Ripe and Raw Pu-Erh Tea: LC-MS Profiling, Antioxidant Capacity and Enzyme Inhibition Activities of Aqueous and Hydro-Alcoholic Extracts
Abstract
:1. Introduction
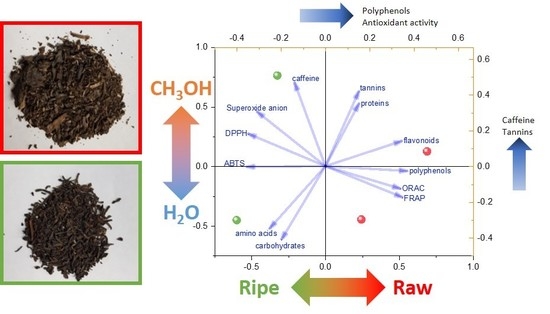
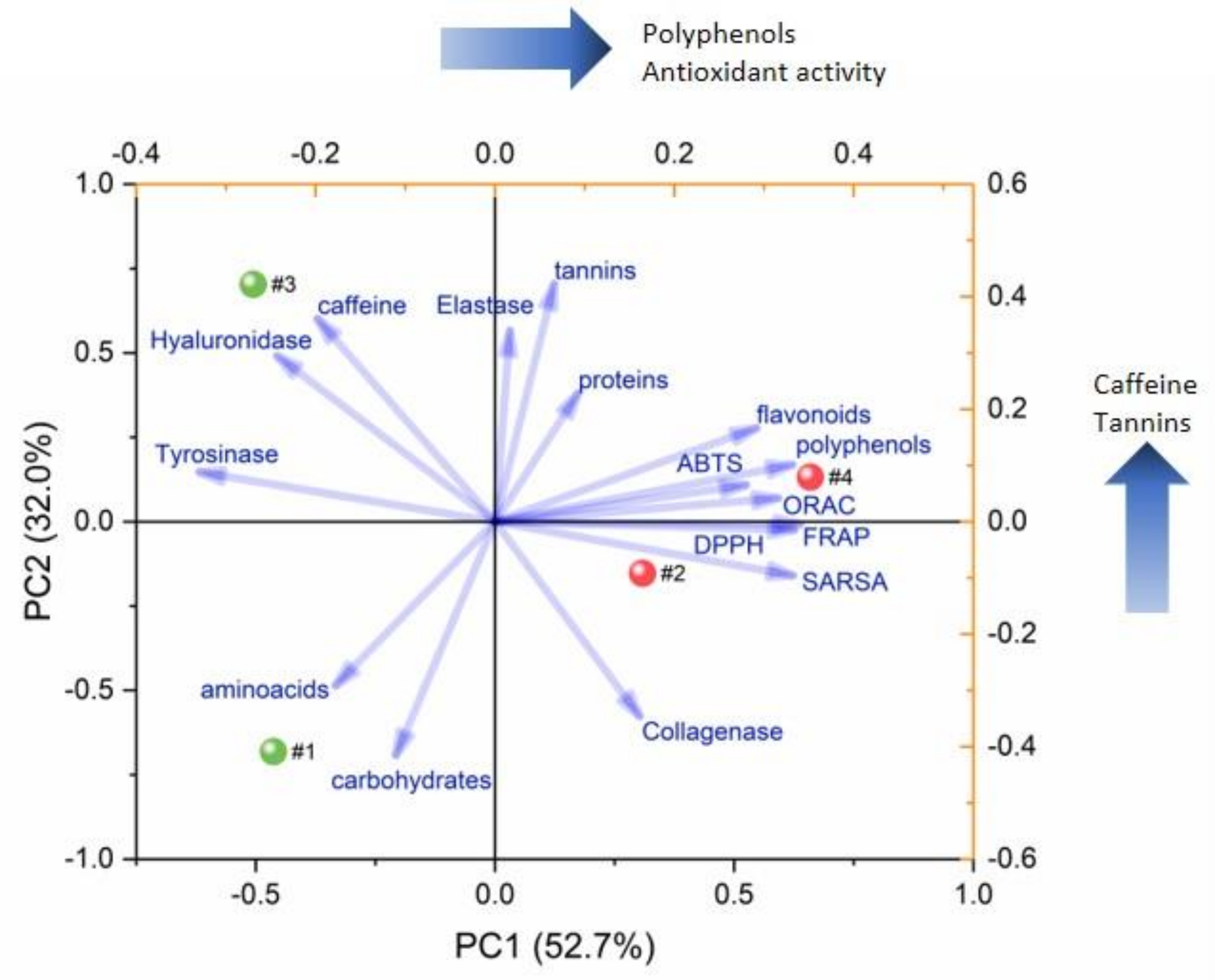
2. Results and Discussion
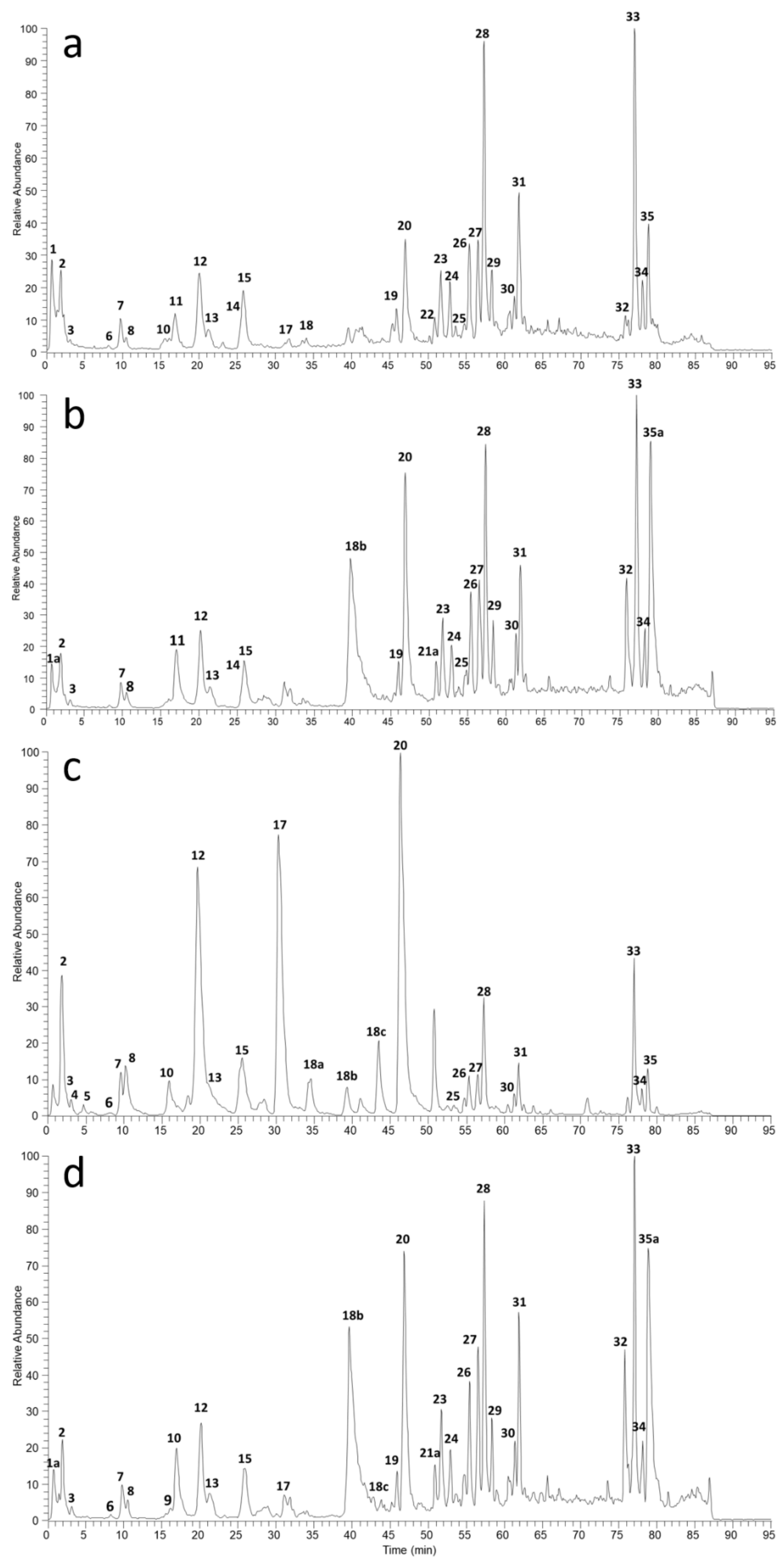
2.1. Polyphenol Characterization by LC/UV/ESI-MS Analysis
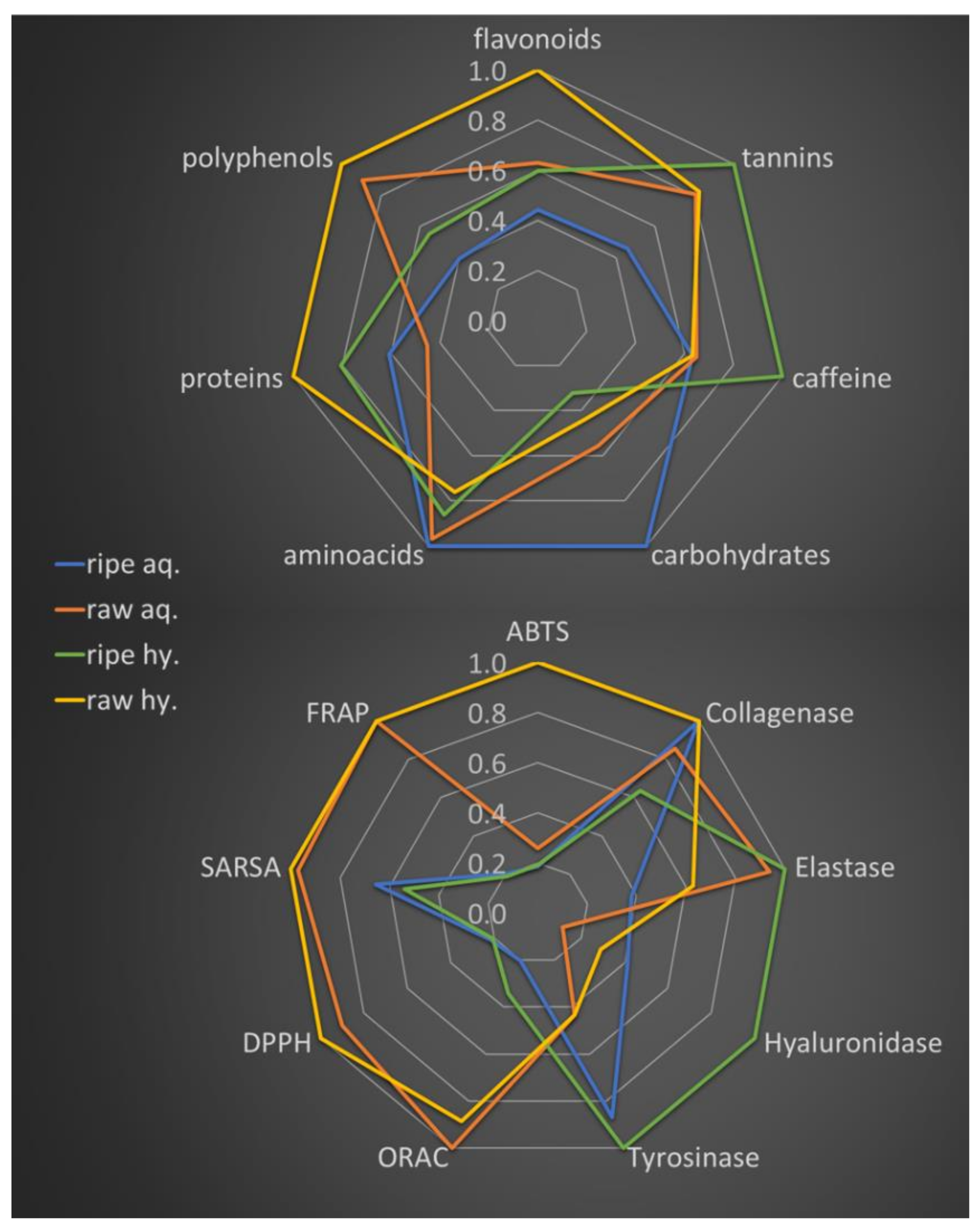
2.2. Tea Extracts Yields, Polyphenol Content, Antioxidant Activity and Anti-Enzyme Activity
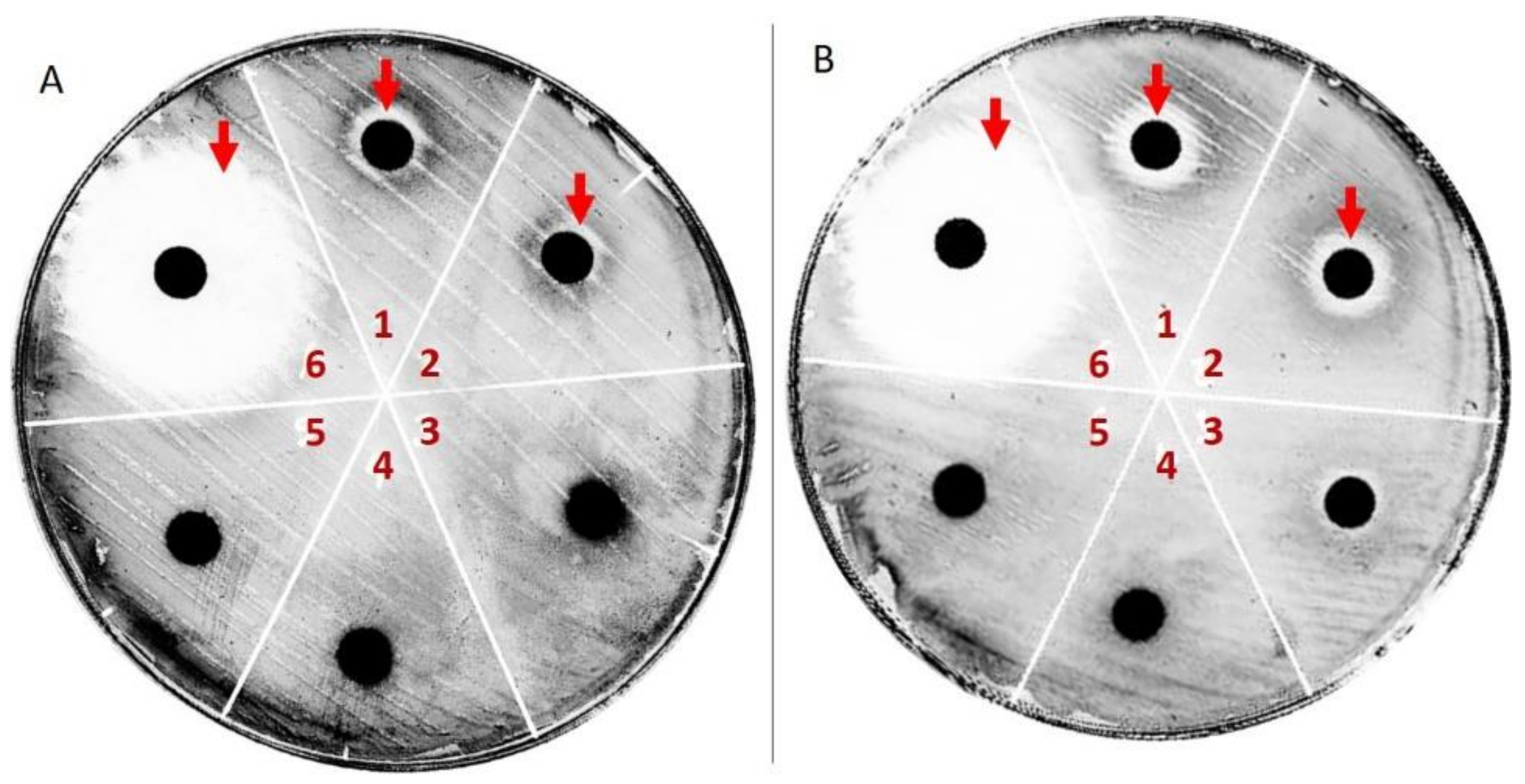
2.3. Antibacterial Activity
3. Materials and Methods
3.1. Starting Materials and Chemicals
3.2. Aqueous Extracts Preparation
3.3. Ethanol Extracts Preparation
3.4. LC-UV/ESI-MS Analysis
3.5. Ripe and Raw Components Characterization by High Resolution Mass Spectrometry
3.6. Determination of the Total Polyphenol Content
3.7. Determination of Total Flavonoid Content
3.8. Determination of Total Tannin Content
3.9. Determination of Free Amino Acid Content
3.10. Determination of Total Protein Content
3.11. Determination of Carbohydrate Content
3.12. Quantitative Determination of Caffeine
3.13. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Assay
3.14. Superoxide Anion Radical Scavenging Activity (SARSA)
3.15. ABTS Radical Cation Decolorization Assay
3.16. Determination of the Oxygen Radical Absorbing Capacity (ORAC)
3.17. FRAP Assay
3.18. Hyaluronidase Inhibition Activity
3.19. Tyrosinase Inhibition Activity
3.20. Elastase Inhibition Activity
3.21. Collagenase Inhibition Activity
3.22. Spectrophotometric Analyses
3.23. Antibacterial Activity
3.24. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.-Y.; Lin-Shiau, S.-Y.; Lin, J.-K. Pu-erh Tea Its Manufacturing and Health Benefits. In Tea and Tea Products Chemistry and Health-Promoting Properties, 1st ed.; Shahidi, F., Lin, J.-K., Ho, C.T., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 9–15. [Google Scholar]
