Lysosome-Targeted Single Fluorescence Probe for Two-Channel Imaging Intracellular SO2 and Biothiols
Abstract
1. Introduction
2. Results and Discussion
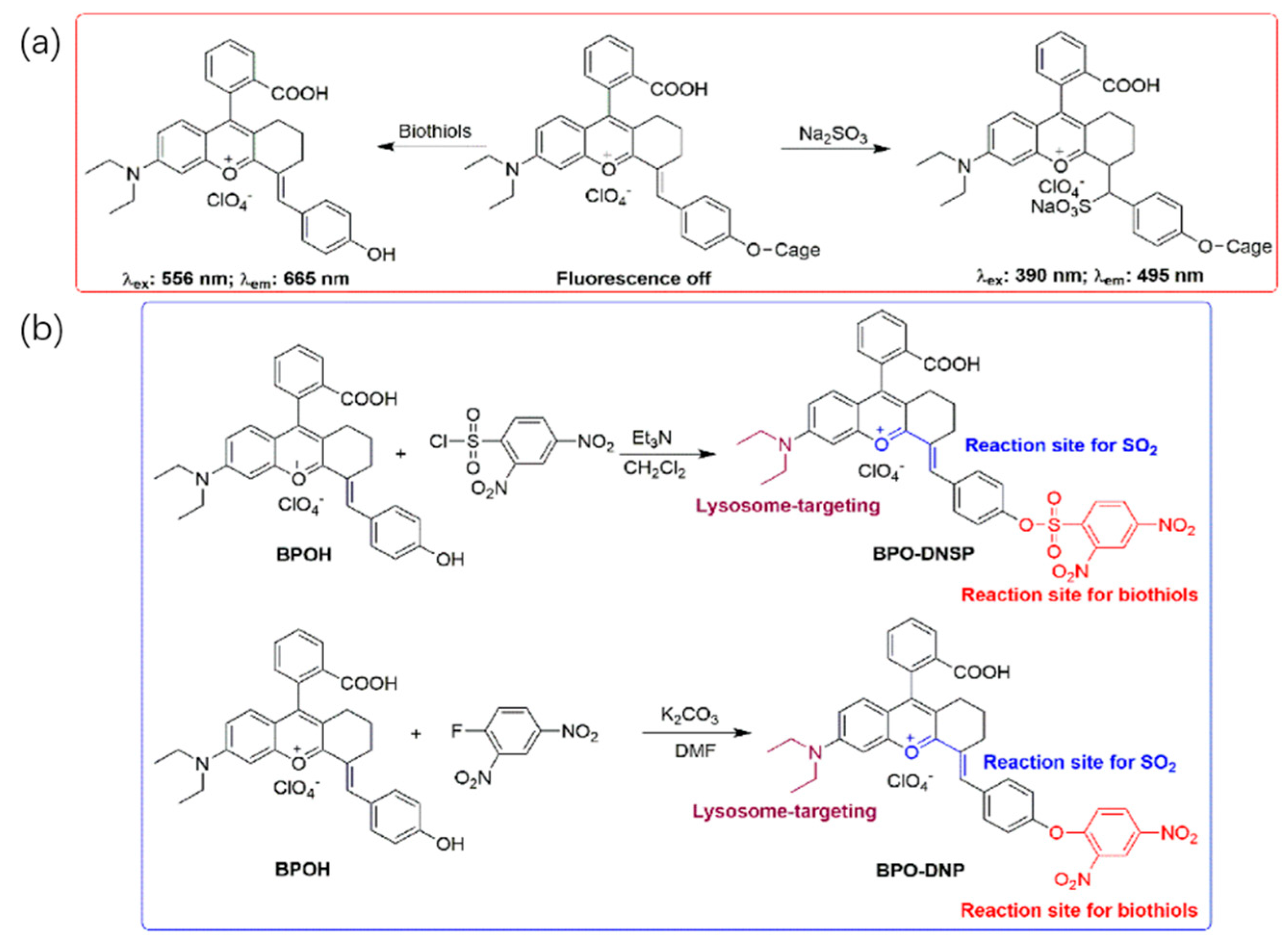
2.1. Design Strategy and Synthesis of Probes
2.2. Separate Response of Probes to SO2 and Biothiols in Different Channels
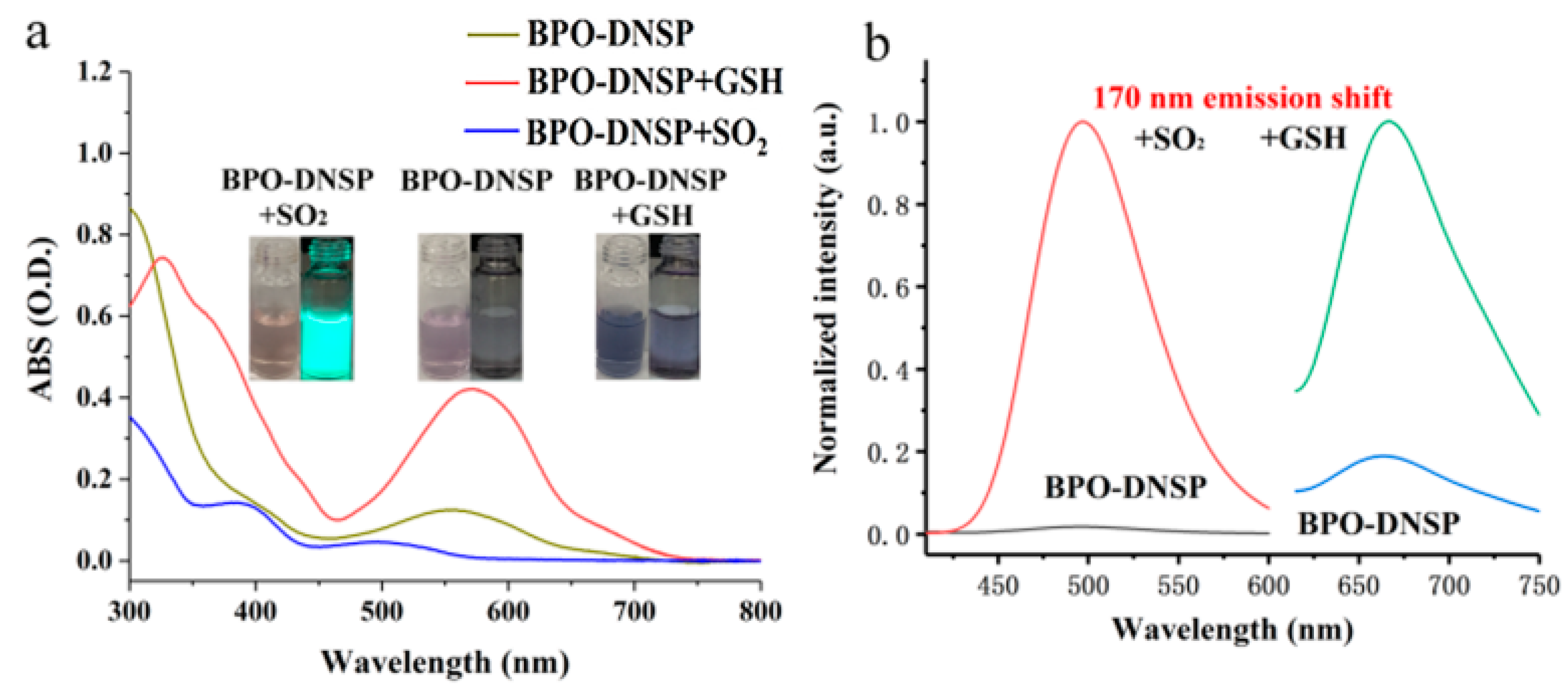
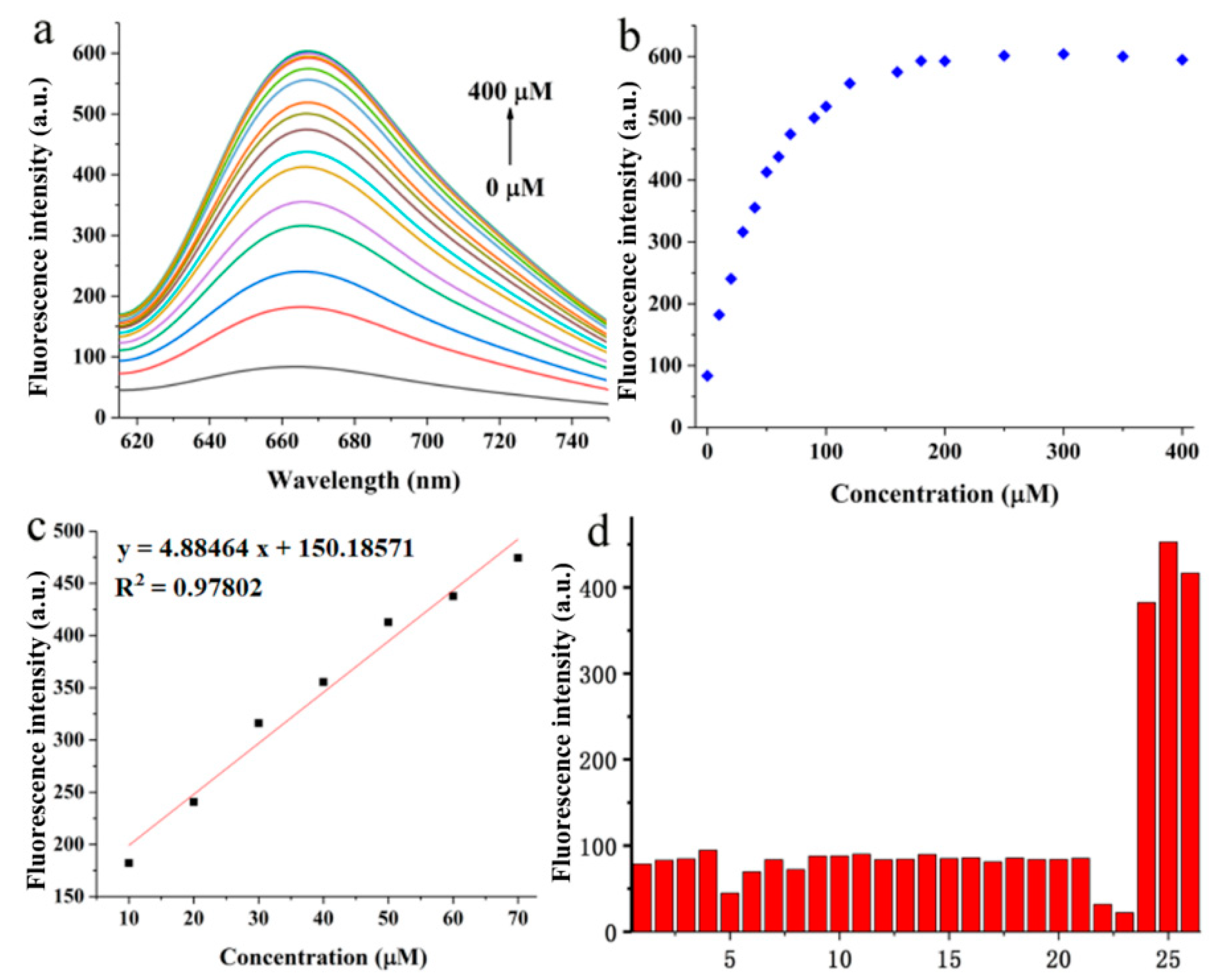
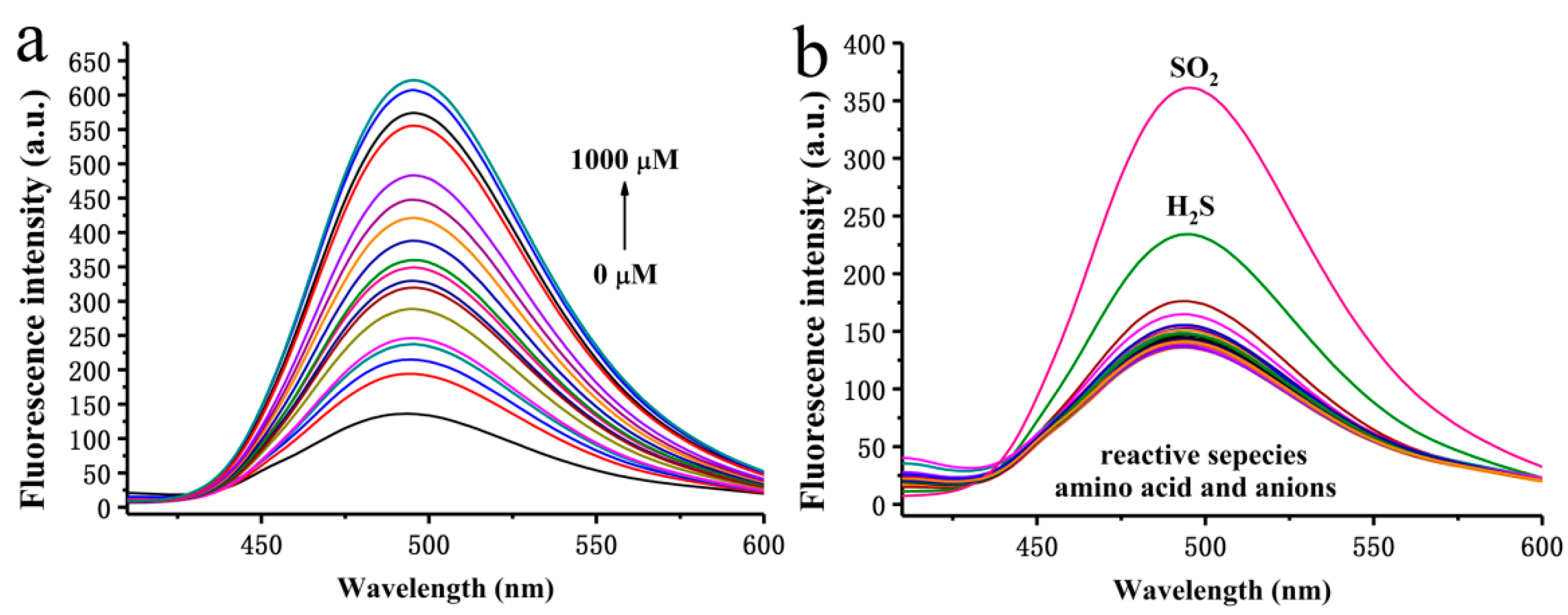
2.3. Optical Response of BPO-DNSP to SO2 and Biothiols
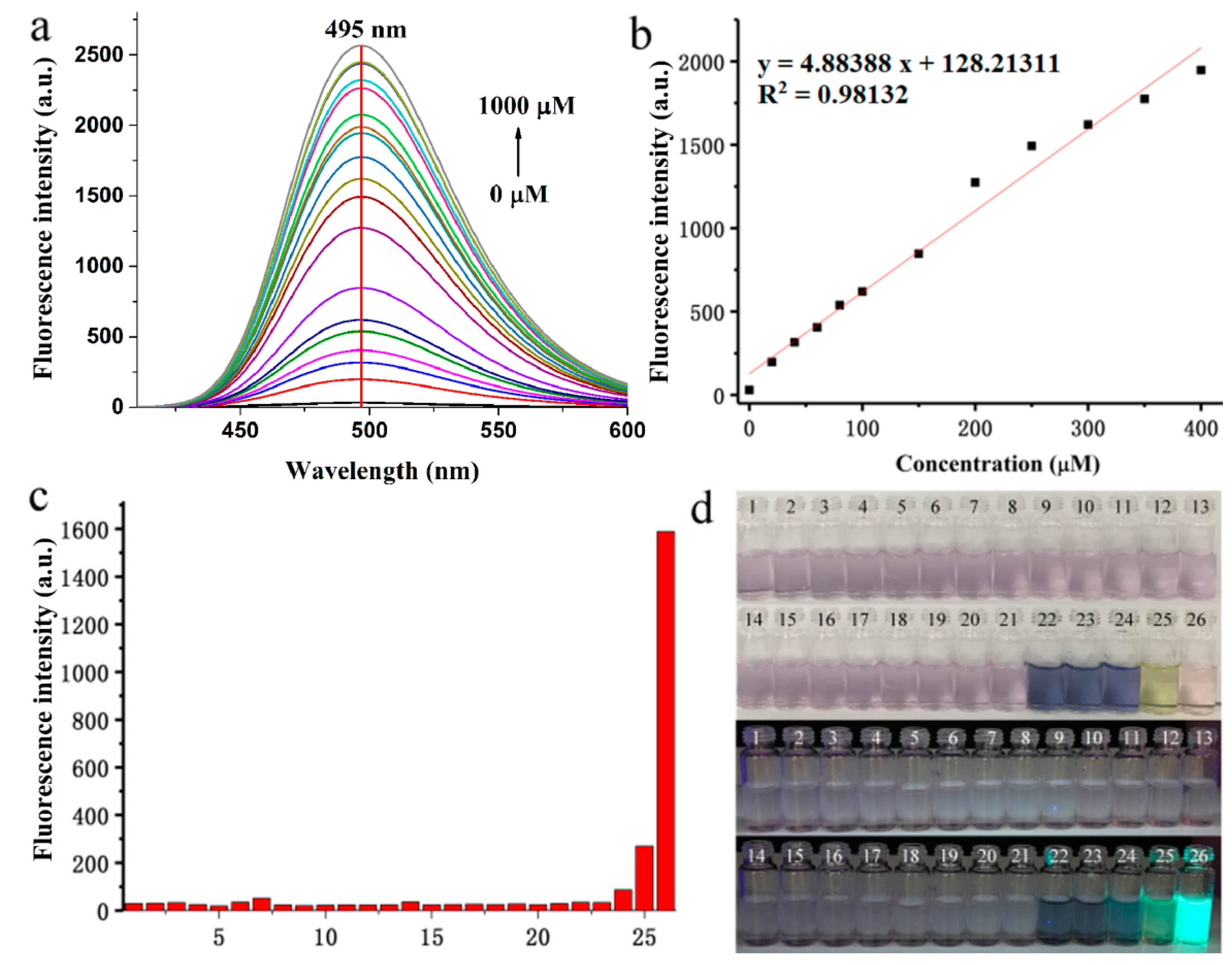
2.4. Optical Response of BPO-DNP to SO2
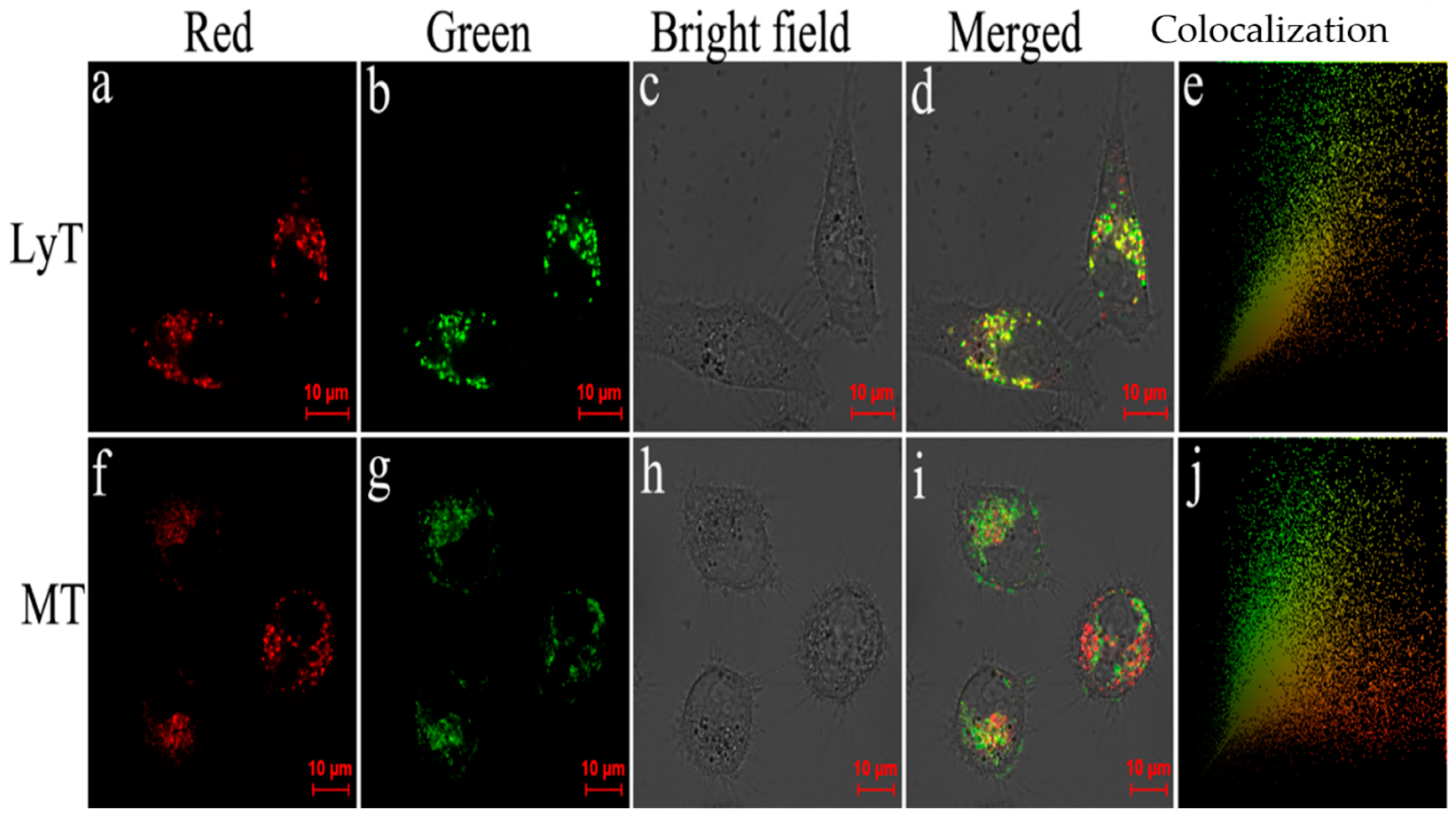
2.5. Fluorescence Imaging of SO2 and Biothiols in Living Cells
3. Experimental
3.1. Materials and Instruments
3.2. Synthesis of BPO-DNSP and BPO-DNP
3.3. Absorption and Fluorescence Spectra Measurement
3.4. Detection Limit
3.5. CCK-8 Assay for the Cell Cytotoxicity
3.6. Cell Culture and Confocal Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.Y.; Ong, C.-N.; Shen, H.-M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Bu, D.; Liu, A.D.; Holmberg, L.; Jia, Y.; Tang, C.; Du, J.; Jin, H. Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signalling. Cell Death Dis. 2014, 5, 1251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Z. The role of sulfur dioxide as an endogenous gaseous vasoactive factor in synergy with nitric oxide. Nitric Oxide 2009, 20, 166–174. [Google Scholar] [CrossRef]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.M.; Gladyshev, V.N. Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J. Mol. Biol. 2010, 404, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The role of reactive sulfur species in oxidative stress. Free Radical Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef]
- Ishanina, M.T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef]
- Shahrokhian, S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 2001, 73, 5972–5978. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, A.N.P. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Li, X.; Bazer, F.W.; Gao, H.; Jobgen, W.; Johnson, G.A.; Li, P.; McKnight, J.R.; Satterfield, M.C.; Spencer, T.E.; Wu, S.G. Amino acids and gaseous signalling. Amino Acids 2009, 37, 65–78. [Google Scholar] [CrossRef]
- Griffith, O.W. Mammalian sulfur amino acid metabolism: An overview. Methods Enzymol. 1987, 143, 366–376. [Google Scholar]
- Sang, N.; Yun, Y.; Li, H.; Hou, L.; Han, M.; Li, G.K. SO2 inhalation contributes to the development and progression of ischemic stroke in the brain. Toxicol. Sci. 2010, 114, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, B.; Tang, Y.; Lin, W. A unique “integration” strategy for the rational design of optically tunable near-infrared fluorophores. Acc. Chem. Res. 2017, 50, 1410–1422. [Google Scholar] [CrossRef]
- Hou, J.-T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.-Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-molecule fluorescent probes for imaging and detection of reactive oxygen, nitrogen, and sulfur species in biological systems. Anal. Chem. 2018, 90, 533–555. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Yin, C.-X.; Xiong, K.-M.; Huo, F.-J.; Salamanca, J.C.; Strongin, R.M. Fluorescent probes with multiple binding sites for the discrimination of Cys, Hcy, and GSH. Angew. Chem. Int. Ed. 2017, 56, 13188–13198. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Chen, X.; Kim, J.S.; Yoon, J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols. Chem. Soc. Rev. 2013, 42, 6019–6031. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–12249. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Huo, F.; Ning, P.; Zhang, Y.; Chao, J.; Meng, X.; Yin, C. Dual-site fluorescent probe for visualizing the metabolism of Cys in living cells. J. Am. Chem. Soc. 2017, 139, 3181–3185. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, P.; Li, K.; Feng, J.; Zou, M.; Yu, X. Dual-site fluorescent probe for highly selective and sensitive detection of sulfite and biothiols. Chine. Chem. Lett. 2018, 29, 992–994. [Google Scholar] [CrossRef]
