Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol
Abstract
1. Introduction
2. Results
2.1. Characteristics of Transfersomes
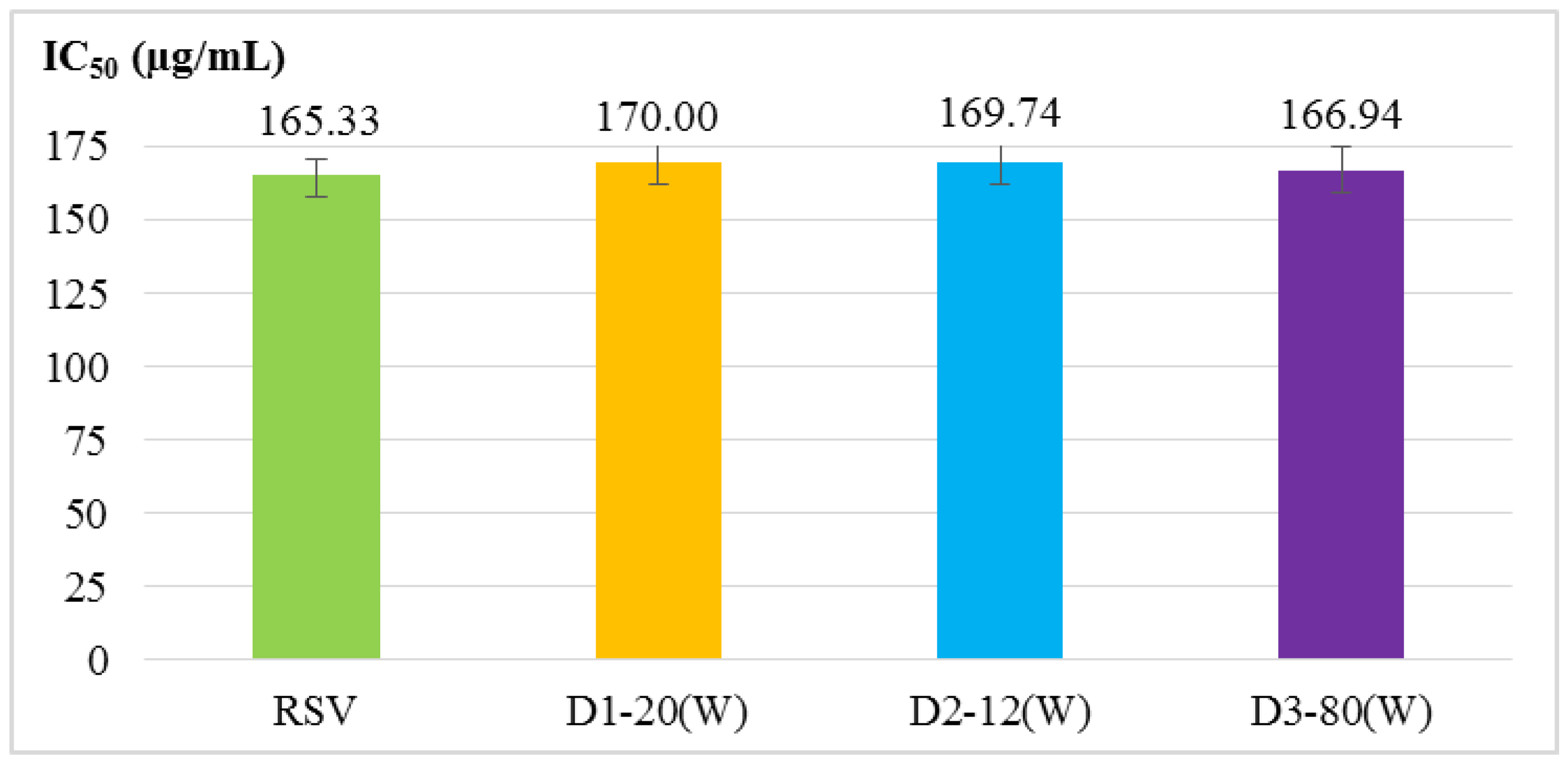
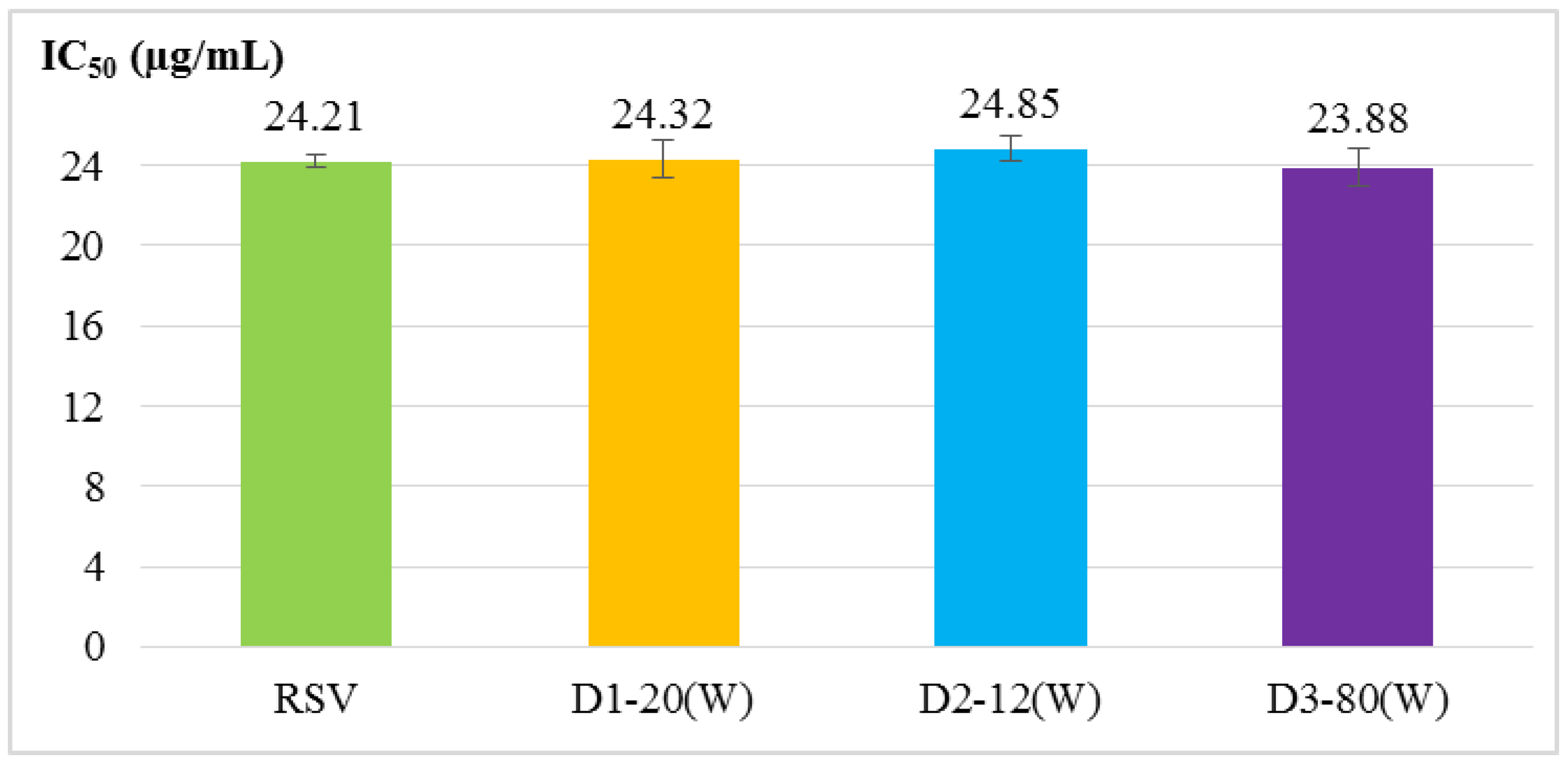
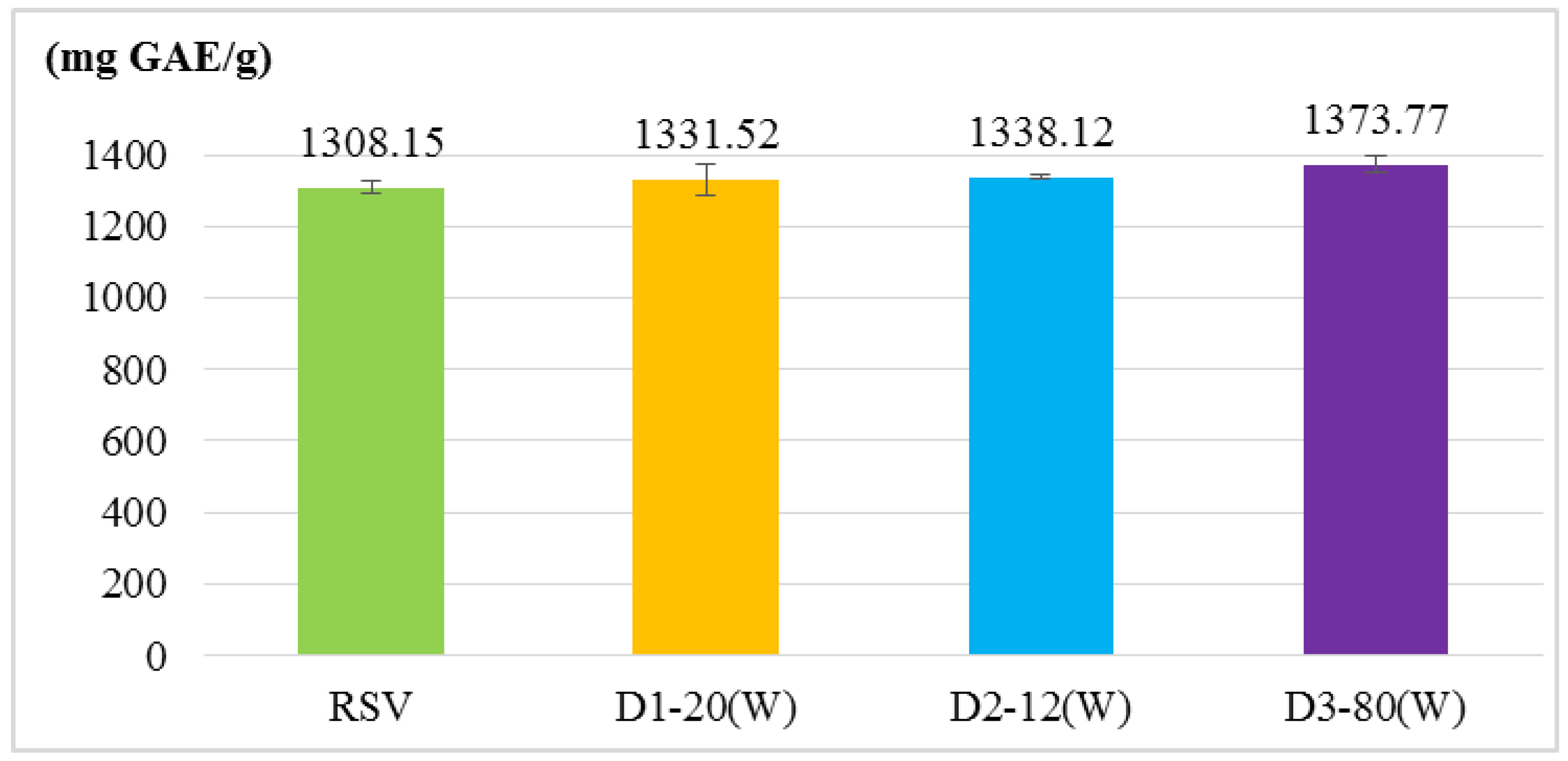
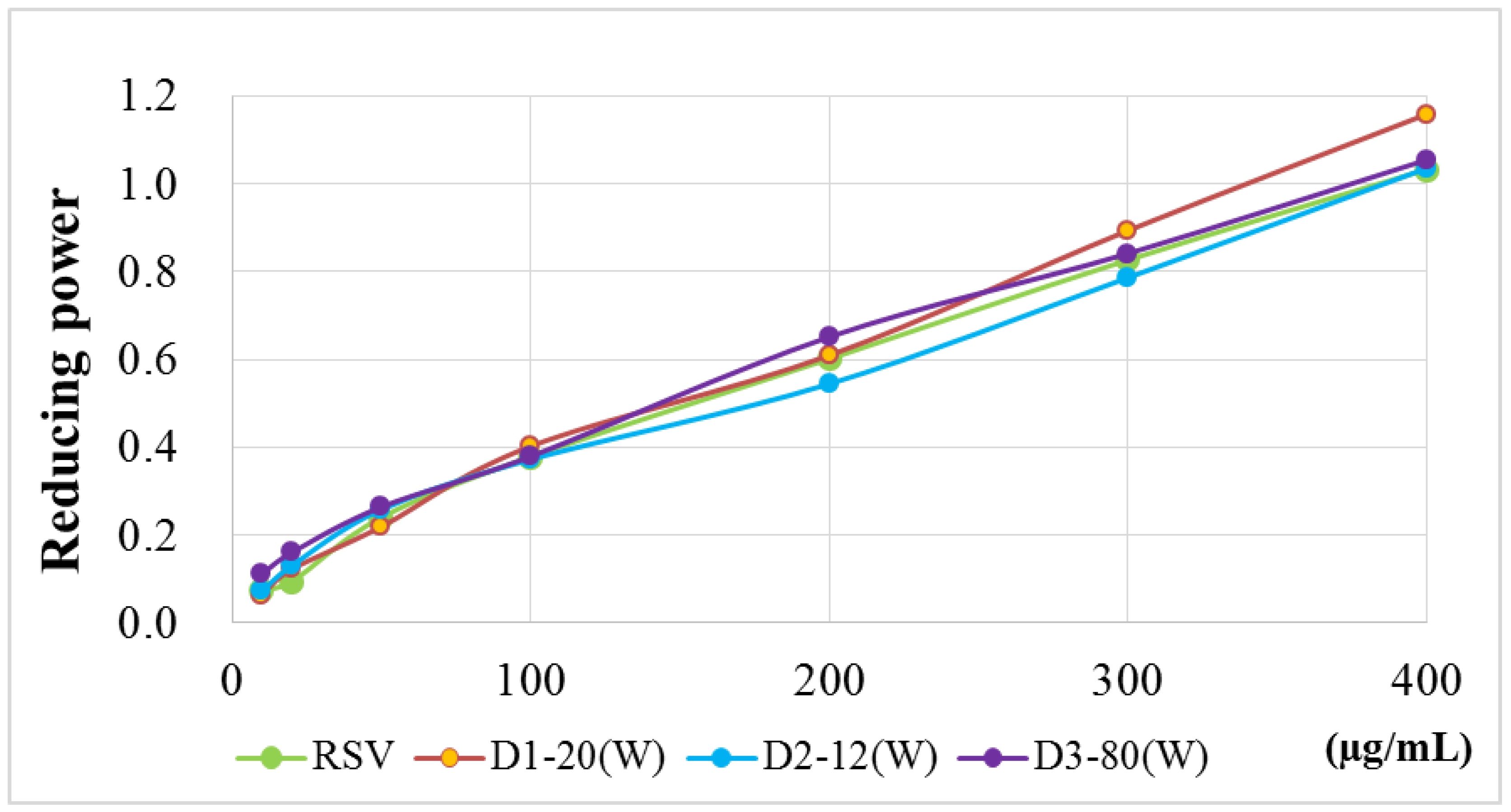
2.2. Antioxidant Activity Assays
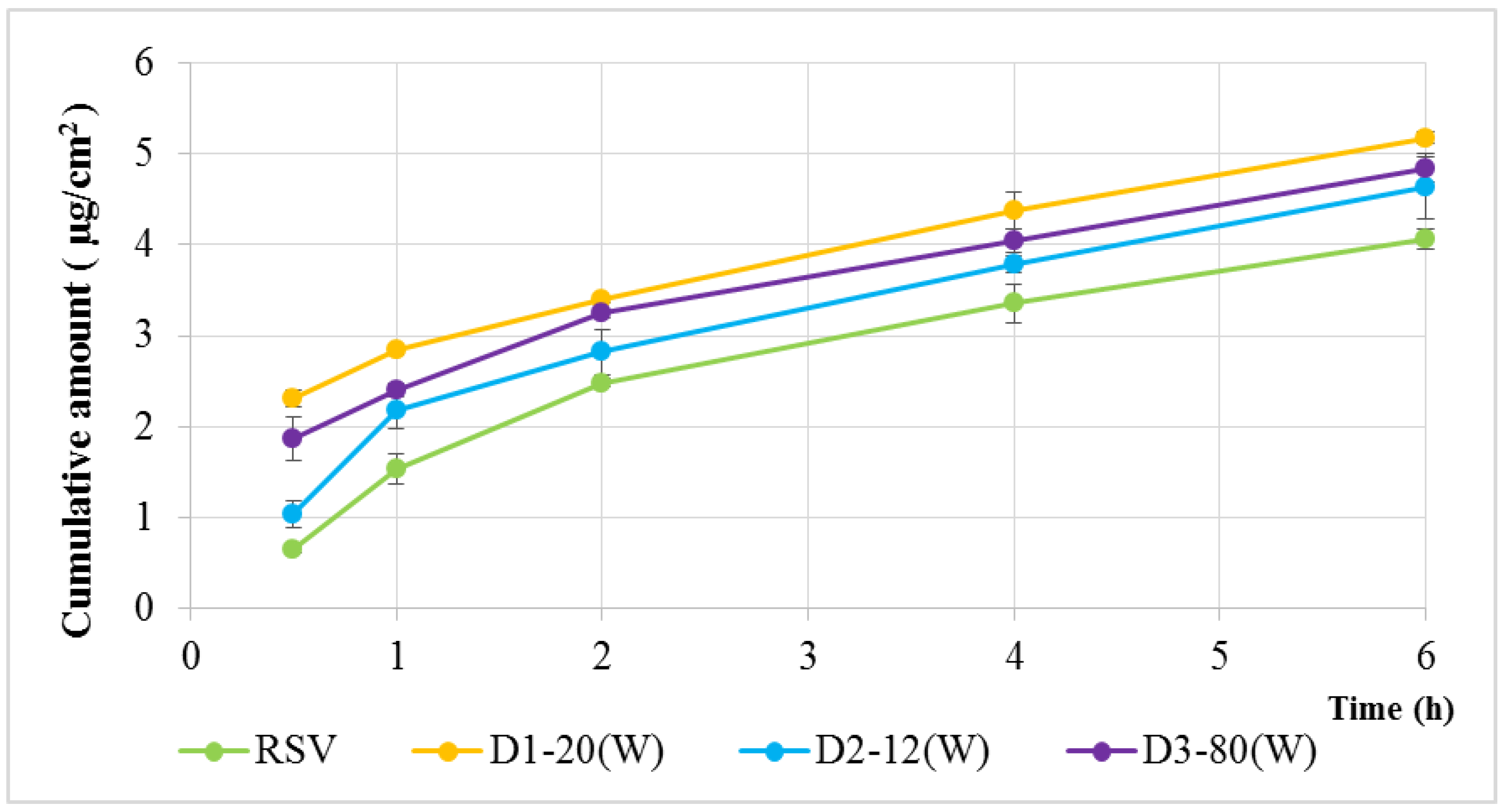
2.3. In Vitro Transdermal Delivery Analysis
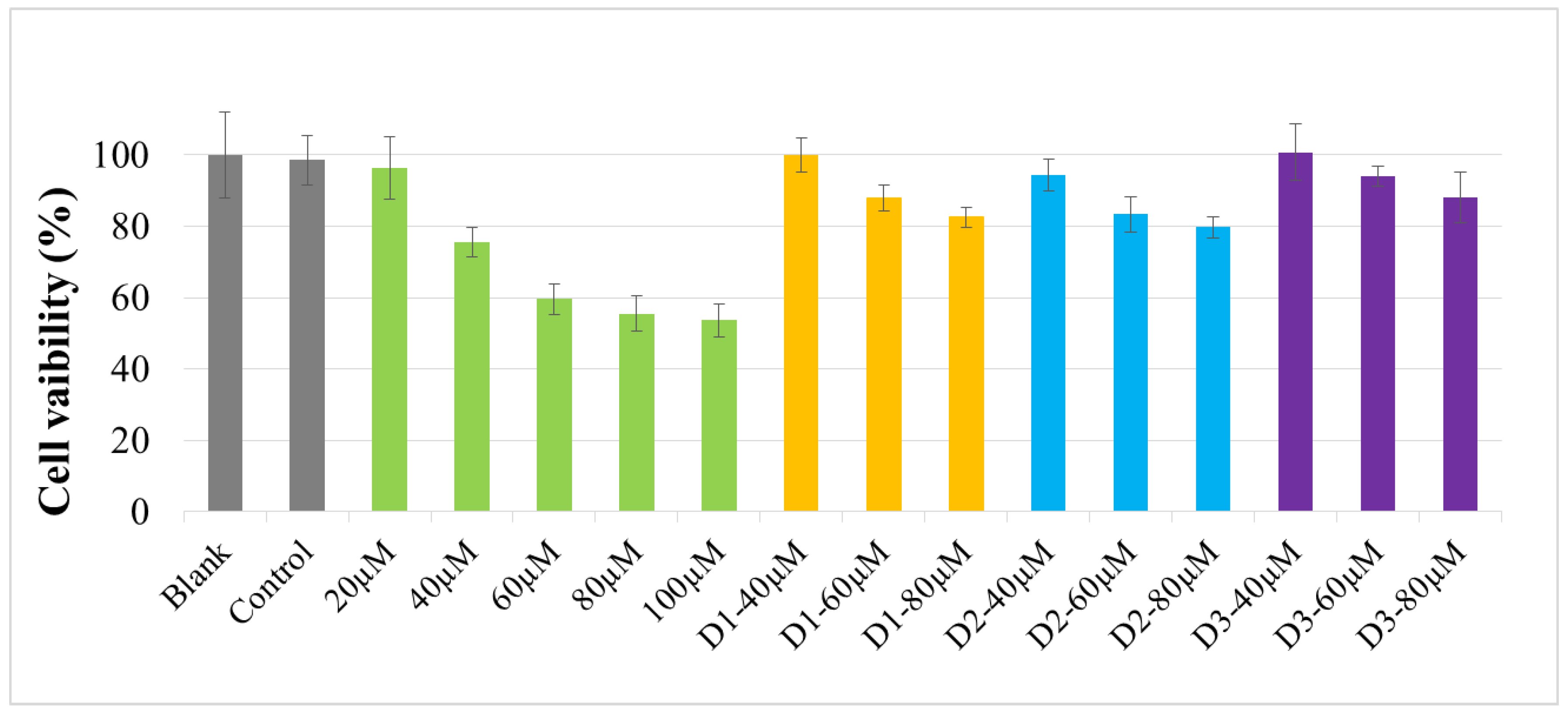
2.4. Cell Viability Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Transfersome
4.3. Particle Sizes and Zeta Potential of Transfersome
4.4. Entrapment Efficiency of Resveratrol
4.5. Stability of Transfersome
4.6. Antioxidant Activity Evaluation
4.6.1. DPPH Radical Scavenging Activity Assay
4.6.2. ABTS+ Radical Cation Scavenging Activity Assay
4.6.3. Total Phenolic Content
4.6.4. Reducing Power
4.7. In Vitro Transdermal Delivery Analysis
4.8. Cell Culture and Cell Viability Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- R Neves, A.; Lucio, M.; LC Lima, J.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.; Gomez-Cabrera, M.; Vina, J. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Švajger, U.; Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Bennett, L.L. Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer mcf7 cells through ros, cell cycle inhibition, caspase 3 and parp cleavage. Biomed. Pharmacother. 2016, 84, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta (BBA)-Mol. Basis Disease 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Cerletti, C.; Guglielmini, G.; Pignatelli, P.; de Gaetano, G.; Violi, F. Effects of resveratrol and other wine polyphenols on vascular function: An update. J. Nutr. Biochem. 2011, 22, 201–211. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of nf-κb activation by resveratrol in lps treated human intestinal cells results in downregulation of pge2 production and cox-2 expression. Toxicol. Vitro 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-formulation studies of resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by ph and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Meybeck, A. Past, present and future of liposome cosmetics. In Liposome Dermatics; Springer: Heidelberg/Berlin, Germany, 1992; pp. 341–345. [Google Scholar]
- Jia, H.J.; Jia, F.Y.; Zhu, B.J.; Zhang, W.P. Preparation and characterization of glycyrrhetinic-acid loaded peg-modified liposome based on peg-7 glyceryl cocoate. Eur. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Pirvu, C.D.; Hlevca, C.; Ortan, A.; Prisada, R. Elastic vesicles as drugs carriers through the skin. Farmacia 2010, 58, 128–135. [Google Scholar]
- Zhang, Y.; Shen, L.; Zhang, K.; Guo, T.; Zhao, J.; Li, N.; Feng, N. Enhanced antioxidation via encapsulation of isooctyl p-methoxycinnamate with sodium deoxycholate-mediated liposome endocytosis. Int. J.Pharm. 2015, 496, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a tool for transdermal and dermal delivery. Drug Discov. Today Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.; Williams, A.C.; Barry, B. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int. J. Pharm. 2004, 276, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, I.; De Stefano, D.; Campani, V.; Mayol, L.; Carnuccio, R.; Fabbrocini, G.; Ayala, F.; La Rotonda, M.I.; De Rosa, G. Nanocarriers for topical administration of resveratrol: A comparative study. Int. J. Pharm. 2013, 440, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.; Kohli, K.; Kumar, V. Nano-transfersomes as a novel carrier for transdermal delivery. Int. J. Pharm. 