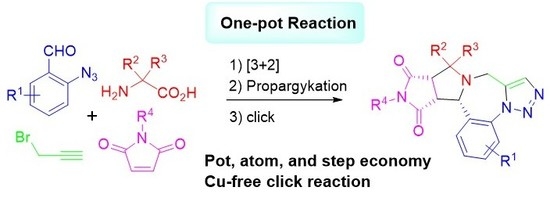
One-Pot Synthesis of Triazolobenzodiazepines Through Decarboxylative [3 + 2] Cycloaddition of Nonstabilized Azomethine Ylides and Cu-Free Click Reactions
Abstract
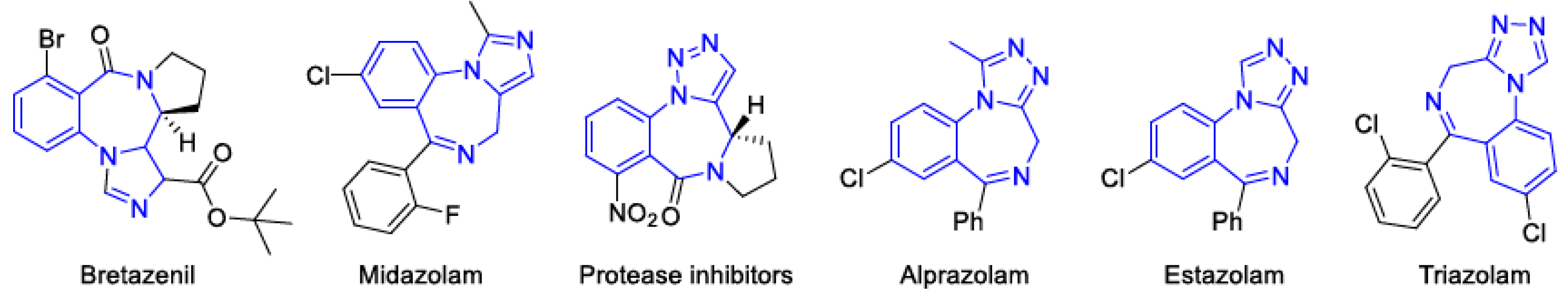
1. Introduction
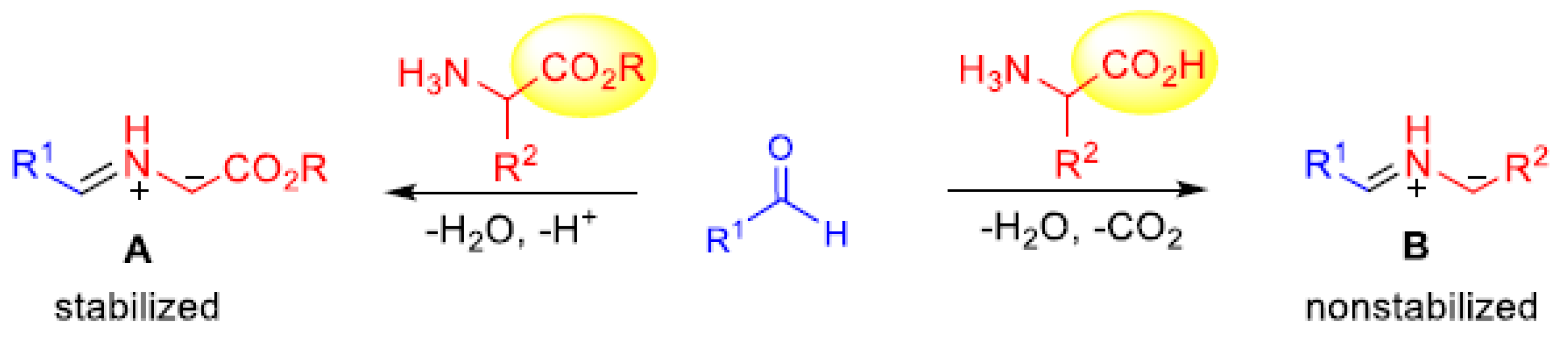
2. Results and Discussions
3. Summary
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References and Notes
- Tashma, Z.; Raveh, L.; Liani, H.; Alkalay, D.; Givoni, S.; Kapon, J.; Cohen, G.; Alcalay, M.; Grauer, E. Bretazenil, a benzodiazepine receptor partial agonist, as an adjunct in the prophylactic treatment of OP poisoning. J. Appl. Toxicol. 2001, 21, S115–S119. [Google Scholar] [CrossRef] [PubMed]
- Djabri, A.; Guy, R.H.; Begona Delgado-Charro, M. Potential of iontophoresis as a drug delivery method for midazolam in pediatrics. Eur. J. Pharm. Sci. 2019, 128, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.K.; Maity, P.K.; Shabab, M.; Khan, M.I. Click chemistry based rapid one-pot synthesis and evaluation for protease inhibition of new tetracyclic triazole fused benzodiazepine derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5241–5245. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ding, M.; Wu, Y.; Li, Y.; Hua, W.; Zhang, F. Electrochemical synthesis of 1,2,4-triazole-fused heterocycles. Green Chem. 2018, 20, 1732–1737. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Sajjad, G.; Ardeshiri, L.H.; Nahad, A. New and mild method for the synthesis of alprazolam and diazepam and computational study of their binding mode to GABAA receptor. Med. Chem. Res. 2016, 25, 1538–1550. [Google Scholar] [CrossRef]
- Santos, F.; Javier, G.; Carlos, P. 1,4-Benzodiazepine N-Nitrosoamidines: Useful Intermediates in the Synthesis of Tricyclic Benzodiazepines. Molecules 2006, 11, 583–588. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Donald, J.R.; Martin, S.F. Synthesis and Diversification of 1,2,3-Triazole-Fused 1,4-Benzodiazepine Scaffolds. Org. Lett. 2011, 13, 852–855. [Google Scholar] [CrossRef]
- Donald, J.R.; Wood, R.R.; Martin, S.F. Application of a Sequential Multicomponent Assembly Process/Huisgen Cycloaddition Strategy to the Preparation of Libraries of 1,2,3-Triazole-Fused 1,4-Benzodiazepines. ACS Comb. Sci. 2012, 14, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Gracias, V.; Darczak, D.; Gasiecki, A.F.; Djuric, S.W. Synthesis of fused triazolo-imidazole derivatives by sequential van Leusen/alkyne–azide cycloaddition reactions. Tetrahedron Lett. 2005, 46, 9053–9056. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Palazzo, T.A.; Kurth, M.J. Facile One-Pot Assembly of Imidazotriazolobenzodiazepines via Indium(III)-Catalyzed Multicomponent Reactions. Org. Lett. 2013, 15, 4492–4495. [Google Scholar] [CrossRef] [PubMed]