- Guo, X.; Xia, L.; Huang, J.; Wang, Y.; Gu, Y.; Wang, P. Novel dual-site fluorescent probe for monitoring cysteine and sulfite in living cells. RSC Adv. 2018, 8, 21047–21054. [Google Scholar] [CrossRef]
- Xie, X.; Yin, C.; Yue, Y.; Chao, J.; Huo, F. Fluorescent probe detect distinguishly sulfite/hydrogen sulfide and thiol via two emission channels in vivo. Sens. Actuators B 2018, 277, 647–653. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Wang, Y.; Liu, Y.-H.; Yu, X.-Q. Dual-site lysosome-targeted fluorescent probe for separate detection of endogenous biothiols and SO2 in living cells. J. Mater. Chem. B 2018, 6, 4232–4238. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Xu, Q.; Wang, J.; Gao, P.; Peng, X. Imaging of lysosomal pH changes with a fluorescent sensor containing a novel lysosome-locating group. Chem. Commun. 2012, 48, 11766–11768. [Google Scholar] [CrossRef]
- Surendran, K.; Vitiello, S.P.; Pearce, D.A. Lysosome dysfunction in the pathogenesis of kidney diseases. Pediatr. Nephrol. 2014, 29, 2253–2261. [Google Scholar] [CrossRef]
- Phan, U.T.; Arunachalam, B.; Cresswell, P. Gamma-interferon-inducible lysosomal thiol reductase (GILT) maturation, activity, and mechanism of action. J. Biol. Chem. 2000, 275, 25907–25914. [Google Scholar] [CrossRef]
