Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide
Abstract
1. Introduction
2. Results and Discussion
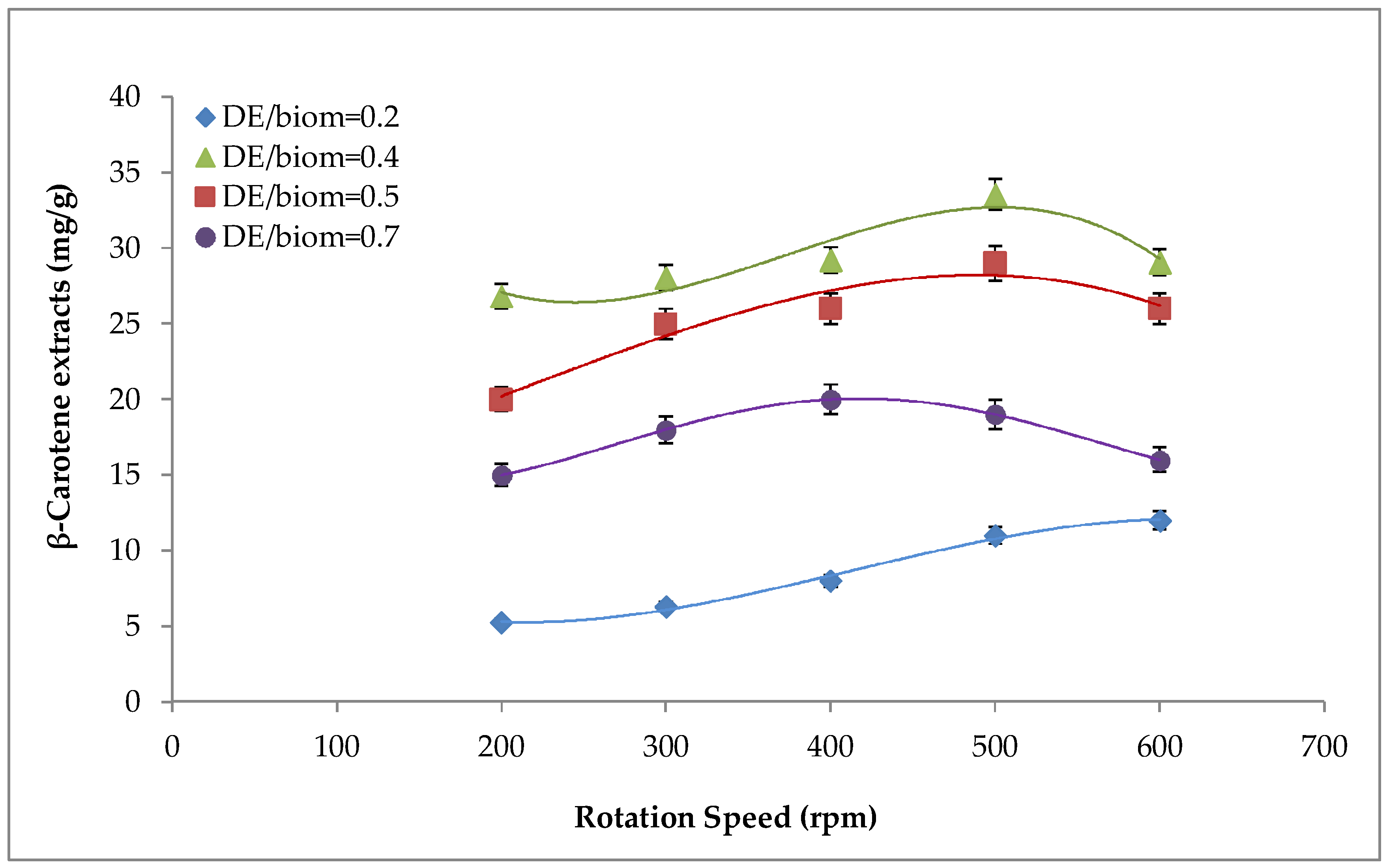
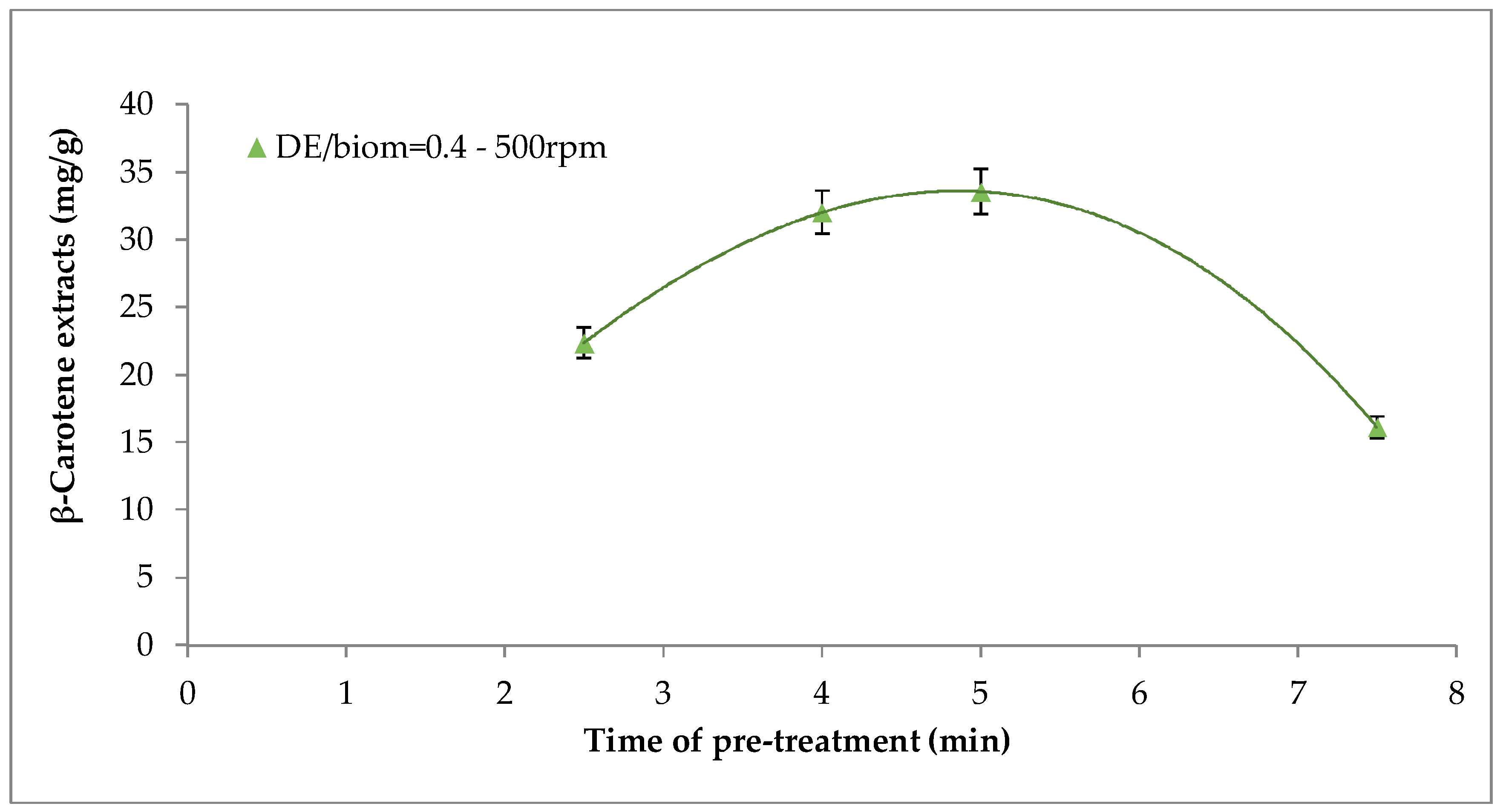
2.1. Effect of Mechanical Pre-Treatment on β-carotene Recovery
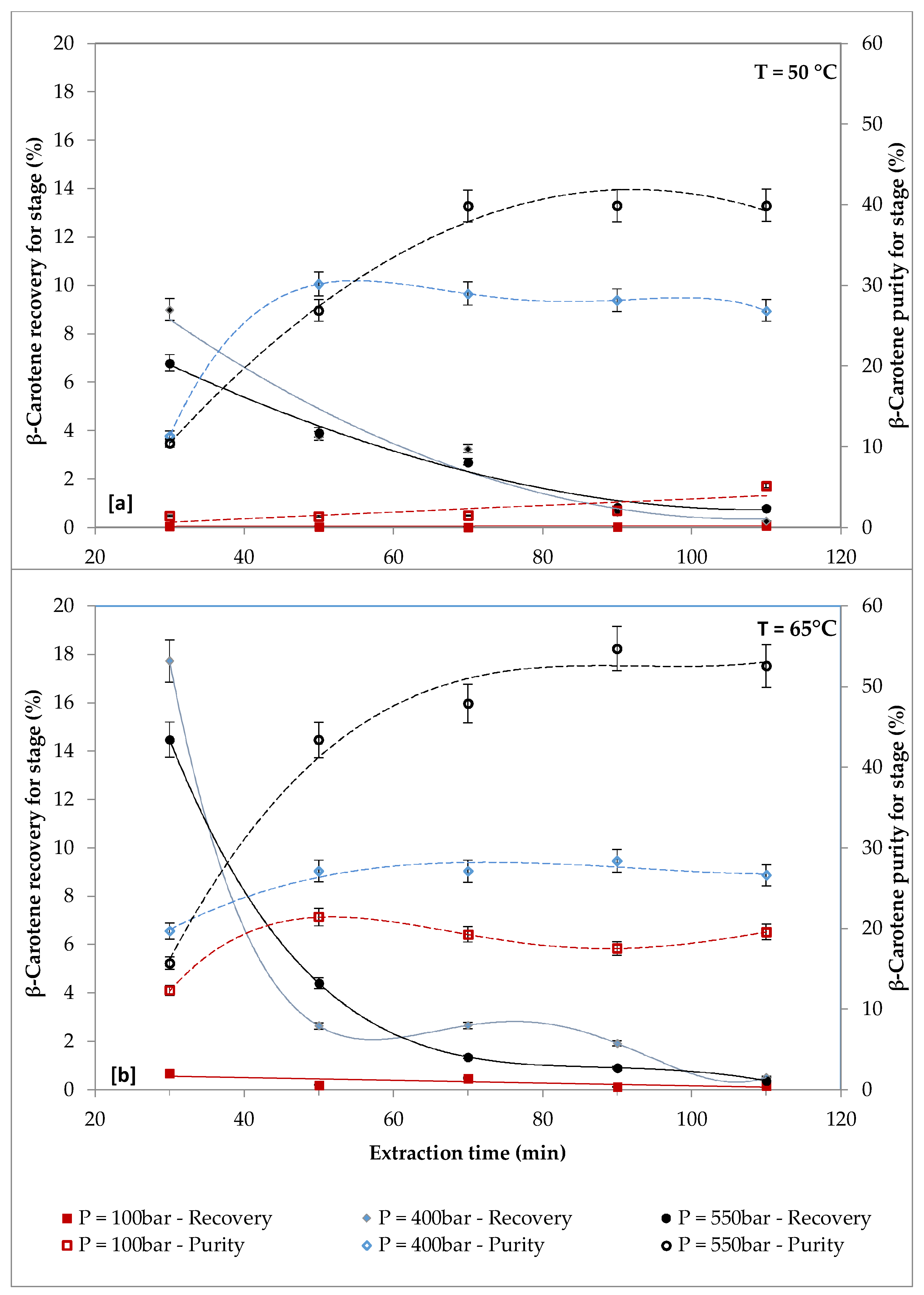
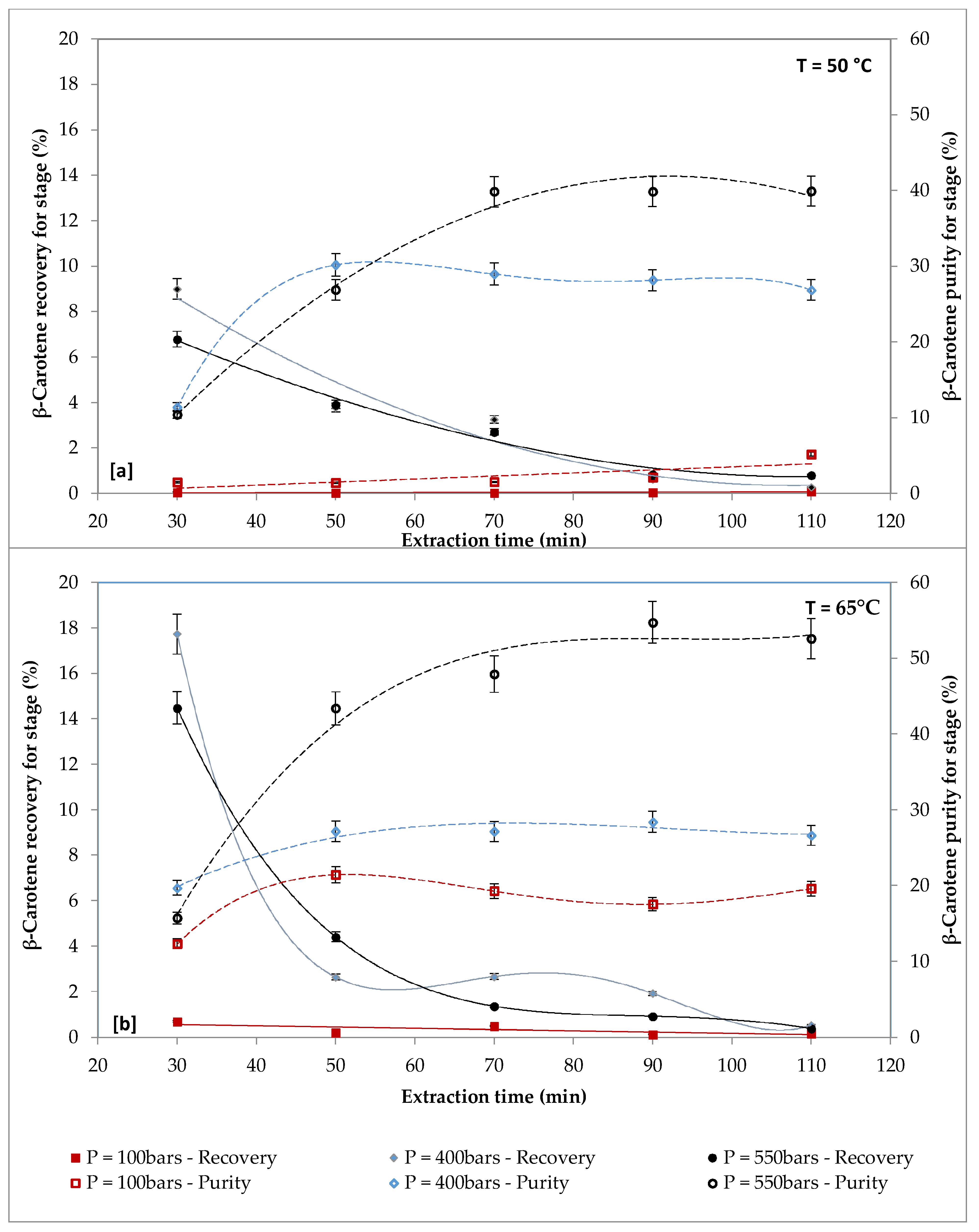
2.2. Effect of Pressure on β-carotene and FAs Extraction
2.3. Effect of Biomass Loading on β-Carotene and on FAs Extraction
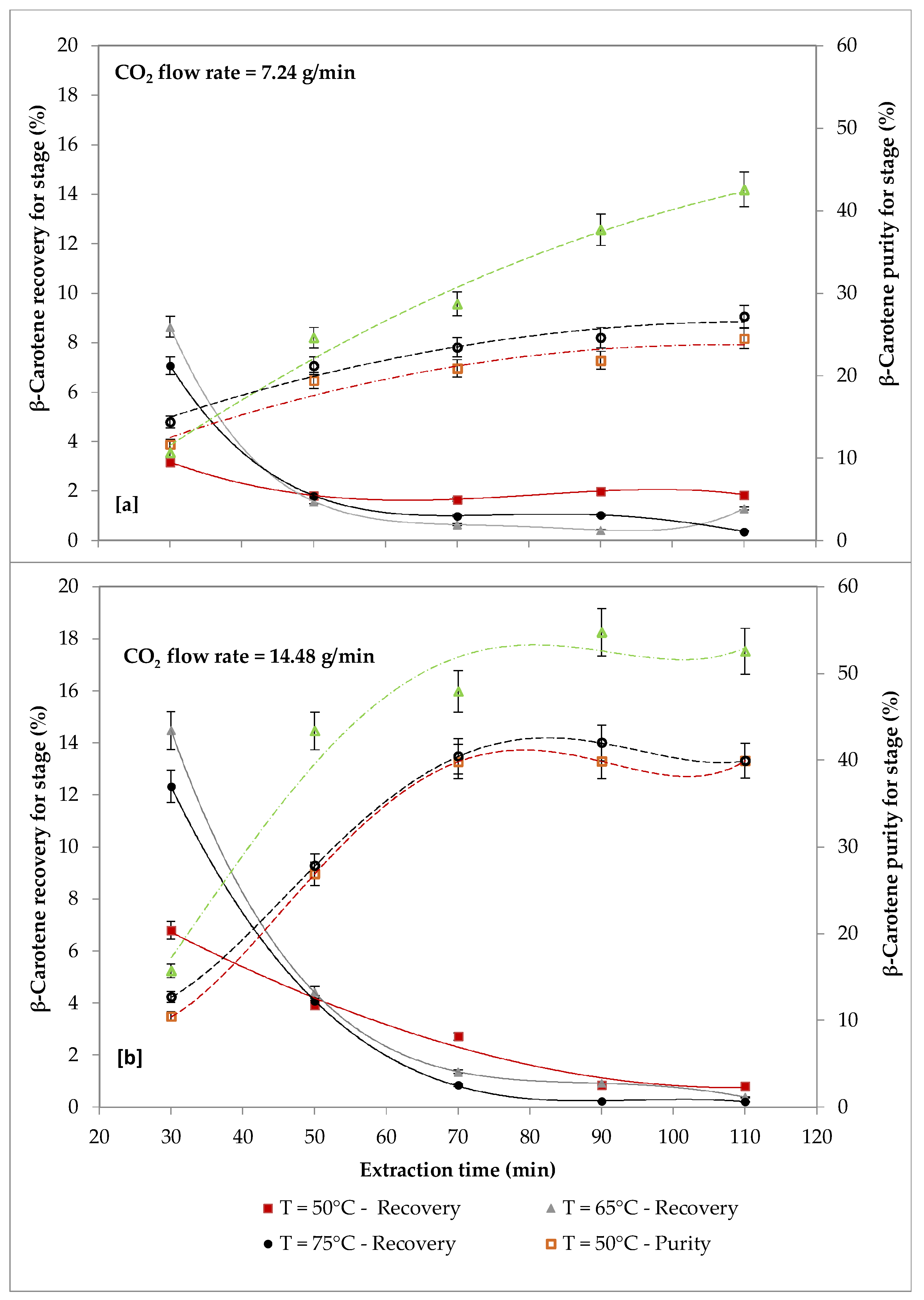
2.4. Effect of Temperature and CO2 Flow Rate on β-carotene and FAs Extraction
3. Materials and Methods
3.1. Microalgal Biomass and Chemicals
3.2. Mechanical Pre-Treatment of Biomass
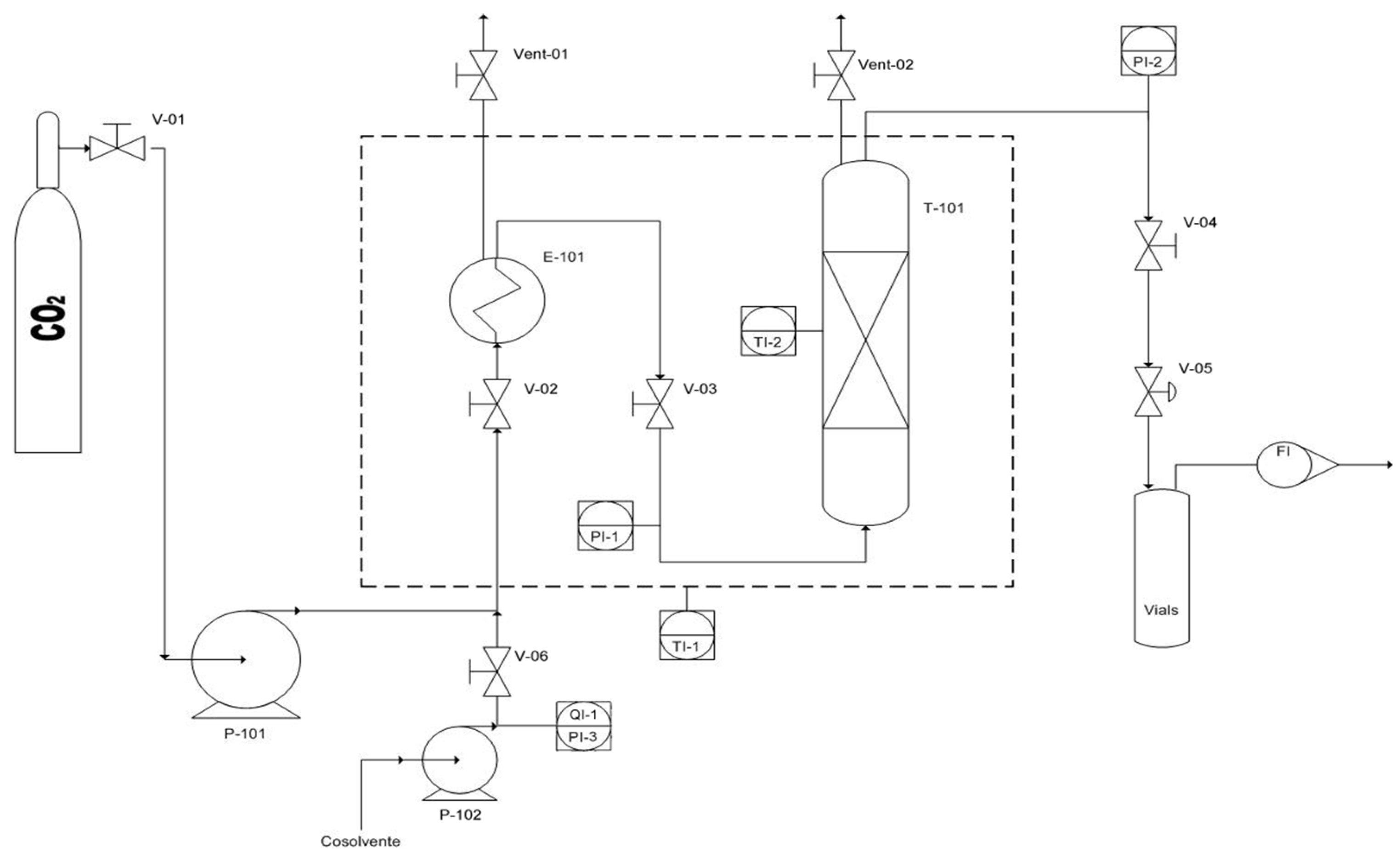
3.3. CO2 Supercritical Extraction Setup
- V-01/06: manual valve;
- P-101: CO2 pump;
- P-102: Co-solvent pump;
- E-101: Pre-Heater (Oven);
- FI: Flow meter;
- Vent-01/02: manual vent;
- T-101: Vessel for the extraction
3.4. Analytical Methods
3.4.1. Analytical Methods for β-Carotene Analysis
3.4.2. Analytical Methods for FAs Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Global Market Insights. Beta Carotene Market Size, Industry Analysis Report, Regional Outlook (U.S.; Germany, UK, Italy, Russia, China, India, Japan, South Korea, Brazil, Mexico, Saudi Arabia, UAE, South Africa), Application Development Potential, Price Trend, Competitive Market; GMI204; Global Market Insights: Selbyville, DE, USA, 2015. [Google Scholar]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octaviant, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011, 5, 7119–7131. [Google Scholar]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.L.; Coelho, J.P.; Fernandes, H.L.; Marrucho, I.J.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Applications of supercritical CO2 extraction to microalgae and plants. J. Chem. Technol. Biotechnol. 1995, 62, 53–59. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Cerdá-Olmedo, E. Fungal carotenoid production. Handb. Fungal Biotechnol. 2004, 20, 367–378. [Google Scholar]