- Zhang, L.; Zhang, Z.Z.; Zhou, Y.B.; Ling, T.J.; Wan, X.C. Chinese dark teas: Postfermentation, chemistry and biological activities. Food Res. Int. 2013, 53, 600–607. [Google Scholar] [CrossRef]
- Hou, Y.; Shao, W.; Xiao, R.; Xu, K.; Ma, Z.; Johnstone, B.H.; Du, Y. Pu-erh tea aqueous extracts lower atherosclerotic risk factors in a rat hyperlipidemia model. Exp. Gerontol. 2009, 44, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yan, X.S. History of Pu’er Tea and comparative study for the effect of its various extracts on lipid-lowering diet. Pak. J. Pharm. Sci. 2014, 27, 1015–1022. [Google Scholar]
- Oi, Y.; Hou, I.C.; Fujita, H.; Yazawa, K. Antiobesity effects of Chinese black tea (Pu-erh tea) extract and gallic acid. Phytother. Res. PTR 2012, 26, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.B.; Hayashi, S.; Fang, C.Y.; Hao, S.M.; Wang, X.J.; Nishiguchi, S.; Tsutsui, H.; Sheng, J. Pu’erh tea extract-mediated protection against hepatosteatosis and insulin resistance in mice with diet-induced obesity is associated with the induction of de novo lipogenesis in visceral adipose tissue. J. Gastroenterol. 2017, 52, 1240–1251. [Google Scholar] [CrossRef]
- Cai, X.; Fang, C.; Hayashi, S.; Hao, S.; Zhao, M.; Tsutsui, H.; Nishiguchi, S.; Sheng, J. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J. Gastroenterol. 2016, 51, 819–829. [Google Scholar] [CrossRef]
- Huang, H.C.; Lin, J.K. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct. 2012, 3, 170–177. [Google Scholar] [CrossRef]
- Shimamura, Y.; Yoda, M.; Sakakibara, H.; Matsunaga, K.; Masuda, S. Pu-erh tea suppresses diet-induced body fat accumulation in C57BL/6J mice by down-regulating SREBP-1c and related molecules. Biosci. Biotechnol. Biochem. 2013, 77, 1455–1460. [Google Scholar] [CrossRef]