- Kand, D.; Saha, T.; Lahiri, M.; Talukdar, P. Lysosome targeting fluorescence probe for imaging intracellular thiols. Org. Biomol. Chem. 2015, 13, 8163–8168. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, L.; Liu, W.; Liu, W. Coumarinocoumarin-based two-photon fluorescent cysteine biosensor for targeting lysosome. Anal. Chem. 2018, 90, 6138–6143. [Google Scholar] [CrossRef] [PubMed]
- Balce, D.R.; Allan, E.R.O.; Mckenna, N.; Yates, R.M. Gamma-interferon-inducible lysosomal thiol reductase (GILT) maintains phagosomal proteolysis in alternatively activated macrophages. J. Biol. Chem. 2014, 289, 31891–31904. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Yue, P.; Meng, X.; Salamanca, J.C.; Escobedo, J.O.; Strongin, R.M.; Yin, C. In situ lysosomal cysteine-specific targeting and imaging during dexamethasone induced apoptosis. Anal. Chem. 2018, 90, 7018–7024. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Han, Z.; Kang, Y.; Peng, X. A two-photon fluorescent probe for lysosomal thiols in live cells and tissues. Sci. Rep. 2016, 6, 19562. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, B.-B.; Si-Tu, X.-M.; Wang, F.-F.; He, H.; Fan, X.-Y.; Jiang, F.-L.; Liu, Y. A lysosome-targeted fluorescent sensor for the detection of glutathione in cells with an extremely fast response. Chem. Commun. 2016, 52, 11579–11582. [Google Scholar]
