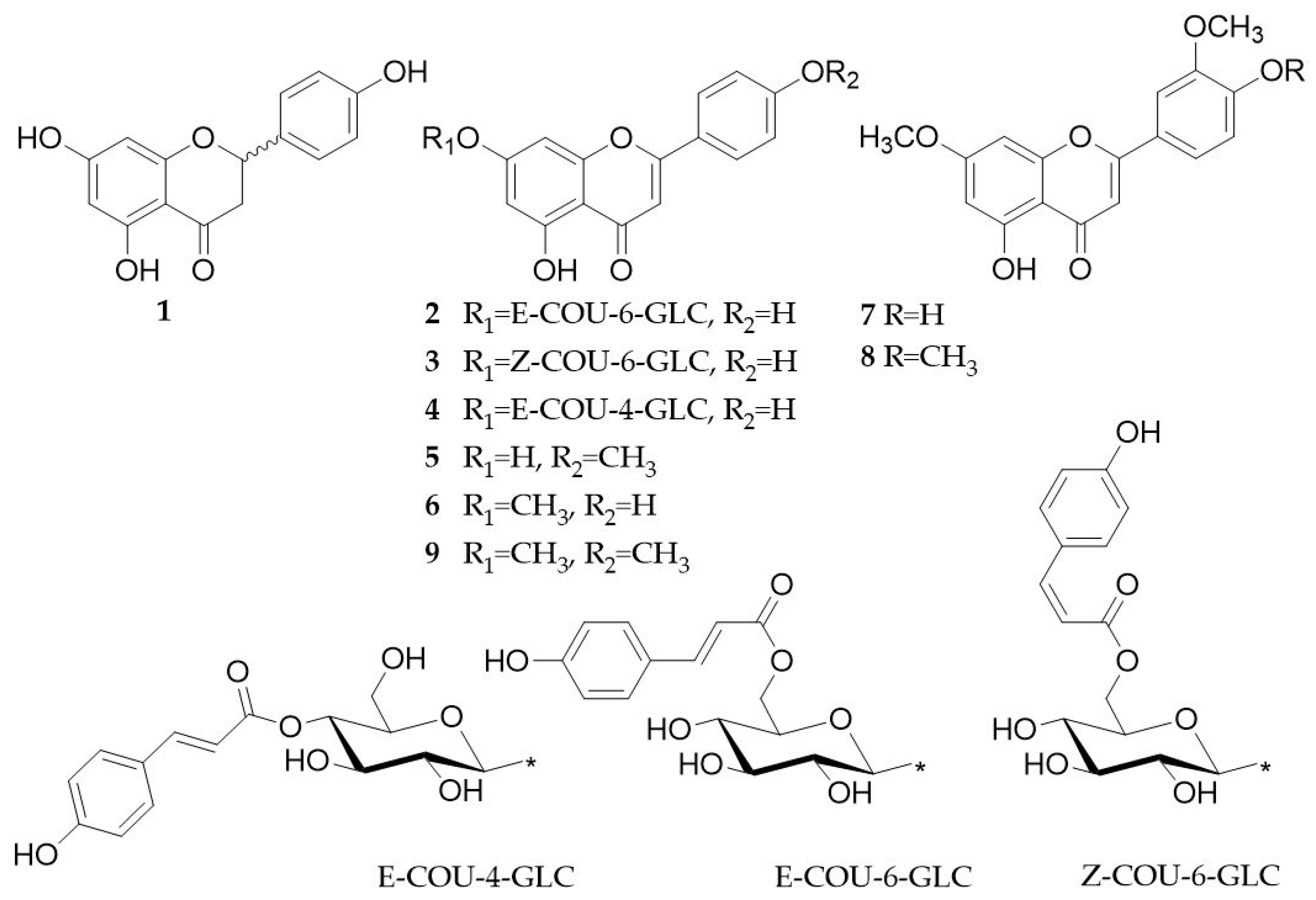
2.3. Isolation of Natural Compounds
2.4. Bioactivity of Isolated Compounds
2.5. Validation of UHPLC-DAD Assay
2.6. Chromatographic Analyses of a Traditional Preparation
3. Discussion
3.1. Bioactivity of Tested Fractions
3.2. Validation
3.3. Traditional Preparation
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Fractionation
4.4. Isolation
4.5. Cytotoxic Assays
4.6. Chromatographic Analyses
4.7. Method Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arbo, M.M.; Espert, S.M. Morphology, phylogeny and biogeography of Turnera L. (Turneraceae). Taxon 2009, 58, 457–467. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Zidorn, C. Ethnobotany, phytochemistry, and bioactivity of the genus Turnera (Passifloraceae) with a focus on damiana—Turnera diffusa. J. Ethnopharmacol. 2014, 152, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Ortiz-López, P.; Gutiérrez-Ortíz, J.; Martínez-Mota, L. Turnera diffusa Wild (Turneraceae) recovers sexual behavior in sexually exhausted males. J. Ethnopharmacol. 2009, 123, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dasmahapatra, A.K.; Khan, S.I.; Khan, I.A. Anti-aromatase activity of the constituents from damiana (Turnera diffusa). J. Ethnopharmacol. 2008, 120, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilar, F.J.; Roman-Ramos, R.; Flores-Saenz, J.L.; Aguirre-Garcia, F. Investigation on the hypoglycaemic effects of extracts of four Mexican medicinal plants in normal and Alloxan-diabetic mice. Phytother. Res. 2002, 16, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Parra-Naranjo, A.; Delgado-Montemayor, C.; Fraga-López, A.; Castañeda-Corral, G.; Salazar-Aranda, R.; Acevedo-Frenández, J.J.; Waksman, N. Acute Hypoglycemic and Antidiabetic Effect of Teuhetenone A Isolated from Turnera diffusa. Molecules 2017, 22, 599. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, J.; Ferreres, F.; Gil-Izquierdo, Á.; Valentão, P.; Andrade, P.B. Medicinal species as MTDLs: Turnera diffusa Willd. Ex Schult inhibits CNS enzymes and delays glutamate excitotoxicity in SH-SY5Y cells via oxidative damage. Food. Chem. Toxicol. 