Influence of Diet Enriched with Cocoa Bean Extracts on Physiological Indices of Laboratory Rats
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Materials
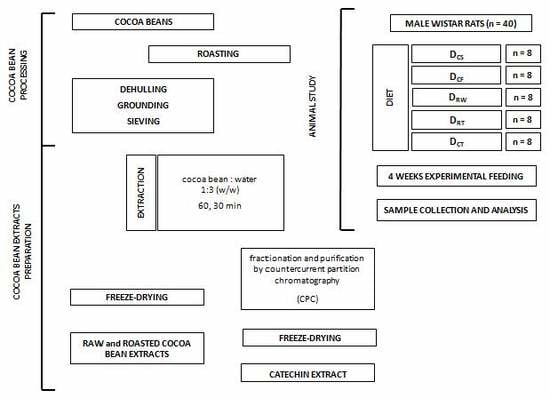
Cocoa Bean Processing
Roasting
Preparation of Cocoa Bean Extracts (CBEs)
Separation and Purification of Cocoa Bioactive Compounds
3.3. Animal Study
3.3.1. General Information on the Tested Subject and Investigated Diets
3.3.2. Sample Collection and Analysis
3.3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakatani, N. Antioxidant activity of resveratrol compared with common food additives. J. Food Prot. 2003, 64, 379–384. [Google Scholar] [CrossRef]
- Brown, J.E.; Kelly, M.F. Inhibition of lipid peroxidation by anthocyanins, anthocyanidins and their phenolic degradation products. Eur. J. Lipid Sci. Tech. 2007, 109, 66–71. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin, and resveratrol in red grapes: content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Khanduja, K.L. Stable free radical scavenging and antiperoxidative properties of resveratrol in vitro compared with some other bioflavonoids. Indian J. Biochem. Biophys. 2003, 40, 416–422. [Google Scholar] [PubMed]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for degenerative disorders: antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe vera. Oxid. Med. Cell. Longev. 2009, 2, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Valls, J.; Millán, S.; Marti, M.P.; Borràs, E.; Arola, L. Advanced separation methods of food anthocyanins, isoflavones, and flavanols. J. Chromatogr. A 2009, 1216, 7143–7172. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavour formation and character in cocoa and chocolate: a critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, E.I.; Akinyeye, R.O.; Ogunlade, I.; Olaofe, O.; Boluwade, J.O. Effect of farm and industrial processing on the amino acid profile of cocoa beans. Food Chem. 2010, 118, 357–363. [Google Scholar] [CrossRef]
- Oracz, J.; Żyżelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Ranneh, Y.; Ismail, A.; Esa, N.M. Identification of phenolic compounds in the polyphenol-rich extract of Malaysian cocoa powder using HPLC-UV-MS/MS and probing their antioxidant properties. J. Food Sci. Tech. 2015, 52, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Davinelli, S.; Di Renzo, L.; De Lorenzo, A.; Olarte, H.H.; Micali, G.; Cicero, A.F.; Gonzalez, S. Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients 2014, 6, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Franch, À.; Castellote, C.; Castell, M.; Pérez-Cano, F.J. Cocoa flavanoid-enriched diet modulates systemic and intestinal immunoglobulin synthesis in adult Lewis rats. Nutrients 2013, 5, 3272–3286. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, Q.R.; Gattward, J.N.; Almoosawi, S.; Silva, M.D.; Dantas, P.A.; De Araujo Júnior, Q.R. Cocoa and human health: from head to foot—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.X.; Oliveira, D.A.; Sodré, G.A.; Gosmann, G.; Brendel, M.; Pungartnik, C. Antimicrobial activity of fermented Theobroma cacao pod husk extract. Genet. Mol. Res. 2014, 13, 7725–7735. [Google Scholar] [CrossRef] [PubMed]