- Wang, K.; Leng, T.; Liu, Y.; Wang, C.; Shi, P.; Shen, Y.; Zhu, W.-H. A novel near-infrared fluorescent probe with a large stokes shift for the detection and imaging of biothiols. Sens. Actuators B 2017, 248, 338–345. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Y.; Ren, M.; Lin, W. Single near-infrared fluorescent probe with high- and low-sensitivity sites for sensing different concentration ranges of biological thiols with distinct modes of fluorescence signals. Chem. Sci. 2016, 7, 1896–19023. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, T.; Miao, J.-Y.; Zhao, B.-X. A ratiometric fluorescent probe with DNBS group for biothiols in aqueous solution. Sens. Actuators B 2016, 223, 274–279. [Google Scholar] [CrossRef]
- Liu, K.; Shang, H.; Kong, X.; Lin, W. A novel near-infrared fluorescent probe with a large Stokes shift for biothiol detection and application in in vitro and in vivo fluorescence imaging. J. Mater. Chem. B 2017, 5, 3836–3841. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Liu, J.; Zhang, J.; Yang, T.; Guo, W. Fluorescent probe for biological gas SO2 derivatives bisulfite and sulphite. Chem. Commun. 2013, 49, 2637–2639. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Li, K.; Li, C.-Y.; Hou, J.-T.; Yu, X.-Q. A water-soluble near-infrared probe for colorimetric and ratiometric sensing of SO2 derivatives in living cells. Chem. Commun. 