- Cao, Z.H.; Yang, H.; He, Z.L.; Luo, C.; Xu, Z.Q.; Gu, D.H.; Jia, J.J.; Ge, C.R.; Lin, Q.Y. Effects of aqueous extracts of raw pu-erh tea and ripened pu-erh tea on proliferation and differentiation of 3T3-L1 preadipocytes. Phytother. Res. PTR 2013, 27, 1193–1199. [Google Scholar] [CrossRef]
- Yamashita, Y.; Wang, L.; Wang, L.; Tanaka, Y.; Zhang, T.; Ashida, H. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct. 2014, 5, 2420–2429. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, J.; Luo, L.; Zhang, D. Effects of Pu-erh tea aqueous extract (PTAE) on blood lipid metabolism enzymes. Food Funct. 2015, 6, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xie, Q.; Kong, P.; Liu, L.; Sun, S.; Xiong, B.; Huang, B.; Yan, L.; Sheng, J.; Xiang, H. Polyphenol- and Caffeine-Rich Postfermented Pu-erh Tea Improves Diet-Induced Metabolic Syndrome by Remodeling Intestinal Homeostasis in Mice. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.H.; Chen, M.Y.; Chen, L.C.; Mao, Y.T.; Ho, C.H.; Lee, W.J.; Lin, Y.K.; Pan, M.H.; Lo, C.Y.; Chen, C.L.; et al. Pu-erh Tea Extract Attenuates Nicotine-Induced Foam Cell Formation in Primary Cultured Monocytes: An in Vitro Mechanistic Study. J. Agric. Food Chem. 2016, 64, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.J.; Wang, L.; Li, Z.; Zhu, D.N.; Guo, S.C.; Xin, W.F.; Yang, Y.F.; Cong, X.; Ma, T.; Shen, P.P.; et al. Inhibition of advanced glycation end product formation by Pu-erh tea ameliorates progression of experimental diabetic nephropathy. J. Agric. Food Chem. 2012, 60, 4102–4110. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.K.; Zeng, Z.; Huang, Y.H.; Zhang, X. Chemical compositions of Pu’er tea fermented by Eurotium Cristatum and their lipid-lowering activity. LWT-Food Sci. Technol. 2018, 98, 204–211. [Google Scholar] [CrossRef]