- Da Costa Cardoso, L.A.; Kanno, K.Y.F.; Karp, S.G. Microbial production of carotenoids: A review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar]
- Schoefs, B. Chlorophyll and carotenoid analysis in food productsProperties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Copper, I.O.M. Dietary Reference Intakes for Vitamin A Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; Institute of Medicine (US) Panel on Micronutrients Washington (DC); National Academies Press (US): Washington, DC, USA, 2001; ISBN 10 0-309-07279-4. ISBN-10 0-309-07290-5. [Google Scholar]
- Isabel Minguez-Mosquera, M.; Hornero-Mendez, D.; Perez-Galvez, A. Carotenoids and Provitamin A in Functional FoodsMethods of Analysis for Functional Foods and Nutraceuticals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 277–335. [Google Scholar]
- Solymosi, K.; Latruffe, N.; Morant-Manceau, A.; Schoefs, B. Food colour additives of natural origin. In Colour Additives for Foods and Beverages; Elsevier: Amsterdam, The Netherlands, 2015; pp. 4–34. [Google Scholar]
- Bayerl, C. Beta-carotene in dermatology: Does it help. Acta Dermatovenerol. Alp. Panonica Adriat. 2008, 17, 160–162. [Google Scholar]
- Weldy, C.; Huesemann, M. Lipid production by Dunaliella salina in batch culture: Effects of nitrogen limitation and light intensity. J. Undergrad. Res. 2007, 7, 115–122. [Google Scholar] [CrossRef]
- Castellani, C.; Edwards, M. Marine Plankton: A Practical Guide to Ecology, Methodology, and Taxonomy; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Shaish, A.; Ben-Amotz, A.; Avron, M.B.T.-M. Biosynthesis of β-carotene. In Carotenoids Part A: Chemistry, Separation, Quantitation, and Antioxidation; Academic Press: Cambridge, MA, USA, 1992; pp. 439–444. [Google Scholar]