- Kosychova, L.; Karalius, A.; Staniulyte, Z.; Sirutkaitis, R.A.; Palaima, A.; Laurynenas, A.; Anusevicius, Z. New 1-(3-nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines: synthesis and computational study. Molecules 2015, 20, 5392–5408. [Google Scholar] [CrossRef] [PubMed]
- Vroemans, R.; Bamba, F.; Winters, J.; Thomas, J.; Jacobs, J.; Meervelt, L.V.; John, J.; Dehaen, W. Sequential Ugi reaction/base-induced ring closing/IAAC protocol toward triazolobenzodiazepine-fused diketopiperazines and hydantoins. Beilstein J. Org. Chem. 2018, 14, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Pearson, W.H. (Eds.) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Kantorowski, E.J.; Kurth, M.J. Dipolar cycloadditions in solid-phase organic synthesis (SPOS). Mol. Divers. 1997, 2, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Nájera, C.; Sansano, J.M. Azomethine Ylides in Organic Synthesis. Curr. Org. Chem. 2003, 7, 1105–1150. [Google Scholar] [CrossRef]
- Coldham, I.; Hufton, R. Intramolecular Dipolar Cycloaddition Reactions of Azomethine Ylides. Chem. Rev. 2005, 105, 2765–2810. [Google Scholar] [CrossRef]
- Rück-Braun, K.; Freysoldt, T.H.E.; Wierschem, F. 1,3-Dipolar cycloaddition on solid supports: nitrone approach towards isoxazolidines and isoxazolines and subsequent transformations. Chem. Soc. Rev. 2005, 34, 507–516. [Google Scholar] [CrossRef]
- Harju, K.; Yli-Kauhaluoma, J. Recent advances in 1,3-dipolar cycloaddition reactions on solid supports. Mol. Divers 2005, 9, 187–207. [Google Scholar] [CrossRef]
- Oderaotoshi, Y.; Cheng, W.; Fujitomi, S.; Kasano, Y.; Minakata, S.; Komatsu, M. exo- and Enantioselective Cycloaddition of Azomethine Ylides Generated from N-Alkylidene Glycine Esters Using Chiral Phosphine−Copper Complexes. Org. Lett. 2003, 5, 5043–5046. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Banerjee, P.; Gadre, S.R. Construction of Enantiopure Pyrrolidine Ring System via Asymmetric [3+2]-Cycloaddition of Azomethine Ylides. Chem. Rev. 2006, 106, 4484–4517. [Google Scholar] [CrossRef] [PubMed]