- Du, W.H.; Peng, S.M.; Liu, Z.H.; Shi, L.; Tan, L.F.; Zou, X.Q. Hypoglycemic effect of the water extract of Pu-erh tea. J. Agric. Food Chem. 2012, 60, 10126–10132. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, W.; Gu, Y.; Dai, J.; Li, X.; Tai, P.; Li, Y.; Ma, X.; Zhang, Y. Protective effect of Pu-erh tea extracts against ethanol-induced gastric mucosal damage in rats. Biomed. Rep. 2018, 8, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, D.; Wu, H. Effects of Pu-erh ripened tea on hyperuricemic mice studied by serum metabolomics. J. Chromatogr. Banal. Technol. Biomed. Life Sci. 2017, 1068–1069, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, G.; Li, C.J.; Zhang, M.; Zhao, H.; Sheng, J.; Shi, W. Pu-erh Tea Reduces Nitric Oxide Levels in Rats by Inhibiting Inducible Nitric Oxide Synthase Expression through Toll-Like Receptor 4. Int. J. Mol. Sci. 2012, 13, 7174–7185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shao, W.F.; Yuan, L.F.; Tu, P.F.; Ma, Z.Z. Decreasing pro-inflammatory cytokine and reversing the immunosenescence with extracts of Pu-erh tea in senescence accelerated mouse (SAM). Food Chem. 2012, 135, 2222–2228. [Google Scholar] [CrossRef]
- Liu, T.; Ding, S.; Yin, D.; Cuan, X.; Xie, C.; Xu, H.; Wang, X.; Sheng, J. Pu-erh Tea Extract Ameliorates Ovariectomy-Induced Osteoporosis in Rats and Suppresses Osteoclastogenesis In Vitro. Front. Pharmacol. 2017, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chai, S.; Ju, Y.; Hou, L.; Zhao, H.; Ma, W.; Li, T.; Sheng, J.; Shi, W. Pu-erh Tea Protects the Nervous System by Inhibiting the Expression of Metabotropic Glutamate Receptor 5. Mol. Neurobiol. 2017, 54, 5286–5299. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hayashi, S.; Cai, X.; Fang, C.; Shi, W.; Tsutsui, H.; Sheng, J. Pu-erh tea extract induces the degradation of FET family proteins involved in the pathogenesis of amyotrophic lateral sclerosis. Biomed. Res. Int. 2014, 2014, 254680. [Google Scholar] [CrossRef]
- Zhao, X.; Song, J.L.; Kim, J.D.; Lee, J.S.; Park, K.Y. Fermented Pu-erh Tea Increases In Vitro Anticancer Activities in HT-29 Cells and Has Antiangiogenetic Effects on HUVECs. J. Env. Pathol. Toxicol. 2013, 32, 275–288. [Google Scholar] [CrossRef]