- García-González, M.; Moreno, J.; Manzano, J.C.; Florencio, F.J.; Guerrero, M.G. Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. J. Biotechnol. 2005, 115, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; Van De Laak, C.C.W.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Pisal, D.S.; Lele, S.S. Carotenoid production from microalga, Dunaliella salina. Indian J. Biotechnol. 2005, 4, 476–483. [Google Scholar]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Chidambara Murthy, K.N.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Paust, J. Recent progress in commercial retinoids and carotenoids. Pure Appl. Chem. 1991, 63, 45–58. [Google Scholar] [CrossRef]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Mobin, S.; Alam, F. Some Promising Microalgal Species for Commercial Applications: A review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, J.F.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, A.D.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F.A. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef]
- Filho, G.L.; De Rosso, V.V.; Meireles, M.A.A.; Rosa, P.T.V.; Oliveira, A.L.; Mercadante, A.Z.; Cabral, F.A. Supercritical CO2 extraction of carotenoids from pitanga fruits (Eugenia uniflora L.). J. Supercrit. Fluids 2008, 46, 33–39. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Di Sanzo, G.; Di Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef]
- Silva, G.F.; Gamarra, F.M.C.; Oliveira, A.L.; Cabral, F.A. Extraction of bixin from annatto seeds using supercritical carbon dioxide. Braz. J. Chem. Eng. 2008, 25, 419–426. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Fernandez-Sevilla, J.M.; Fernández, F.G.A.; García, M.C.C.; Grima, E.M. Supercritical fluid extraction of carotenoids from Scenedesmus almeriensis. Food Chem. 2010, 123, 928–935. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E.; Lubián, L.M.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. J. Food Eng. 2005, 66, 245–251. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.-A.A.; Badens, E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluids 2013, 77, 7–16. [Google Scholar] [CrossRef]
- Feller, R.; Matos, Â.P.; Mazzutti, S.; Moecke, E.H.S.; Tres, M.V.; Derner, R.B.; Oliveira, J.V.; Junior, A.F. Polyunsaturated ω-3 and ω-6 fatty acids, total carotenoids and antioxidant activity of three marine microalgae extracts obtained by supercritical CO2 and subcritical n–butane. J. Supercrit. Fluids 2017, 133, 437–443. [Google Scholar] [CrossRef]
- Novello, Z.; Scapinello, J.; Magro, J.D.; Zin, G.; Luccio, M.; Di Tres, M.V.; Oliveira, J.V. Extraction, chemical characterization and antioxidant activity of andiroba seeds oil obtained from pressurized n-butane. Ind. Crops Prod. 2015, 76, 697–701. [Google Scholar] [CrossRef]
- Awaluddin, S.A.; Thiruvenkadam, S.; Izhar, S.; Hiroyuki, Y.; Danquah, M.K.; Harun, R. Subcritical water technology for enhanced extraction of biochemical compounds from Chlorella vulgaris. BioMed Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Steenson, D.F.; Min, D.B. Effects of β-carotene and lycopene thermal degradation products on the oxidative stability of soybean oil. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 1153–1160. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Liau, B.C.; Shen, C.T.; Liang, F.P.; Hong, S.E.; Hsu, S.L.; Jong, T.T.; Chang, C.M.J. Supercritical fluids extraction and anti-solvent purification of carotenoids from microalgae and associated bioactivity. J. Supercrit. Fluids 2010, 55, 169–175. [Google Scholar] [CrossRef]