- Potowski, M.; Bauer, J.O.; Strohmann, C.; Antonchick, A.P.; Waldmann, H. Highly Enantioselective Catalytic [6+3] Cycloadditions of Azomethine Ylides. Angew. Chem. Int. Ed. 2012, 51, 9512–9516. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.; Carretero, J.C. Recent advances in the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides. Chem. Commun. 2014, 50, 12434–12446. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Potowski, M.; Jia, Z.J.; Antonchick, A.P.; Waldmann, H. Catalytic Enantioselective 1,3-Dipolar Cycloadditions of Azomethine Ylides for Biology-Oriented Synthesis. Acc. Chem. Res. 2014, 47, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Maruoka, K. Recent Advances of Catalytic Asymmetric 1,3-Dipolar Cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Progress in 1,3-dipolar cycloadditions in the recent decade: an update to strategic development towards the arsenal of organic synthesis. Tetrahedron 2016, 72, 1603–1644. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, H.Y.; Jin, Q.W.; Zheng, C.W.; Zhao, G.; Shang, Y.J. Thiourea–Quaternary Ammonium Salt Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Imino Esters to Construct Spiro[pyrrolidin-3,3′-oxindoles]. Org. Lett. 2016, 18, 4774–4777. [Google Scholar] [CrossRef]
- Esteban, F.; Cieslik, W.; Arpa, E.M.; Guerrero-Corella, A.; Diaz-Tendero, S.; Perles, J.; Fernandez-Salas, J.A.; Fraile, A.; Aleman, J. Intramolecular Hydrogen Bond Activation: Thiourea-Organocatalyzed Enantioselective 1, 3-Dipolar Cycloaddition of Salicylaldehyde-Derived Azomethine Ylides with Nitroalkenes. Acs Catal. 2018, 8, 1884–1890. [Google Scholar]
- Zhang, W.; Lu, Y.; Chen, C.H.-T.; Zeng, L.; Kassel, D.B. Fluorous Mixture Synthesis of Two Libraries with Hydantoin-, and Benzodiazepinedione-Fused Heterocyclic Scaffolds. J. Comb. Chem. 2006, 8, 687–695. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhi, S.J.; Wang, W.; Liu, S.; Jasinski, J.P.; Zhang, W. A pot-economical and diastereoselective synthesis involving catalyst-free click reaction for fused-triazolobenzodiazepines. Green Chem. 2016, 18, 2642–2646. [Google Scholar] [CrossRef]
- Zhang, X.F.; Legris, M.; Muthengi, A.; Zhang, W. [3 + 2] Cycloaddition-based one-pot synthesis of 3,9-diazabicyclo[4.2.1]nonane-containing scaffold. Chem. Heterocycl. Com. 2017, 53, 468–473. [Google Scholar] [CrossRef]
- Muthengi, A.; Zhang, X.F.; Dhawan, G.; Zhang, W.S.; Corsinia, F.; Zhang, W. Sequential (3 + 2) cycloaddition and (5 + n) annulation for modular synthesis of dihydrobenzoxazines, tetrahydrobenzoxazepines and tetrahydrobenzoxazocines. Green Chem. 2018, 20, 3134–3139. [Google Scholar] [CrossRef]