2013, 454, 367–380. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Preparation of liposomes using supercritical carbon dioxide technology: Effects of phospholipids and sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Banu, R.; Gerding, J.; Franklin, C.; Sikazwe, D.; Horton, W.; Török, M.; Davis, J.; Cheng, K.; Nakazwe, M.; Mochona, B. 4,5-dimethoxy-2-nitrobenzohydrazides and 1-(1-benzylpiperidin-4-yl)ethan-1-ones as potential antioxidant/cholinergic endowed small molecule leads. Sci. Pharm. 2018, 86, 2. [Google Scholar] [CrossRef]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity in vitro of quercetin glycoside obtained in beauveria bassiana culture and its interaction with liposome membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Novel, M.; Ayu, S.; Berlian, G.; Tandrasasmita, O.; Tjandrawinata, R.; Anggadiredja, K. The in vitro–in vivo safety confirmation of peg-40 hydrogenated castor oil as a surfactant for oral nanoemulsion formulation. Sci. Pharm 2017, 85, 18. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Sharma, V.; Pathak, K. Nanovesicles for transdermal delivery of felodipine: Development, characterization, and pharmacokinetics. Int. J. Pharm. Investig. 2014, 4. [Google Scholar] [CrossRef]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Physical and chemical stability of anthocyanin-rich black carrot extract-loaded liposomes during storage. Food Res. Int. 2018, 108, 491–497. [Google Scholar] [CrossRef] [PubMed]
- de Kanter, M.; Meyer-Kirschner, J.; Viell, J.; Mitsos, A.; Kather, M.; Pich, A.; Janzen, C. Enabling the measurement of particle sizes in stirred colloidal suspensions by embedding dynamic light scattering into an automated probe head. Measurement 2016, 80, 92–98. [Google Scholar] [CrossRef]
- Sanna, V.; Roggio, A.M.; Pala, N.; Marceddu, S.; Lubinu, G.; Mariani, A.; Sechi, M. Effect of chitosan concentration on plga microcapsules for controlled release and stability of resveratrol. Int.J. Biol. Macromol. 2015, 72, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT-Food Sci. Technol. 2017, 85, 37–44. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| - | Particle Size (nm) | PDI | EE (%) | |||
|---|---|---|---|---|---|---|
| - | 0 Days | 14 Days | 0 Days | 14 Days | 0 Days | 14 Days |