- Vertuani, S.; Scalambra, E.; Vittorio, T.; Bino, A.; Malisardi, G.; Baldisserotto, A.; Manfredini, S. Evaluation of antiradical activity of different cocoa and chocolate products: relation with lipid and protein composition. J. Med. Food 2014, 17, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Urpi-Sarda, M.; Lamuela-Raventos, R.M.; Estruch, R. Cardioprotective effects of cocoa: clinical evidence from randomized clinical intervention trials in humans. Mol. Nutr. Food Res. 2013, 57, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Bruna, C.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Bioactive compounds and antioxidant activity of cocoa hulls (Theobroma cacao L.) from different origins. J. Appl. Bot. Food Qual. 2009, 83, 9–13. [Google Scholar] [CrossRef]
- Cienfuegos-Jovellanos, E.; Quiñones, M.M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.; Guardia-Llorens, M.; Hidalgo, S.; Rozan, P. Protective effect of Acticoa powder, a cocoa polyphenolic extract, on prostate carcinogenesis in Wistar-Unilever rats. Eur. J. Cancer Prev. 2008, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Cocchi, C.A.; Lanfredini, M.; Keen, C.L.; Gershwin, M.E. Chocolate at heart: the anti-inflammatory impact of cocoa flavanols. Mol. Nutr. Food Res. 2008, 52, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, T.; Itoh, F.; Nozaki, A.; Nakano, Y.; Katsuda, S.I.; Osakabe, N.; Tubone, H.; Kondo, K.; Itakura, H. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J. Atheroscler. Thromb. 2005, 12, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.; Paglieroni, T.G.; Pearson, D.A.; Wun, T.; Schmitz, H.H.; Gosselin, R.; Keen, C.L. Cocoa and wine polyphenols modulate platelet activation and function. J. Nutr. 2000, 130, 2120S–2126S. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo Brito, B.D.N.; Campos Chisté, R.; da Silva Pena, R.; Abreu Gloria, M.B.; Santos Lopez, A. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Simpson Budu, A.; Kwesi Saalia, F. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Tech. 2013, 50, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.S.; Santana, L.R.R.; Soares, S.E.; Bispo, E.S. Effect of the roasting temperature and time of cocoa beans on the sensory characteristics and acceptability of chocolate. Food Sci. Tech. 2017, 37, 522–530. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Oracz, J.; Krysiak, W.; Budryn, G.; Nebesny, E. Effects of various roasting conditions on acrylamide, acrolein and polycyclic aromatic hydrocarbons content in cocoa bean and the derived chocolates. Drying Tech. 2017, 35, 363–374. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavor profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Krysiak, W.; Oracz, J.; Sosnowska, D.; Budryn, G.; Nebesny, E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res. Int. 2016, 89, 918–929. [Google Scholar] [CrossRef]