- Zhang, X.F.; Qiu, W.Q.; Ma, X.M.; Evans, J.; Kaur, M.; Jasinski, J.P.; Zhang, W. One-Pot Double [3 + 2] Cycloadditions for Diastereoselective Synthesis of Pyrrolidine-Based Polycyclic Systems. J. Org. Chem. 2018, 83, 13536–13542. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, O.; Kanemasa, S.; Ohe, M.; Takenaka, S. Simple Generation of Nonstabilized Azomethine Ylides through Decarboxylative Condensation of α-Amino acids with Carbonyl Compounds via 5- Oxazolidinone Intermediates. Bull. Chem. Soc. Jpn. 1987, 60, 4079–4089. [Google Scholar] [CrossRef]
- Grigg, R.; Idle, J.; McMeekin, P.; Vipond, D. The Decarboxylative Route to Azomethine Ylides. Mechanism of 1,3-Dipoie Formation. J. Chem. Soc. Chem. Commun. 1987, 2, 49–51. [Google Scholar] [CrossRef]
- Aly, M.F.; Grigg, R.; Thianpatanagul, S.; Sridharan, V. X=Y–ZH systems as potential 1,3-dipoles. Part 10. The decarboxylative route to azomethine ylides. Background and relevance to pyridoxal decarboxylases. J. Chem. Soc. Perkin Trans. I 1988, 4, 949–955. [Google Scholar] [CrossRef]
- Kanemasa, S. The Chemistry of Azomethine Ylides Developed in the Institute. Rep. Inst. Adv. Mater. Study Kynshu Univ. 1988, 2, 149–177. [Google Scholar] [CrossRef]
- Kanemasa, S.; Sakamoto, K.; Tsuge, O. Nonstabilized Azomethine Ylides Generated by Decarboxylative Condensation of α-Amino acids. Structural Variation, Reactivity and Stereoselectivity. Bull. Chem. Soc. Jpn. 1989, 62, 1960–1968. [Google Scholar] [CrossRef]
- Rehn, S.; Bergman, J.; Stensland, B. The Three-Component Reaction between Isatin, α-Amino Acids, and Dipolarophiles. Eur. J. Org. Chem. 2004, 413–418. [Google Scholar] [CrossRef]
- Kudryavtsev, K.V. Three-component synthesis of tricyclic lactams containing the pyrrolizidin-3-one moiety. Russ. Chem. Bull. 2008, 57, 2364–2372. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, M.; Zhang, W.S.; Legris, M.; Zhang, W. Synthesis of trifluoromethylated pyrrolidines via decarboxylative [3+2] cycloaddition of non-stabilized N-unsubstituted azomethine ylides. J. Fluorine Chem. 2017, 204, 18–22. [Google Scholar] [CrossRef]
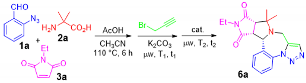
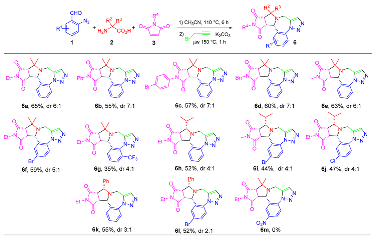
- General procedure for the one-pot synthesis: A solution of 2-azidebenzaldehyde 1 (1.0 mmol), amino acid 2 (1.2 mmol) and maleimide 3 (1.0 mmol) in 3.0 mL of CH3CN was heated at 110 °C for 6 h in a sealed tube. Upon the completion of the reaction as monitored by LC-MS, propargyl bromide solution (80% in toluene, 5.0 mmol) and K2CO3 (2.5 mmol) were added to the reaction mixture and then heated under microwaves at 150 °C for 1 h. The concentrated reaction mixture was loaded onto a semi-preparative HPLC with a C18 column to afford purified major diastereomer of product 6.