- Xie, J.; Yu, H.; Song, S.; Fang, C.; Wang, X.; Bai, Z.; Ma, X.; Hao, S.; Zhao, H.Y.; Sheng, J. Pu-erh Tea Water Extract Mediates Cell Cycle Arrest and Apoptosis in MDA-MB-231 Human Breast Cancer Cells. Front. Pharmacol. 2017, 8, 190. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, S.; Chen, H.; Wang, Y.; Wang, Y.; Hochstetter, D.; Xu, P. Studies on the bioactivity of aqueous extract of pu-erh tea and its fractions: In vitro antioxidant activity and alpha-glycosidase inhibitory property, and their effect on postprandial hyperglycemia in diabetic mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 53, 75–83. [Google Scholar] [CrossRef]
- Zheng, X.N.; Wang, X.W.; Li, L.Y.; Xu, Z.W.; Huang, H.Y.; Zhao, J.S.; Zhang, D.; Yin, X.; Sheng, J.; Tang, J.T. Pu-erh tea powder preventive effects on cisplatin-induced liver oxidative damage in Wistar rats. Asian Pac. J. Cancer Prev. 2014, 15, 7389–7394. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, C.F.; Shen, S.M.; Wang, G.L.; Liu, P.; Liu, Z.M.; Wang, Y.Y.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Antioxidant phenolic compounds from Pu-erh tea. Molecules 2012, 17, 14037–14045. [Google Scholar] [CrossRef]
- Hsieh, S.K.; Xu, J.R.; Lin, N.H.; Li, Y.C.; Chen, G.H.; Kuo, P.C.; Chen, W.Y.; Tzen, J.T.C. Antibacterial and laxative activities of strictinin isolated from Pu’er tea (Camellia sinensis). J. Food Drug Anal. 2016, 24, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Lin, Y.L.; Hsu, W.L.; Hsieh, S.K.; Tzen, J.T.C. Significant elevation of antiviral activity of strictinin from Pu’er tea after thermal degradation to ellagic acid and gallic acid. J. Food Drug Anal. 2015, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Kim, J.; Park, H.J.; Liu, K.H.; Lee, C.H. Application of Metabolomics in the Analysis of Manufacturing Type of Pu-erh Tea and Composition Changes with Different Postfermentation Year. J. Agric. Food Chem. 2010, 58, 345–352. [Google Scholar] [CrossRef]