- Patil, P.D.; Dandamudi, K.P.R.; Wang, J.; Deng, Q.; Deng, S. Extraction of bio-oils from algae with supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2018, 135, 60–68. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.S.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Vechpanich, J.; Shotipruk, A. Recovery of Free Lutein from Tagetes erecta: Determination of Suitable Saponification and Crystallization Conditions. Sep. Sci. Technol. 2010, 46, 265–271. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |

| Class of Fatty Acids | Operative Temperature (°C) | |||||
|---|---|---|---|---|---|---|
| 50 | 65 | |||||
| Operative Pressure (bar) | ||||||
| 100 | 400 | 550 | 100 | 400 | 550 | |
| Lipids (mg/g) | 1.65 ± 0.07 | 6.37 ± 0.26 | 5.70 ± 0.23 | 1.24 ± 0.05 | 7.91 ± 0.32 | 0.88 ± 0.04 |
| % of FAME | 94.06 ± 4.33 | 91.73 ± 4.22 | 98.66 ± 4.54 | 89.38 ± 4.11 | 95.88 ± 4.41 | 96.21 ± 4.43 |
| FAME (mg/g) | 1.55 ± 0.05 | 5.84 ± 0.19 | 5.62 ± 0.18 | 1.11 ± 0.04 | 7.58 ± 0.24 | 0.84 ± 0.03 |
| SFAs (mg/g) | 0.79 ± 0.02 | 3.00 ± 0.07 | 3.30 ± 0.08 | 1.00 ± 0.02 | 4.12 ± 0.10 | 0.55 ± 0.01 |
| MUFAs (mg/g) | 0.62 ± 0.01 | 2.17 ± 0.09 | 1.79 ± 0.07 | 0.11 ± 0.00 | 2.69 ± 0.11 | 0.23 ± 0.01 |
| PUFAs (mg/g) | 0.14 ± 00 | 0.67 ± 0.02 | 0.53 ± 0.02 | <Ldl | 0.78 ± 0.03 | 0.06 ± 0.00 |
| SFAs | ||||||
| Palmitic acid (% of FAME) | 44.15 ± 2.21 | 43.54 ± 2.18 | 50.12 ± 2.51 | 85.83 ± 4.29 | 45.29 ± 2.26 | 65.58 ± 3.28 |
| Palmitic acid (mg/g) | 0.68 ± 0.03 | 2.54 ± 0.11 | 2.81 ± 0.12 | 0.95 ± 0.04 | 3.43 ± 0.14 | 0.55 ± 0.02 |
| Stearic acid (% of FAME) | 6.89 ± 0.28 | 7.83 ± 0.32 | 8.50 ± 0.35 | 4.47 ± 0.18 | 8.96 ± 0.37 | <Ldl |
| Stearic acid (mg/g) | 0.11 ± 00 | 0.46 ± 0.02 | 0.49 ± 0.02 | 0.05 ± 0.00 | 0.69 ± 0.03 | <Ldl |
| MUFAs | ||||||