- Guggenheim, K.G.; Toru, H.; Kurth, M.J. One-Pot, Two-Step Cascade Synthesis of Quinazolinotriazolobenzodiazepines. Org. Lett. 2012, 14, 3732–3735. [Google Scholar] [CrossRef] [PubMed]
- De Moliner, F.; Bigatti, M.; Banfi, L.; Riva, R.; Basso, A. OPHA (Oxidation−Passerini−Hydrolysis−Alkylation) Strategy: A Four- Step, One-Pot Improvement of the Alkylative Passerini Reaction. Org. Lett. 2014, 16, 2280–2283. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


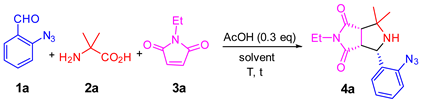
| ntry | Solvent | T (°C) | t (h) | 4a (%) b | dr (%) c |
|---|---|---|---|---|---|
| 1 | 2-Me THF | 80 | 4 | trace | — |
| 2 | MePh | 110 | 4 | trace | — |
| 3 | EtOH | 80 | 4 | 82 | 5:1 |
| 4 | EtOH | 110 | 6 | 93 | 6:1 |
| 5 | CH3CN | 110 | 4 | 92 | 6:1 |
| 6 | CH3CN | 110 | 6 | 93 | 6:1 |
| 7 | CH3CN | 125 | 12 | 88 | 6:1 |

| Entry | Solvent | T1 (°C) | t1 (h) | 5a (%) b | Cat. | T2 (°C) | t2 (h) | 6a (%) b |
|---|---|---|---|---|---|---|---|---|
| 1 | EtOH | 80 | 2 | trace | ||||
| 2 | CH3CN | 80 | 2 | 94 | CuCl | 100 | 3 | 35 |
| 3 | CH3CN | 80 | 2 | 94 | CuBr | 100 | 3 | 60 |
| 4 | CH3CN | 80 | 2 | 94 | CuI | 100 | 3 | 89 |
| 5 | CH3CN | 80 | 2 | 94 | — | 100 | 3 | 5 |
| 6 c | CH3CN | 110 | 0.5 | 93 | — | 150 | 1 | 88 (dr 6:1) |
| Entry | T1 (°C) | t1 (h) | Cat. | T2 (°C) | t2 (h) | 6a (%) b |
|---|---|---|---|---|---|---|
| 1 | 110 | 0.5 | — | 150 | 1 | 75 |
| 2 | 150 | 0.5 | — | 150 | 1 | 51 |
| 3 | 150 | 1 | — | 150 | 1 | 76 (dr 6:1) |
| 4 | 110 | 0.5 | CuI | 110 | 1 | 70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. One-Pot Synthesis of Triazolobenzodiazepines Through Decarboxylative [3 + 2] Cycloaddition of Nonstabilized Azomethine Ylides and Cu-Free Click Reactions. Molecules 2019, 24, 601. https://doi.org/10.3390/molecules24030601
Ma X, Zhang X, Qiu W, Zhang W, Wan B, Evans J, Zhang W. One-Pot Synthesis of Triazolobenzodiazepines Through Decarboxylative [3 + 2] Cycloaddition of Nonstabilized Azomethine Ylides and Cu-Free Click Reactions. Molecules. 2019; 24(3):601. https://doi.org/10.3390/molecules24030601
Chicago/Turabian StyleMa, Xiaoming, Xiaofeng Zhang, Weiqi Qiu, Wensheng Zhang, Bruce Wan, Jason Evans, and Wei Zhang. 2019. "One-Pot Synthesis of Triazolobenzodiazepines Through Decarboxylative [3 + 2] Cycloaddition of Nonstabilized Azomethine Ylides and Cu-Free Click Reactions" Molecules 24, no. 3: 601. https://doi.org/10.3390/molecules24030601
APA StyleMa, X., Zhang, X., Qiu, W., Zhang, W., Wan, B., Evans, J., & Zhang, W. (2019). One-Pot Synthesis of Triazolobenzodiazepines Through Decarboxylative [3 + 2] Cycloaddition of Nonstabilized Azomethine Ylides and Cu-Free Click Reactions. Molecules, 24(3), 601. https://doi.org/10.3390/molecules24030601