- Tian, L.W.; Tao, M.K.; Xu, M.; Hu, J.; Zhu, H.T.; Xiong, W.Y.; Wang, D.; Yang, C.R.; Zhang, Y.J. Carboxymethyl- and carboxyl-catechins from ripe Pu-er tea. J. Agric. Food Chem. 2014, 62, 12229–12234. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.K.; Xu, M.; Zhu, H.T.; Cheng, R.R.; Wang, D.; Yang, C.R.; Zhang, Y.J. New phenylpropanoid-substituted flavan-3-ols from Pu-er ripe tea. Nat. Prod. Commun. 2014, 9, 1167–1170. [Google Scholar] [PubMed]
- Kim, M.J.; Kim, S.S.; Lee, S.I. Quality Characteristics and Content of Polysaccharides in Green Tea Fermented by Monascus pilosus. Prev. Nutr. Food Sci. 2012, 17, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Li, X.; Yang, H.; Wang, F.; Kong, J.; Qiu, D.; Li, Z. Mass spectrometry-based metabolomics and chemometric analysis of Pu-erh teas of various origins. Food Chem. 2018, 268, 271–278. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, N.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Plant Resources, Chemical Constituents and Bioactivities of Tea Plants from the Genus Camellia Section Thea. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef]
- Pedan, V.; Rohn, S.; Holinger, M.; Huhn, T.; Chetschik, I. Bioactive Compound Fingerprint Analysis of Aged Raw Pu’er Tea and Young Ripened Pu’er Tea. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Luo, Y.H.; Wang, P.P.; Zhao, M.Y.; Li, L.; Hu, X.S.; Chen, F. Simultaneous determination of free amino acids in Pu-erh tea and their changes during fermentation. Food Chem. 2016, 194, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cui, F.; Li, H.; Sheng, J.; Lv, J. Metabolic changes during the pu-erh tea pile-fermentation revealed by a liquid chromatography tandem mass-spectrometry-based metabolomics approach. J. Food Sci. 2013, 78, C1665–C1672. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Ma, Z.Z.; Tu, P.F. Comparison of the chemical constituents of aged pu-erh tea, ripened pu-erh tea, and other teas using HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2011, 59, 8754–8760. [Google Scholar] [CrossRef]
