Characteristics of Free Amino Acids (the Quality Chemical Components of Tea) under Spatial Heterogeneity of Different Nitrogen Forms in Tea (Camellia sinensis) Plants
Abstract
:1. Introduction
2. Results and Discussion
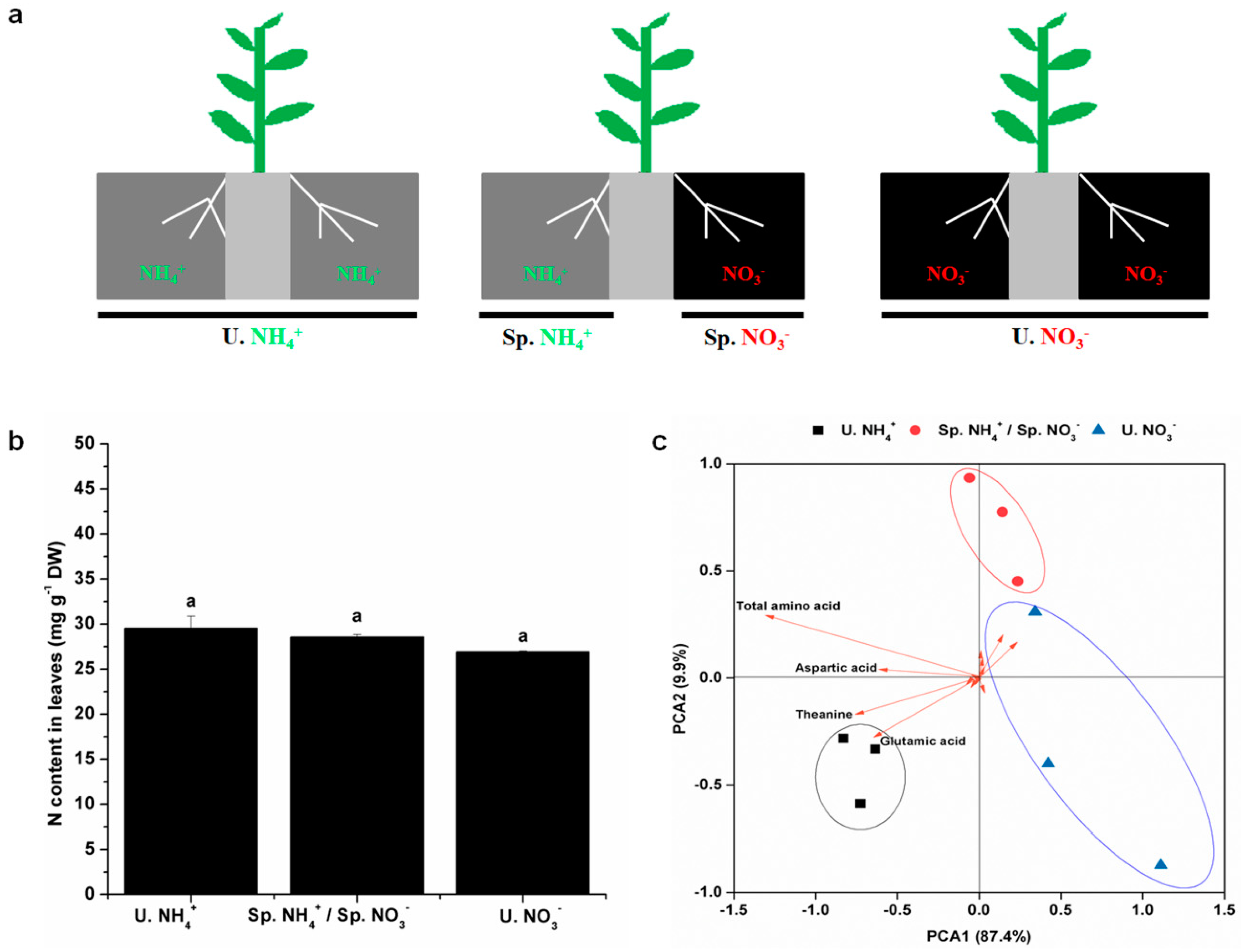