2017, 106, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Avelino-Flores, M.D.C.; Cruz-López, M.D.C.; Jiménez-Montejo, F.E.; Reyes-Leyva, J. Cytotoxic Activity of the Methanolic Extract of Turnera diffusa Willd on Breast Cancer Cells. J. Med. Food 2015, 18, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The açai flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Stavroullakis, A.; Oliveira, T.; Prakki, A. Cytotoxicity and potential anti-inflammatory activity of velutin on RAW 264.7 cell line differentiation: Implications in periodontal bone loss. Arch. Oral Biol. 2017, 83, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.; Jossang, A.; Bodo, B. DNA Topoisomerase I Inhibitors: Cytotoxic Flavones from Lethedon tannaensis. J. Nat. Prod. 1996, 59, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, H.; Wang, Q.; Wang, G. Synthesis and antiproliferative activity of novel aminoalkylated flavones. Chem. Heterocycl. Com. 2017, 53, 871–875. [Google Scholar] [CrossRef]
- Köhler, I.; Jenett-Siems, K.; Siems, K.; Hernández, M.A.; Ibarra, R.A.; Berendsohn, W.G.; Bienzle, U.; Eich, E. In vitro Antiplasmodial Investigation of Medicinal Plants from El Salvador§. Z. Naturforsch. 2002, 57, 277–281. [Google Scholar] [CrossRef]
- Boege, F.; Straub, T.; Kehr, A.; Boesenberg, C.; Christiansen, K.; Andersen, A.; Jakob, F.; Köhrle, J. Selected Novel Flavones Inhibit the DNA Binding or the DNA Religation Step of Eukaryotic Topoisomerase I. J. Biol. Chem. 1996, 271, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.; Mehta, R.; Runyan, C.; Rao, K.; Vaughan, A.; Moon, R. Flavonoids as DNA Topoisomerase Antagonists and Poisons: Structure-Activity Relationships. J. Nat. Prod. 1995, 58, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Fang, X. Apigenin, Chrysin, and Luteolin Selectively Inhibit Chymotrypsin-Like and Trypsin-Like Proteasome Catalytic Activities in Tumor Cells. Planta Med. 2010, 76, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Zhu, H.Y.; Zhang, X.H.; Du, Z.Y.; Xu, Y.J.; Yu, X.D. Apigenin Inhibits Proliferation and Induces Apoptosis in Human Multiple Myeloma Cells through Targeting the Trinity of CK2, Cdc37 and Hsp90. Mol. Cancer 2011, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Perez-Meseguer, J.; Garza-Juarez, A.; Salazar-Aranda, R.; Salazar-Cavazos, M.L.; Rodriguez, Y.C.D.L.T.; Rivas-Galindo, V.; de Torres, N.W. Development and Validation of an HPLC-DAD Analytical Procedure for Quality Control of Damiana (Turnera diffusa), Using an Antioxidant Marker Isolated from the Plant. J. AOAC Int. 2010, 93, 1161–1168. [Google Scholar] [PubMed]
- Wang, I.-K.; Lin-Shiau, S.-Y.; Lin, J.-K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar] [CrossRef]