2014, 50, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Chen, S.; Song, X.; Kang, J. A real-time colorimetric and ratiometric fluorescent probe for rapid detection of SO2 derivatives in living cells based on a near-infrared benzopyrylium dye. RSC Adv. 2015, 5, 25409–25415. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Wu, J.; Wang, Y.; Liu, Y.-H.; Yu, X.-Q. A Novel Colorimetric Fluorescent Probe for SO2 and Its Application in Living Cells Imaging. Molecules 2018, 23, 871. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.; Sun, H.; Wang, S.; Kong, F. Construction of a novel near infrared fluorescent probe with multiple fluorescence emission and its application for SO2 derivative detection in cells and living zebrafish. J. Mat. Chem. B 2018, 6, 7060–7065. [Google Scholar] [CrossRef]
- Shang, H.; Liu, K.; Lin, W. Construction of a novel ratiometric near-infrared fluorescent probe for SO2 derivatives and its application for biological imaging. Anal. Methods 2017, 9, 3790–3794. [Google Scholar]
- Zhang, X.; Wang, B.; Wang, C.; Chen, L.; Xiao, Y. Monitoring lipid peroxidation within foam cells by lysosome-targetable and ratiometric probe. Anal. Chem. 2015, 87, 8292–8300. [Google Scholar] [CrossRef]
- He, D.-D.; Liu, W.; Sun, R.; Fan, C.; Xu, Y.-J.; Ge, J.-F. N-Pyridineium-2-yl Darrow Red analogue: Unique near-infrared lysosome-biomarker for the detection of cancer cells. Anal. Chem. 2015, 87, 1499–1502. [Google Scholar] [CrossRef]