| Cis-9-Octadecenoic acid (% of FAME) | 39.80 ± 1.79 | 37.14 ± 1.67 | 31.89 ± 1.44 | 9.70 ± 0.44 | 35.44 ± 1.59 | 27.41 ± 1.23 |
| Cis-9-Octadecenoic acid (mg/g) | 0.62 ± 0.01 | 2.17 ± 0.03 | 1.79 ± 0.03 | 0.11 ± 0.00 | 2.69 ± 0.12 | 0.23 ± 0.00 |
| PUFAs | ||||||
| Linoleic acid-ω-6 (% of FAME) | 9.16 ± 2.5 | 11.49 ± 0.29 | 9.49 ± 0.24 | <Ldl | 10.31 ± 0.26 | 7.00 ± 0.18 |
| Linoleic acid-ω-6 (mg/g) | 0.14 ± 1.5 | 0.67 ± 0.01 | 0.53 ± 0.01 | <Ldl | 0.78 ± 0.04 | 0.06 ± 0.00 |
| γ-Linolenic acid-ω-6 (% of FAME) | <Ldl | <Ldl | <Ldl | <Ldl | <Ldl | <Ldl |
| γ-Linolenic acid-ω-6 (mg/g) | <Ldl | <Ldl | <Ldl | <Ldl | <Ldl | <Ldl |
| Class of Fatty Acids | Biomass Loading (g) | |
|---|---|---|
| 2.45 | 7.53 | |
| Lipids (mg/g) | 5.70 ± 0.23 | 3.73 ± 0.15 |
| % of FAME | 98.66 ± 4.54 | 89.61 ± 1.97 |
| FAME (mg/g) | 5.62 ± 0.18 | 3.34 ± 0.13 |
| SFAs (mg/g) | 3.30 ± 0.08 | 2.44 ± 0.10 |
| MUFAs (mg/g) | 1.79 ± 0.07 | 0.18 ± 0.01 |
| PUFAs (mg/g) | 0.53 ± 0.02 | 0.72 ± 0.03 |
| SFAs | ||
| Palmitic acid (% of FAME) | 50.12 ± 2.51 | 73.20 ± 2.64 |
| Palmitic acid (mg/g) | 2.81 ± 0.12 | 2.44 ± 0.10 |
| Stearic acid(% of FAME) | 8.50 ± 0.35 | <Ldl |
| Stearic acid (mg/g) | 0.49 ± 0.02 | <Ldl |
| MUFAs | ||
| Cis-9-Octadecenoic acid (% of FAME) | 31.89 ± 1.44 | 5.34 ± 0.19 |
| Cis-9-Octadecenoic acid (mg/g) | 1.79 ± 0.03 | 0.18 ± 0.01 |
| PUFAs | ||
| Linoleic acid-ω-6 (% of FAME) | 9.49 ± 0.24 | <Ldl |
| Linoleic acid-ω-6 (mg/g) | 0.53 ± 0.01 | <Ldl |
| γ-Linolenic acid-ω-6 (% of FAME) | <Ldl | 21.46 ± 0.54 |
| γ-Linolenic acid-ω-6 (mg/g) | 5.70 ± 0.23 | 0.72 ± 0.03 |
| Class of Fatty Acids | Operative CO2 Flow Rate at 550 bars | |||||
|---|---|---|---|---|---|---|
| 7.24 g/min | 14.48 g/min | |||||
| Operative Temperature (°C) | ||||||
| 50 | 65 | 75 | 50 | 65 | 75 | |
| Lipids (mg/g) | 3.05 ± 0.15 | 8.18 ± 0.34 | 4.45 ± 0.14 | 5.70 ± 0.23 | 7.75 ± 0.24 | 8.47 ± 0.34 |