- Krysiak, W.; Adamski, R.; Żyżelewicz, D. Factors influencing the color of the roasted cocoa bean. J. Food Qual. 2013, 36, 21–31. [Google Scholar] [CrossRef]
- Ramli, N.; Hassan, O.; Said, M.; Samsudin, W.; Idris, N.A. Influence of roasting conditions on volatile flavor of roasted Malaysian cocoa beans. J. Food Process. Pres. 2006, 30, 280–298. [Google Scholar] [CrossRef]
- MacLeod, N.A.; Ørskov, E.R. Absorption and utilization of volatile fatty acids in ruminants. Can.J. Anim.Sci. 1984, 64, 354–355. [Google Scholar] [CrossRef]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Let. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Krysiak, W.; Budryn, G.; Oracz, J.; Nebesny, E. Tocopherols in cocoa butter obtained from cocoa bean roasted in different forms and under various process parameters. Food Res. Int. 2014, 63, 390–399. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Zakłos-Szyda, M.; Juśkiewicz, J.; Bojczuk, M.; Oracz, J.; Budryn, G.; Miśkiewicz, K.; Krysiak, W.; Zduńczyk, Z.; Jurgoński, A. Cocoa bean (Theobroma cacao L.) phenolic extracts as PTP1B inhibitors, hepatic HepG2 and pancreatic β-TC3 cell cytoprotective agents and their influence on oxidative stress in rats. Food Res. Int. 2016, 89, 946–957. [Google Scholar] [CrossRef]
- Bojczuk, M.; Żyżelewicz, D.; Hodurek, P. Centrifugal partition chromatography – a review of recent applications and some classic references. J. Sep. Sci. 2017, 40, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, J.; Castagnino, C.; Chèze, C.; Vercauteren, J. Preparative isolation of polyphenolic compounds from Vitis vinifera by centrifugal partition chromatography. J. Chromatogr. A 2002, 964, 123–128. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H.; et al. Recommendations for euthanasia of experimental animals: Part 2. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hofirek, B.; Haas, D. Comparative studies of ruminal fluid collected by oral tube or by puncture of the caudoventral ruminal sac. Acta Vet. Brno 2001, 70, 27–33. [Google Scholar] [CrossRef]
- Djouzi, Z.; Andrieux, C. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with a human faecal flora. Br. J. Nutr. 1997, 78, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Juśkiewicz, J.; Zduńczyk, Z. Lactulose-induced diarrhea in rats: effect on caecal development and activities of microbial enzymes. Comp. Biochem. Physiol. 2002, 133A, 411–417. [Google Scholar] [CrossRef]