| A1–20(B) | 48.34 ± 0.19 | 49.27 ± 0.47 | 0.145 ± 0.007 | 0.166 ± 0.005 | 39.76 ± 2.70 | 25.19 ± 1.26 |
| A2–12(B) | 56.35 ± 0.31 | 58.53 ± 0.66 | 0.128 ± 0.016 | 0.119 ± 0.018 | 33.31 ± 0.87 | 24.85 ± 2.27 |
| A3–80(B) | 64.53 ± 0.41 | 66.32 ± 0.30 | 0.042 ± 0.011 | 0.041 ± 0.003 | 48.27 ± 2.29 | 30.30 ± 0.43 |
| B1–20(B) | 72.03 ± 0.27 | 73.37 ± 0.16 | 0.072 ± 0.017 | 0.074 ± 0.012 | 62.23 ± 3.65 | 52.01 ± 0.45 |
| B2–12(B) | 55.46 ± 0.55 | 62.43 ± 1.04 | 0.222 ± 0.013 | 0.197 ± 0.038 | 62.21 ± 1.56 | 56.60 ± 0.51 |
| B3–80(B) | 58.48 ± 0.19 | 57.90 ± 0.11 | 0.304 ± 0.010 | 0.270 ± 0.016 | 67.96 ± 1.85 | 60.17 ± 0.81 |
| C1–20(B) | 49.85 ± 0.14 | 50.02 ± 0.35 | 0.127 ± 0.013 | 0.111 ± 0.014 | 43.50 ± 0.96 | 26.72 ± 1.99 |
| C2–12(B) | 73.72 ± 15.54 | 60.56 ± 2.66 | 0.233 ± 0.085 | 0.268 ± 0.006 | 42.06 ± 2.00 | 24.70 ± 1.21 |
| C3–80(B) | 68.85 ± 1.77 | 67.10 ± 0.27 | 0.424 ± 0.044 | 0.373 ± 0.003 | 44.00 ± 0.56 | 31.70 ± 1.88 |
| D1–20(W) | 64.28 ± 0.60 | 66.43 ± 0.21 | 0.206 ± 0.008 | 0.177 ± 0.004 | 59.93 ± 0.99 | 50.60 ± 1.33 |
| D2–12(W) | 43.07 ± 0.21 | 61.67 ± 1.93 | 0.312 ± 0.039 | 0.557 ± 0.024 | 56.13 ± 1.52 | 47.82 ± 0.54 |