| % of FAME | 96.59 ± 2.51 | 95.65 ± 1.34 | 94.06 ± 1.50 | 98.66 ± 4.54 | 97.07 ± 0.87 | 95.96 ± 1.54 |
| FAME (mg/g) | 2.94 ±0.12 | 7.82 ± 0.38 | 4.18 ± 0.16 | 5.62 ± 0.18 | 7.52 ± 0.32 | 8.12 ± 0.26 |
| SFAs (mg/g) | 1.81 ± 0.08 | 4.62 ± 0.22 | 2.54 ± 0.11 | 3.30 ± 0.08 | 4.34 ± 0.19 | 4.93 ± 0.17 |
| MUFAs (mg/g) | 0.42 ± 0.02 | 2.22 ± 0.10 | 1.26 ± 0.06 | 1.79 ± 0.07 | 2.32 ± 0.08 | 2.46 ± 0.11 |
| PUFAs (mg/g) | 0.71 ± 0.03 | 0.99 ± 0.04 | 0.38 ± 0.02 | 0.53 ± 0.02 | 0.87 ± 0.03 | 0.73 ± 0.03 |
| SFAs | ||||||
| Palmitic acid (% of FAME) | 56.48 ± 1.19 | 47.53 ± 0.52 | 54.42 ± 0.98 | 50.12 ± 2.51 | 50.06 ± 0.75 | 53.71 ± 0.86 |
| Palmitic acid (mg/g) | 1.66 ± 0.08 | 3.72 ± 0.17 | 2.27 ± 0.11 | 2.81 ± 0.12 | 3.76 ± 0.18 | 4.36 ± 0.18 |
| Stearic acid (% of FAME) | 5.02 ± 0.24 | 11.50 ± 0.48 | 6.46 ± 0.23 | 8.50 ± 0.35 | 7.71 ± 0.78 | 7.02 ± 0.34 |
| Stearic acid (mg/g) | 0.15 ± 0.01 | 0.90 ± 0.04 | 0.27 ± 0.01 | 0.49 ± 0.02 | 0.58 ± 0.03 | 0.57 ± 0.03 |
| MUFAs | ||||||
| Cis-9-Octadecenoic acid (% of FAME) | 14.28 ± 0.50 | 28.38 ± 0.54 | 30.20 ± 1.06 | 31.89 ± 1.44 | 30.81 ± 0.77 | 30.30 ± 1.36 |
| Cis-9-Octadecenoic acid (mg/g) | 0.42 ± 0.02 | 2.22 ± 0.08 | 1.26 ± 0.05 | 1.79 ± 0.03 | 2.32 ± 0.10 | 2.46 ± 0.04 |
| PUFAs | ||||||
| Linoleic acid-ω-6 (% of FAME) | 24.22 ± 0.36 | 12.59 ± 0.23 | 9.17 ± 0.23 | 9.49 ± 0.24 | 10.77 ± 0.48 | 8.98 ± 0.22 |
| Linoleic acid-ω-6 (mg/g) | 0.71 ± 0.03 | 0.99 ± 0.04 | 0.38 ± 0.02 | 0.53 ± 0.01 | 0.81 ± 0.04 | 0.73 ± 0.04 |
| γ-Linolenic acid-ω-6 (% of FAME) | <Ldl | <Ldl | <Ldl | <Ldl | 0.76 ± 0.04 | <Ldl |
| γ-Linolenic acid-ω-6 (mg/g) | <Ldl | <Ldl | <Ldl | <Ldl | 0.06 ± 0.00 | <Ldl |
| Class of Fatty Acids | Theoretical Content |
|---|---|
| Lipids (mg/g) | 34.87 ± 1.05 |
| % of FAME | 90.51 ± 3.62 |
| FAME (mg/g) | 31.56 ± 1.10 |