- Jarosławska, J.; Juśkiewicz, J.; Wróblewska, M.; Jurgoński, A.; Król, B.; Zduńczyk, Z. Polyphenol-rich strawberry pomace reduces serum and liver lipids and alters gastrointestinal metabolite formation in fructose-fed rats. J. Nutr. 2011, 141, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A. Method for assay of intestinal disaccharidases. Anal. Вiochem. 1964, 7, 18–25. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Diet | p-Value | |||||
|---|---|---|---|---|---|---|
| DCS | DCF | DRW | DRT | DCT | ||
| Small intestine | ||||||
| Weight with content 1 | 1.98 ± 0.09 a | 2.10 ± 0.11 b | 2.16 ± 0.12 ab | 2.30 ± 0.10 a | 2.05 ± 0.09 b | 0.002 |
| Content pH | 7.33 ± 0.06 | 7.18 ± 0.04 | 6.92 ± 0.06 | 7.32 ± 0.05 | 7.17 ± 0.04 | 0.053 |
| Sucrase activity 2 | 6.53 ± 0.47 a | 3.69 ± 0.39 b | 4.83 ± 0.39 b | 3.97 ± 0.47 b | 4.39 ± 0.48 b | 0.004 |
| Maltase activity 2 | 35.8 ± 0.78 a | 22.6 ± 0.86 b | 35.4 ± 0.82 a | 27.7 ± 0.68 ab | 28.5 ± 0.71 ab | 0.009 |
| Lactase activity 2 | 1.56 ± 0.11 ab | 0.816 ± 0.09c | 1.73 ± 0.12 a | 1.33 ± 0.11 abc | 1.16 ± 0.12 bc | 0.001 |
| Cecum | ||||||
| Tissue 1 | 0.201 ± 0.002 a | 0.182 ± 0.003 ab | 0.192 ± 0.003 ab | 0.197 ± 0.001 a | 0.173 ± 0.002 b | 0.010 |
| Content 1 | 0.586 ± 0.012 a | 0.436 ± 0.020 ab | 0.495 ± 0.019 ab | 0.509 ± 0.025 ab | 0.386 ± 0.021 b | 0.016 |
| DM (%) | 24.0 ± 0.41 ab | 24.5 ± 0.46 ab | 22.5 ± 0.39 b | 23.7 ± 0.40 ab | 26.1 ± 0.41 a | 0.010 |
| NH3 (mg/g) | 0.227 ± 0.007 ab | 0.254 ± 0.009 a | 0.213 ± 0.008 ab | 0.188 ± 0.009 b | 0.210 ± 0.005 ab | 0.016 |
| Content pH | 7.21 ± 0.04 | 7.34 ± 0.05 | 7.34 ± 0.04 | 7.41 ± 0.04 | 7.44 ± 0.05 | 0.197 |
| Colon | ||||||
| Tissue 1 | 0.325 ± 0.018 | 0.279 ± 0.013 | 0.292 ± 0.012 | 0.290 ± 0.013 | 0.285 ± 0.010 | 0.055 |
| Content 1 | 0.205 ± 0.012 | 0.222 ± 0.011 | 0.230 ± 0.015 | 0.238 ± 0.015 | 0.195 ± 0.018 | 0.502 |
| Content pH | 7.42 ± 0.05 ab | 7.50 ± 0.04 ab | 7.24 ± 0.08 b | 7.65 ± 0.05 a | 7.48 ± 0.04 ab | 0.049 |
| Diet | p-Value | |||||
|---|---|---|---|---|---|---|
| DCS | DCF | DRW | DRT | DCT | ||
| β-glucosidase | ||||||
| day 0 | 39.0 ± 1.4 | 40.6 ± 1.5 | 35.1 ± 1.6 | 37.3 ± 1.7 | 38.6 ± 1.4 | 0.499 |
| day 1 | 40.9 ± 2.7 c | 111.0 ± 3.2 a | 101.0 ± 4.5 a | 71.7 ± 3.9 b | 58.2 ± 2.7 bc | <0.001 |
| day 4 | 19.0 ± 1.1 bc | 24.0 ± 1.3 ab | 20.8 ± 1.4 ab | 26.7 ± 1.4 a | 13.6 ± 1.3 c | <0.001 |
| day 8 | 10.5 ± 0.7 b | 12.7 ± 0.5 ab | 14.7 ± 0.8 ab | 16.5 ± 0.6 a | 13.1 ± 0.8 ab | 0.015 |
| day 11 | 7.19 ± 0.78 b | 9.02 ± 0.83 b | 14.10 ± 0.86 a | 14.20 ± 0.79 a | 10.40 ± 0.71 ab | 0.009 |
| β-galactosidase | ||||||
| day 0 | 168.0 ± 1.9 | 164.0 ± 1.8 | 161.0 ± 1.7 | 168.0 ± 1.6 | 168.0 ± 1.8 | 0.326 |
| day 1 | 213.0 ± 5.0 d | 528.0 ± 6.0 a | 400.0 ± 9.0 abc | 480.0 ± 7.0 ab | 290.0 ± 8.0 cd | <0.001 |
| day 4 | 134 ± 9.0 c | 238.0 ± 7.0 a | 206.0 ± 8.0 ab | 164.0 ± 6.0 bc | 170.0 ± 8.0 bc | <0.001 |