| D3–80(W) | 40.13 ± 0.63 | 45.67 ± 0.45 | 0.266 ± 0.009 | 0.201 ± 0.009 | 59.01 ± 1.02 | 51.17 ± 1.72 |
| E1–20(W) | 81.39 ± 0.41 | 67.56 ± 0.21 | 0.146 ± 0.009 | 0.097 ± 0.008 | 61.69 ± 0.29 | 31.76 ± 0.74 |
| E2–20(W) | 75.88 ± 0.59 | 73.03 ± 0.74 | 0.119 ± 0.008 | 0.095 ± 0.022 | 56.71 ± 0.86 | 24.66 ± 2.29 |
| - | Zeta (mV) | Zeta (mV) | |
|---|---|---|---|
| B1-20(B) | −1.93 ± 0.24 | D1-20(W) | −19.53 ± 0.91 |
| B2-12(B) | −6.29 ± 0.47 | D2-12(W) | −59.90 ± 0.20 |
| B3-80(B) | −0.54 ± 0.08 | D3-80(W) | −23.93 ± 0.31 |
| Composition(%W/V) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | RSV | Emulmetik 900 | Tween-20 | 1200UP | Tween-80 | Ethanol | PBS Buffer | Distilled Water |
| A1-20(B) | 0.2 | 3.33 | 1.67 | 5.00 | q.s. to 100 | |||
| A2-12(B) | 0.2 | 3.33 | 1.67 | 5.00 | q.s. to 100 | |||
| A3-80(B) | 0.2 | 3.33 | - | - | 1.67 | 5.00 | q.s. to 100 | |
| B1-20(B) | 0.2 | 3.75 | 1.25 | 5.00 | q.s. to 100 | - | ||
| B2-12(B) | 0.2 | 3.75 | 1.25 | 5.00 | q.s. to 100 | |||
| B3-80(B) | 0.2 | 3.75 | - | - | 1.25 | 5.00 | q.s. to 100 | |
| C1-20(B) | 0.2 | 4.00 | 1.00 | 5.00 | q.s. to 100 | - | ||
| C2-12(B) | 0.2 | 4.00 | 1.00 | 5.00 | q.s. to 100 | |||