| SFAs (mg/g) | 15.33 ± 0.69 |
| MUFAs (mg/g) | 05.68 ± 0.28 |
| PUFAs (mg/g) | 10.56 ± 0.31 |
| SFAs | |
| Palmitic acid (% of FAME) | 30.57 ± 1.25 |
| Palmitic acid (mg/g) | 09.65 ± 0.44 |
| Stearic acid (% of FAME) | 17.99 ± 0.58 |
| Stearic acid (mg/g) | 05.68 ± 0.14 |
| MUFAs | |
| Cis-9-Octadecenoic acid (% of FAME) | 17.98 ± 0.52 |
| Cis-9-Octadecenoic acid (mg/g) | 05.68 ± 0.12 |
| PUFAs | |
| Linoleic acid-ω-6 (% of FAME) | 16.47 ± 0.68 |
| Linoleic acid-ω-6 (mg/g) | 05.2 ± 0.22 |
| γ-Linolenic acid-ω-6 (% of FAME) | 16.99 ± 0.70 |
| γ-Linolenic acid-ω-6 (mg/g) | 05.36 ± 0.17 |
| Biomass Loading (g) | DE (g) | Glass Spheres (g) | Bulk Density (g/mL) | Bed Height (cm) | Porosity [-] |
|---|---|---|---|---|---|
| 2.45 | 0.98 | 44.0 | 0.087 | 19.7 | 0.46 |
| 7.53 | 3.01 | 44.0 | 0.223 | 23.6 | 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molino, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Marino, T.; Karatza, D.; Musmarra, D. Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules 2019, 24, 782. https://doi.org/10.3390/molecules24040782
Molino A, Larocca V, Di Sanzo G, Martino M, Casella P, Marino T, Karatza D, Musmarra D. Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules. 2019; 24(4):782. https://doi.org/10.3390/molecules24040782
Chicago/Turabian StyleMolino, Antonio, Vincenzo Larocca, Giuseppe Di Sanzo, Maria Martino, Patrizia Casella, Tiziana Marino, Despina Karatza, and Dino Musmarra. 2019. "Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide" Molecules 24, no. 4: 782. https://doi.org/10.3390/molecules24040782
APA StyleMolino, A., Larocca, V., Di Sanzo, G., Martino, M., Casella, P., Marino, T., Karatza, D., & Musmarra, D. (2019). Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules, 24(4), 782. https://doi.org/10.3390/molecules24040782