- Ruela-de-Sousa, R.R.; Fuhler, G.M.; Blom, N.; Ferreira, C.V.; Aoyama, H.; Peppelenbosch, M.P. Cytotoxicity of apigenin on leukemia cell lines: Implications for prevention and therapy. Cell Death Dis. 2010, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.-I.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory Effects of Naringenin on Tumor Growth in Human Cancer Cell Lines and Sarcoma S-180-Implanted Mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Shen, S.-C.; Lin, H.-Y. Rutinoside at C7 attenuates the apoptosis-inducing activity of flavonoids. Biochem. Pharm. 2003, 66, 1139–1150. [Google Scholar] [CrossRef]
- Chen, R.; Hollborn, M.; Grosche, A.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol. Vis. 2014, 20, 242–258. [Google Scholar] [PubMed]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of Flavonoids toward Cultured Normal Human Cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pawar, R.S.; Ali, Z.; Khan, I.A. Phytochemical Investigation of Turnera diffusa. J. Nat. Prod. 2007, 70, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.; El-Mousallamy, A.M.D.; Barakat, H.H.; Buddrus, J.; Linscheid, M. Flavonoid lactates from leaves of Marrubium vulgare. Phytochemistry 1989, 28, 3201–3206. [Google Scholar] [CrossRef]
- Venturella, P.; Bellino, A.; Marino, M.L. Three acylated flavone glycosides from Sideritis syriaca. Phytochemistry 1995, 38, 527–530. [Google Scholar] [CrossRef]
- Nath, L.R.; Gorantla, J.N.; Joseph, S.M.; Antony, J.; Thankachan, S.; Menon, D.B.; Sankar, S.; Lankalapalli, R.S.; Anto, R.J. Kaempferide, the most active among the four flavonoids isolated and characterized from Chromolaena odorata, induces apoptosis in cervical cancer cells while being pharmacologically safe. RSC Adv. 2015, 5, 100912–100922. [Google Scholar] [CrossRef]
- Suleimenov, E.M.; Jose, R.A.; Rakhmadieva, S.B.; De Borggraeve, W.; Dehaen, W. Flavonoids from Senecio viscosus. Chem. Nat. Compd. 2009, 45, 731–732. [Google Scholar] [CrossRef]
- Tang, J.; Li, H.-L.; Li, Y.-L.; Zhang, W.-D. Flavonoids from rhizomes of Veratrum dahuricum. Chem. Nat. Compd. 2007, 43, 696–697. [Google Scholar] [CrossRef]
- Kou, L.-Q.; Cheng, X.-L.; Zhang, Z.-T. Syntheses and Crystal Structures of Two Apigenin Alkylation Derivatives. J. Chem. Crystallogr. 2008, 38, 21–25. [Google Scholar] [CrossRef]
- Nakasugi, T.; Komai, K. Antimutagens in the Brazilian Folk Medicinal Plant Carqueja (Baccharis trimera Less.). J. Agric. Food Chem. 1998, 46, 2560–2564. [Google Scholar] [CrossRef]
- Koopman, G.; Reutelingsperger, C.P.M.; Kuijten, G.A.M.; Keehnen, R.M.J.; Pals, S.T.; van Oers, M.H.J. Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds 5 + 6 and 7 are available from the authors. |