| C3-80(B) | 0.2 | 4.00 | - | - | 1.00 | 5.00 | q.s. to 100 | - |
| D1-20(W) | 0.2 | 3.75 | 1.25 | 5.00 | q.s. to 100 | |||
| D2-12(W) | 0.2 | 3.75 | 1.25 | 5.00 | q.s. to 100 | |||
| D3-80(W) | 0.2 | 3.75 | - | - | 1.25 | 5.00 | - | q.s. to 100 |
| E1-20(W) | 0.2 | 3.75 | 1.25 | 10.00 | - | q.s. to 100 | ||
| E2-20(W) | 0.2 | 3.75 | 1.25 | - | - | 20.00 | - | q.s. to 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-S.; Li, Y.-S.; Kuo, Y.-C.; Tsai, S.-J.J.; Lin, C.-C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600. https://doi.org/10.3390/molecules24030600
Wu P-S, Li Y-S, Kuo Y-C, Tsai S-JJ, Lin C-C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules. 2019; 24(3):600. https://doi.org/10.3390/molecules24030600
Chicago/Turabian StyleWu, Pey-Shiuan, Yu-Syuan Li, Yi-Ching Kuo, Suh-Jen Jane Tsai, and Chih-Chien Lin. 2019. "Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol" Molecules 24, no. 3: 600. https://doi.org/10.3390/molecules24030600
APA StyleWu, P.-S., Li, Y.-S., Kuo, Y.-C., Tsai, S.-J. J., & Lin, C.-C. (2019). Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules, 24(3), 600. https://doi.org/10.3390/molecules24030600