| day 8 | 81.6 ± 7.4 b | 130.0 ± 8.2 a | 170.0 ± 7.9 a | 140.0 ± 7.5 a | 157.0 ± 7.1 a | 0.001 |
| day 11 | 84.9 ± 7.4 b | 131.0 ± 7.1 a | 146.0 ± 6.8 a | 134.0 ± 7.2 a | 151.0 ± 7.5 a | 0.008 |
| β-glucuronidase | ||||||
| day 0 | 97.0 ± 2.5 | 100.0 ± 1.8 | 93.9 ± 2.7 | 94.2 ± 1.9 | 94.4 ± 2.5 | 0.615 |
| day 1 | 157.0 ± 5.2 c | 271.0 ± 6.9 a | 253.0 ± 7.1 a | 226.0 ± 4.8 b | 173.0 ± 8.1 c | <0.001 |
| day 4 | 48.2.0 ± 3.9 c | 70.4 ± 4.2 b | 114.0 ± 3.8 a | 77.6 ± 4.5 b | 46.5 ± 3.7 c | <0.001 |
| day 8 | 53.1 ± 2.6 bc | 60.8 ± 3.9 bc | 86.7 ± 3.2 a | 68.0 ± 3.5 ab | 46.1 ± 3.8 c | <0.001 |
| day 11 | 34.3 ± 3.1 b | 39.9 ± 2.9 b | 60.2 ± 3.1 a | 60.8 ± 3.2 a | 47.4 ± 2.8 ab | 0.010 |
| Diet | p-Value | |||||
|---|---|---|---|---|---|---|
| DCS | DCF | DRW | DRT | DCT | ||
| α-glucosidase | ||||||
| Extracellular 1 | 20.4 ± 0.5 a | 17.0 ± 0.7 ab | 17.8 ± 0.5 ab | 13.6 ± 0.6 b | 17.3 ± 0.7 ab | 0.007 |
| Intracellular 1 | 4.12 ± 0.8 b | 1.61 ± 0.9 b | 12.30 ± 0.9 a | 9.16 ± 0.7 a | 2.22 ± 0.8 b | <0.001 |
| total 1 | 24.6 ± 0.9 ab | 18.6 ± 1.1 b | 30.1 ± 0.9 a | 22.8 ± 0.8 b | 19.5 ± 1.2 b | 0.002 |
| release degree 2 | 84.5 ± 1.5 a | 91.0 ± 0.9 a | 61.4 ± 1.9 b | 61.0 ± 0.8 b | 88.6 ± 1.8 a | <0.001 |
| β-glucosidase | ||||||
| Extracellular 1 | 3.63 ± 0.20 a | 3.13 ± 0.12 a | 2.76 ± 0.13 ab | 2.50 ± 0.19 ab | 1.84 ± 0.15 b | 0.007 |
| Intracellular 1 | 3.02 ± 0.21 a | 0.61 ± 0.31 b | 3.68 ± 0.26 a | 4.08 ± 0.17 a | 1.02 ± 0.32 b | <0.001 |
| Total 1 | 6.65 ± 0.36 a | 3.74 ± 0.39 b | 6.44 ± 0.39 a | 6.58 ± 0.35 a | 2.85 ± 0.41 b | <0.001 |
| release degree 2 | 56.1 ± 2.7 b | 81.9 ± 3.1 a | 42.3 ± 3.6 c | 39.0 ± 2.7 c | 65.2 ± 3.1 b | <0.001 |
| α-galactosidase | ||||||
| Extracellular 1 | 10.80 ± 0.71 | 11.00 ± 0.61 | 8.54 ± 0.54 | 11.30 ± 0.59 | 7.72 ± 0.61 | 0.134 |
| Intracellular 1 | 7.90 ± 1.1 b | 5.98 ± 0.9 b | 14.40 ± 0.9 a | 19.40 ± 1.1 a | 5.70 ± 1.0 b | <0.001 |
| Total 1 | 18.7 ± 1.5 bc | 17.0 ± 1.7 bc | 22.9 ± 1.4 b | 30.7 ± 1.5 a | 13.4 ± 1.6 c | <0.001 |
| release degree 2 | 57.7 ± 1.9 a | 66.5 ± 2.7 a | 38.1 ± 1.9 b | 34.5 ± 2.5 b | 59.2 ± 1.9 a | <0.001 |
| β-galactosidase | ||||||
| Extracellular 1 | 44.4 ± 2.5 b | 71.0 ± 2.9 a | 60.9 ± 2.7 a | 40.6 ± 3.1 b | 62.0 ± 2.9 a | <0.001 |
| Intracellular 1 | 20.7 ± 1.7 ab | 21.3 ± 1.5 ab | 29.2 ± 1.6 a | 25.1 ± 1.7 ab | 18.0 ± 1.6 b | 0.041 |
| total 1 | 65.1 ± 3.9 b | 92.3 ± 4.1 a | 90.1 ± 3.9 a | 65.7 ± 3.5 b | 80.0 ± 3.4 ab | 0.026 |
| release degree 2 | 69.3 ± 1.5 ab | 77.8 ± 1.7 a | 67.3 ± 1.6 b | 62.6 ± 1.5 b | 77.2 ± 1.7 a | 0.003 |
| β-glucuronidase | ||||||
| Extracellular 1 | 21.4 ± 0.9 | 22.0 ± 1.1 | 20.0 ± 0.9 | 17.2 ± 1.2 | 18.7 ± 0.9 | 0.240 |
| Intracellular 1 | 12.00 ± 0.76 | 7.22 ± 0.81 | 11.20 ± 0.84 | 10.50 ± 0.86 | 6.97 ± 0.89 | 0.109 |
| Total 1 | 33.3 ± 1.4 | 29.2 ± 1.5 | 31.2 ± 1.6 | 27.6 ± 1.7 | 25.7 ± 1.4 | 0.155 |