- Aldini, G.; Regazzoni, L.; Pedretti, A.; Carini, M.; Cho, S.M.; Park, K.M.; Yeum, K.J. An integrated high resolution mass spectrometric and informatics approach for the rapid identification of phenolics in plant extract. J. Chromatogr. A 2011, 1218, 2856–2864. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Anwar, L.N.; Mordi, M.N.; Ramanathan, S.; Mansor, S.M. Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacogn. Res. 2010, 2, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.O.; Sui, J.; Young, A.B.; Whittal, R.M. Interferences and contaminants encountered in modern mass spectrometry. Anal. Chim. Acta 2008, 627, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.Z.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.S.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Q.; Zhang, Z.Z.; Wan, X.C. A novel colorimetric determination of free amino acids content in tea infusions with 2,4-dinitrofluorobenzene. J. Food Compos. Anal. 2009, 22, 137–141. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Leyva, A.; Quintana, A.; Sanchez, M.; Rodriguez, E.N.; Cremata, J.; Sanchez, J.C. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biol. J. Int. Assoc. Biol. Stand. 2008, 36, 134–141. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. LEB 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Valentao, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J. Agric. Food Chem. 2001, 49, 3476–3479. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.H.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Reissig, J.L.; Storminger, J.L.; Leloir, L.F. A modified colorimetric method for the estimation of N-acetylamino sugars. J. Biol. Chem. 1955, 217, 959–966. [Google Scholar] [PubMed]