| Compound | Regression Curve | R2 | LOD 1 | LOQ 1 |
|---|---|---|---|---|
| 4 | 0.9985 | 1.39 | 4.60 | |
| 5 + 6 | 0.9955 | 0.162 | 0.535 | |
| 7 | 0.9956 | 0.236 | 0.780 | |
| 8 | 0.9924 | 0.120 | 0.396 |
| Compound | Repeatability 1 | Intra-day 1 1 | Intra-day 2 1 | Inter-day 1 |
|---|---|---|---|---|
| 2 | 12.9 (0.138) | 10.9 (4.95) | 10.6 (4.90) | 10.8 (4.82) |
| 3 | 2.16 (2.29) | 1.73 (5.52) | 1.68 (4.20) | 1.71 (4.97) |
| 4 | 8.38 (1.19) | 7.73 (4.18) | 7.29 (4.83) | 7.51 (5.25) |
| 5 + 6 | 1.96 (1.62) | 1.72 (3.02) | 1.55 (5.48) | 1.64 (6.89) |
| 7 | 2.12 (1.05) | 1.63 (6.89) | 1.71 (9.01) | 1.67 (7.82) |
| 8 | 1.08 (0.389) | 0.907 (3.37) | 0.897 (3.66) | 0.902 (3.40) |
| 9 | 1.73 (0.169) | 1.39 (4.87) | 1.39 (5.34) | 1.39 (4.88) |
| Compound | Sample Concentration 1 | Spiked Amount 2 | Total Concentration 1 | Recovery 3 |
|---|---|---|---|---|
| 4 | 6.56 | 3.08 | 4.72 | 105 |
| 7.69 | 12.6 | 113 | ||
| 15.4 | 18.7 | 112 | ||
| 23.1 | 24.7 | 111 | ||
| 5 + 6 | 1.38 | 0.401 | 0.746 | 102 |
| 1.07 | 2.10 | 99.7 | ||
| 2.14 | 2.83 | 106 | ||
| 3.21 | 3.55 | 107 | ||
| 7 | 1.52 | 0.585 | 0.964 | 102 |
| 1.04 | 2.18 | 100 | ||
| 2.08 | 2.84 | 102 | ||
| 3.12 | 3.50 | 99.2 | ||
| 8 | 0.798 | 0.297 | 0.497 | 109 |
| 0.793 | 1.39 | 109 | ||
| 1.59 | 1.99 | 109 | ||
| 2.09 | 2.29 | 127 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willer, J.; Jöhrer, K.; Greil, R.; Zidorn, C.; Çiçek, S.S. Cytotoxic Properties of Damiana (Turnera diffusa) Extracts and Constituents and A Validated Quantitative UHPLC-DAD Assay. Molecules 2019, 24, 855. https://doi.org/10.3390/molecules24050855
Willer J, Jöhrer K, Greil R, Zidorn C, Çiçek SS. Cytotoxic Properties of Damiana (Turnera diffusa) Extracts and Constituents and A Validated Quantitative UHPLC-DAD Assay. Molecules. 2019; 24(5):855. https://doi.org/10.3390/molecules24050855
Chicago/Turabian StyleWiller, Johanna, Karin Jöhrer, Richard Greil, Christian Zidorn, and Serhat Sezai Çiçek. 2019. "Cytotoxic Properties of Damiana (Turnera diffusa) Extracts and Constituents and A Validated Quantitative UHPLC-DAD Assay" Molecules 24, no. 5: 855. https://doi.org/10.3390/molecules24050855
APA StyleWiller, J., Jöhrer, K., Greil, R., Zidorn, C., & Çiçek, S. S. (2019). Cytotoxic Properties of Damiana (Turnera diffusa) Extracts and Constituents and A Validated Quantitative UHPLC-DAD Assay. Molecules, 24(5), 855. https://doi.org/10.3390/molecules24050855