| release degree 2 | 64.8 ± 1.9 ab | 78.1 ± 2.4 a | 64.6 ± 2.5 ab | 61.7 ± 1.7 ab | 73.5 ± 1.9 ab | 0.046 |
| Diet | p-Value | |||||
|---|---|---|---|---|---|---|
| DCS | DCF | DRW | DRT | DCT | ||
| VFA (µmol/g content) | ||||||
| acetic | 73.1 ± 1.9 | 71.4 ± 2.2 | 86.7 ± 1.9 | 75.9 ± 2.3 | 77.5 ± 2.1 | 0.052 |
| propionic | 15.8 ± 0.5 ab | 13.8 ± 0.6 b | 19.2 ± 0.7 a | 19.0 ± 0.5 a | 16.5 ± 0.6 ab | 0.012 |
| iso-butyric | 1.84 ± 0.07 a | 1.37 ± 0.06 ab | 1.53 ± 0.07 ab | 1.21 ± 0.05 b | 1.40 ± 0.08 ab | 0.014 |
| butyric | 10.70 ± 0.51 a | 8.70 ± 0.48 ab | 7.52 ± 0.51 b | 6.16 ± 0.46 b | 7.31 ± 0.53 b | 0.005 |
| iso-valeric | 1.82 ± 0.06 a | 1.26 ± 0.04 b | 1.34 ± 0.05 b | 1.06 ± 0.05 b | 1.18 ± 0.07 b | <0.001 |
| valeric | 3.23 ± 0.25 | 1.78 ± 0.19 | 1.91 ± 0.21 | 1.92 ± 0.23 | 1.75 ± 0.21 | 0.062 |
| Sum of iso-butyric, iso-valeric and valeric acid | 6.89 ± 0.21 a | 4.42 ± 0.27 b | 4.77 ± 0.24 b | 4.20 ± 0.28 b | 4.33 ± 0.23 b | 0.002 |
| VFA total | 107.0 ± 2.6 | 98.3 ± 2.9 | 118.0 ± 3.1 | 105.0 ± 3.2 | 106.0 ± 3.1 | 0.071 |
| VFA pool (µmol/100 g BW) | ||||||
| acetic | 43.1 ± 1.5 | 31.6 ± 1.9 | 42.4 ± 2.2 | 38.8 ± 2.3 | 29.9 ± 1.8 | 0.069 |
| propionic | 9.45 ± 0.46 ab | 6.21 ± 0.51 b | 9.40 ± 0.54 ab | 9.65 ± 0.53 a | 6.30 ± 0.55 ab | 0.049 |
| butyric | 6.41 ± 0.32 a | 3.90 ± 0.31 b | 3.66 ± 0.29 b | 3.14 ± 0.38 b | 2.73 ± 0.36 b | 0.002 |
| Sum of iso-butyric, iso-valeric and valeric acid | 4.01 ± 0.21 a | 1.97 ± 0.19 b | 2.34 ± 0.18 b | 2.18 ± 0.22 b | 1.69 ± 0.20 b | <0.001 |
| VFA total | 63.0 ± 2.5 a | 43.7 ± 3.4 ab | 57.8 ± 2.8 ab | 53.8 ± 2.7 ab | 40.6 ± 2.9 b | 0.032 |
| VFA profile (% total) | ||||||
| acetic | 68.8 ± 0.5 b | 73.3 ± 0.5 a | 73.4 ± 0.6 a | 72.1 ± 0.6 a | 73.1 ± 0.5 a | 0.011 |
| propionic | 15.0 ± 0.5 ab | 13.2 ± 0.4 b | 16.3 ± 0.6 ab | 18.0 ± 0.5 a | 15.9 ± 0.4 ab | 0.005 |
| butyric | 9.88 ± 0.34 a | 8.90 ± 0.39 a | 6.31 ± 0.41 b | 5.96 ± 0.36 b | 6.86 ± 0.38 b | 0.001 |
| Diet | p-Value | |||||
|---|---|---|---|---|---|---|
| DCS | DCF | DRW | DRT | DCT | ||
| WBC (103/μL) | 5.70 ± 0.19 ab | 6.11 ± 0.21 ab | 6.54 ± 0.20 a | 6.23 ± 0.19 ab | 4.97 ± 0.22 b | 0.032 |
| LYM (103/μL) | 4.67 ± 0.21 ab | 4.97 ± 0.16 ab | 5.48 ± 0.17 a | 5.06 ± 0.19 ab | 4.10 ± 0.18 b | 0.040 |
| MID (103/μL) | 0.361 ± 0.018 a | 0.347 ± 0.014 a | 0.171 ± 0.019 b | 0.256 ± 0.025 ab | 0.242 ± 0.021 ab | 0.015 |
| GRA (103/μL) | 0.665 ± 0.049 | 0.807 ± 0.051 | 0.890 ± 0.055 | 0.917 ± 0.048 | 0.681 ± 0.051 | 0.108 |
| LYM (%) | 82.1 ± 0.9 | 81.1 ± 0.8 | 82.8 ± 0.9 | 81.6 ± 0.7 | 82.3 ± 1.1 | 0.626 |
| MID (%) | 6.18 ± 0.32 a | 5.68 ± 0.38 ab | 2.76 ± 0.34 c | 3.86 ± 0.38 bc | 4.74 ± 0.36 abc | 0.002 |
| GRA (%) | 11.8 ± 0.8 | 13.3 ± 0.9 | 14.5 ± 0.7 | 14.6 ± 0.8 | 13.0 ± 0.9 | 0.347 |
| RBC (103/μL) | 7.47 ± 0.05 | 7.59 ± 0.07 | 7.76 ± 0.08 | 7.83 ± 0.05 | 7.76 ± 0.03 | 0.054 |