- Dou, Y.; Gu, X.; Ying, S.; Zhu, S.; Yu, S.; Sheng, W.; Zhu, Q. A novel lysosome-targeted fluorogenic probe based on 5-triazole-quinoline for the rapid detection of hydrogen sulfide in living cells. Org. Biomol. Chem. 2018, 16, 712–716. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Chen, H. Analogs of Changsha near-infrared dyes with large Stokes Shifts for bioimaging. Biomaterials 2013, 34, 9566–9571. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, L.; Zhou, X.-L.; Wu, M.-Y. Lysosome-Targeted Single Fluorescence Probe for Two-Channel Imaging Intracellular SO2 and Biothiols. Molecules 2019, 24, 618. https://doi.org/10.3390/molecules24030618
Wang Y, Liu L, Zhou X-L, Wu M-Y. Lysosome-Targeted Single Fluorescence Probe for Two-Channel Imaging Intracellular SO2 and Biothiols. Molecules. 2019; 24(3):618. https://doi.org/10.3390/molecules24030618
Chicago/Turabian StyleWang, Yue, Li Liu, Xian-Li Zhou, and Ming-Yu Wu. 2019. "Lysosome-Targeted Single Fluorescence Probe for Two-Channel Imaging Intracellular SO2 and Biothiols" Molecules 24, no. 3: 618. https://doi.org/10.3390/molecules24030618
APA StyleWang, Y., Liu, L., Zhou, X.-L., & Wu, M.-Y. (2019). Lysosome-Targeted Single Fluorescence Probe for Two-Channel Imaging Intracellular SO2 and Biothiols. Molecules, 24(3), 618. https://doi.org/10.3390/molecules24030618