- Masamoto, Y.; Ando, H.; Murata, Y.; Shimoishi, Y.; Tada, M.; Takahata, K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci. Biotechnol. Abiochem. 2003, 67, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Kraunsoe, J.A.; Claridge, T.D.; Lowe, G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 1996, 35, 9090–9096. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Raw materials of ripe and raw pu-erh teas are available from the authors. |
| Time | Water % | Acetonitrile % | Formic Acid % |
|---|---|---|---|
| 0 | 5 | 0.1 | |
| 50 | 17 | 0.1 | |
| 80 | 32 | 0.1 | |
| 83 | 40 | 0.1 | |
| 95 | 5 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, G.; Marinello, C.; Grassi, A.; Picozzi, C.; Aldini, G.; Carini, M.; Regazzoni, L. Ripe and Raw Pu-Erh Tea: LC-MS Profiling, Antioxidant Capacity and Enzyme Inhibition Activities of Aqueous and Hydro-Alcoholic Extracts. Molecules 2019, 24, 473. https://doi.org/10.3390/molecules24030473
Roda G, Marinello C, Grassi A, Picozzi C, Aldini G, Carini M, Regazzoni L. Ripe and Raw Pu-Erh Tea: LC-MS Profiling, Antioxidant Capacity and Enzyme Inhibition Activities of Aqueous and Hydro-Alcoholic Extracts. Molecules. 2019; 24(3):473. https://doi.org/10.3390/molecules24030473
Chicago/Turabian StyleRoda, Gabriella, Cristina Marinello, Anita Grassi, Claudia Picozzi, Giancarlo Aldini, Marina Carini, and Luca Regazzoni. 2019. "Ripe and Raw Pu-Erh Tea: LC-MS Profiling, Antioxidant Capacity and Enzyme Inhibition Activities of Aqueous and Hydro-Alcoholic Extracts" Molecules 24, no. 3: 473. https://doi.org/10.3390/molecules24030473
APA StyleRoda, G., Marinello, C., Grassi, A., Picozzi, C., Aldini, G., Carini, M., & Regazzoni, L. (2019). Ripe and Raw Pu-Erh Tea: LC-MS Profiling, Antioxidant Capacity and Enzyme Inhibition Activities of Aqueous and Hydro-Alcoholic Extracts. Molecules, 24(3), 473. https://doi.org/10.3390/molecules24030473