| HGB (g/dL) | 13.8 ± 0.1 c | 14.1 ± 0.1 abc | 14.6 ± 0.1 a | 14.4 ± 0.1 ab | 14.0 ± 0.1 bc | 0.010 |
| HCT (%) | 40.2 ± 0.3 b | 40.8 ± 0.2 ab | 42.6 ± 0.4 a | 42.6 ± 0.3 a | 41.1 ± 0.2 ab | 0.020 |
| MCV (fL) | 53.7 ± 0.2 ab | 53.8 ± 0.3 ab | 54.9 ± 0.2 a | 54.4 ± 0.3 ab | 52.9 ± 0.1 b | 0.035 |
| MCH (pg) | 18.4 ± 0.1 | 18.6 ± 0.2 | 18.8 ± 0.1 | 18.5 ± 0.1 | 18.0 ± 0.2 | 0.121 |
| MCHC (g/dL) | 34.3 ± 0.2 | 34.6 ± 0.1 | 34.3 ± 0.1 | 33.8 ± 0.2 | 34.0 ± 0.1 | 0.121 |
| RDWc (%) | 17.5 ± 0.1 bc | 17.3 ± 0.1 c | 17.6 ± 0.1 abc | 18.0 ± 0.1 a | 17.8 ± 0.1 ab | 0.004 |
| PLT (103/μL) | 482 ± 4 | 468 ± 6 | 520 ± 8 | 514 ± 10 | 485 ± 9 | 0.087 |
| PCT (%) | 0.372 ± 0.010 | 0.367 ± 0.005 | 0.400 ± 0.006 | 0.381 ± 0.007 | 0.371 ± 0.009 | 0.227 |
| MPV (fL) | 7.73 ± 0.07 ab | 7.83 ± 0.06 a | 7.33 ± 0.04 c | 7.41 ± 0.05 bc | 7.68 ± 0.06 abc | 0.013 |
| PDWc (%) | 33.8 ± 0.2 ab | 35.0 ± 0.1 a | 33.2 ± 0.3 b | 33.8 ± 0.2 ab | 35.0 ± 0.1 a | 0.007 |
| (%) | Diet 1 | ||||
|---|---|---|---|---|---|
| DCS | DCF | DRW | DRT | DCT | |
| Casein | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| DL-methionine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Rapeseed oil | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Palm oil | - | 14.0 | 14.0 | 14.0 | 14.0 |
| Cellulose | 5.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Sucrose | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mineral blend | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin blend | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Raw cocoa extract | - | - | 2.25 | - | - |
| Roasted cocoa extract | - | - | - | 2.45 | - |
| Monomeric flavan-3-ols fraction | - | - | - | - | 0.114 |
| Corn starch | 53.0 | 42.0 | 39.75 | 39.55 | 41.886 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żyżelewicz, D.; Bojczuk, M.; Budryn, G.; Jurgoński, A.; Zduńczyk, Z.; Juśkiewicz, J.; Oracz, J. Influence of Diet Enriched with Cocoa Bean Extracts on Physiological Indices of Laboratory Rats. Molecules 2019, 24, 825. https://doi.org/10.3390/molecules24050825
Żyżelewicz D, Bojczuk M, Budryn G, Jurgoński A, Zduńczyk Z, Juśkiewicz J, Oracz J. Influence of Diet Enriched with Cocoa Bean Extracts on Physiological Indices of Laboratory Rats. Molecules. 2019; 24(5):825. https://doi.org/10.3390/molecules24050825
Chicago/Turabian StyleŻyżelewicz, Dorota, Małgorzata Bojczuk, Grażyna Budryn, Adam Jurgoński, Zenon Zduńczyk, Jerzy Juśkiewicz, and Joanna Oracz. 2019. "Influence of Diet Enriched with Cocoa Bean Extracts on Physiological Indices of Laboratory Rats" Molecules 24, no. 5: 825. https://doi.org/10.3390/molecules24050825
APA StyleŻyżelewicz, D., Bojczuk, M., Budryn, G., Jurgoński, A., Zduńczyk, Z., Juśkiewicz, J., & Oracz, J. (2019). Influence of Diet Enriched with Cocoa Bean Extracts on Physiological Indices of Laboratory Rats. Molecules, 24(5), 825. https://doi.org/10.3390/molecules24050825