2.1. Nitrogen (N) Content and Diversity of Free Amino Acids (FAAs) in Leaves
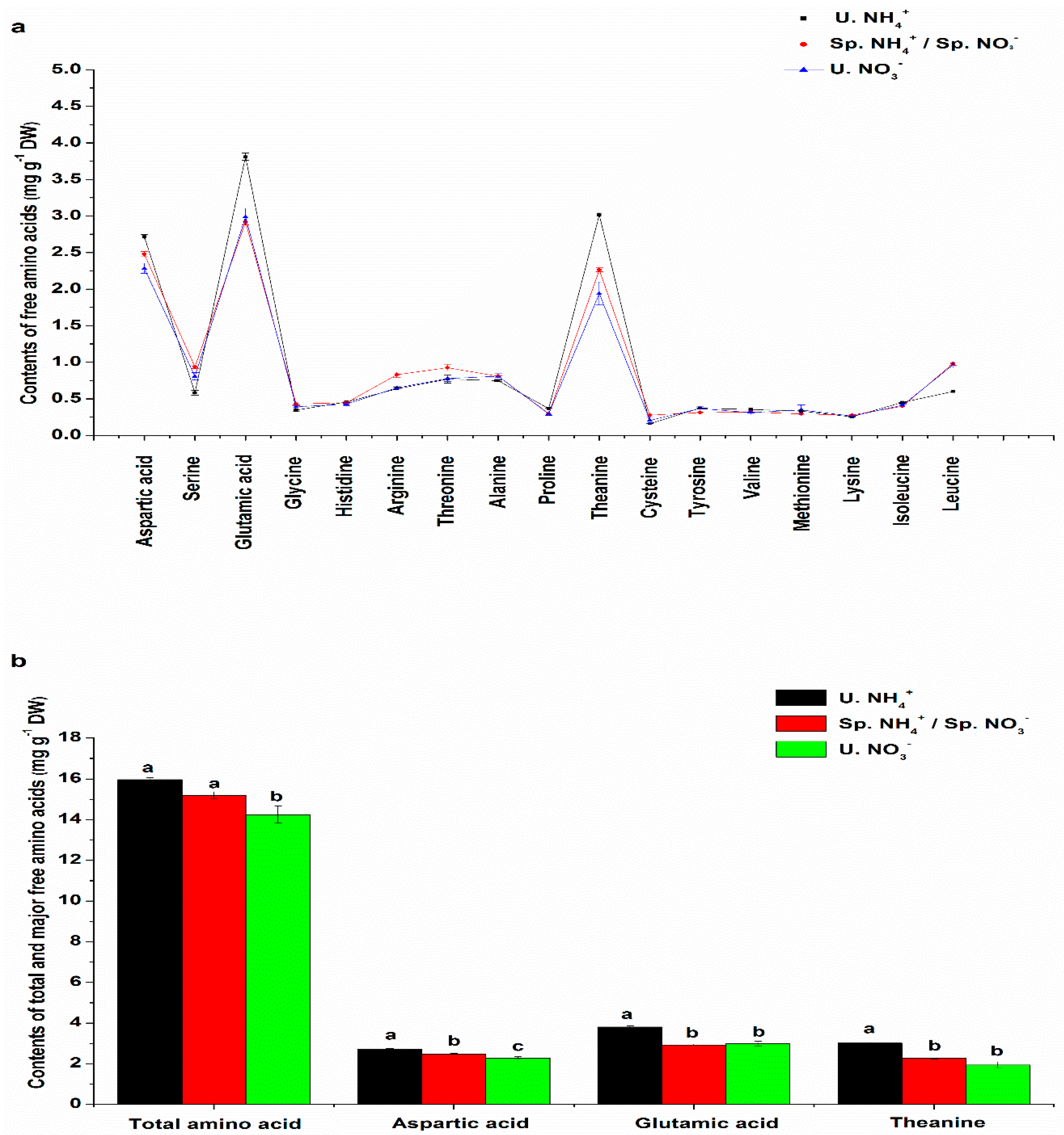
2.2. Free Amino Acid (FAA) Content Characteristics in Leaves
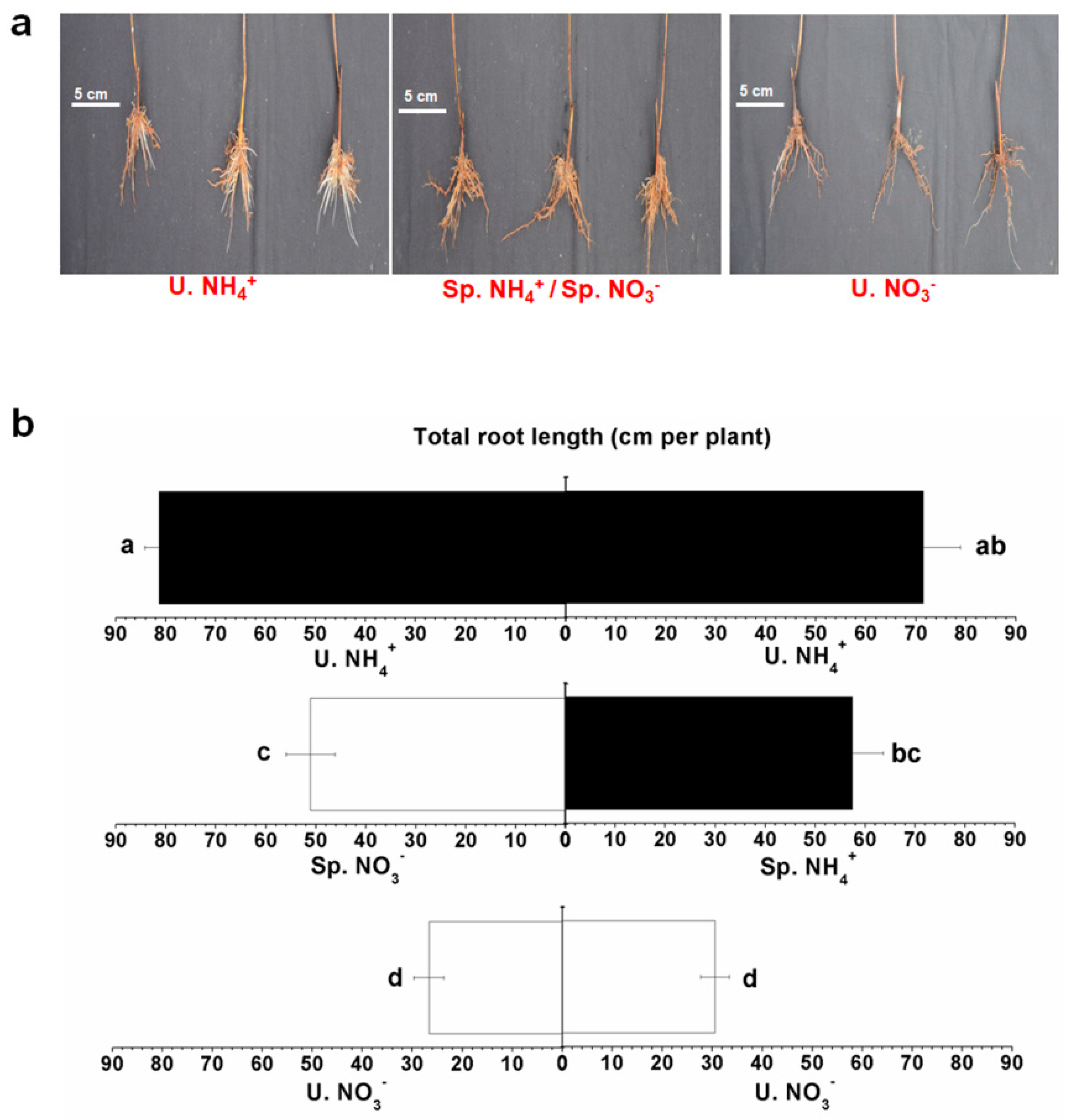
2.3. Root Characteristics under Split-Root System with Different N Forms
3. Materials and Methods
3.1. Plant Materials and Split-Root System
3.2. Determinations of N and Free Amino Acid (FAA) Contents
3.3. Determination of Root Length
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ruan, L.; Wei, K.; Wang, L.; Cheng, H.; Zhang, F.; Wu, L.; Bai, P.; Zhang, C. Characteristics of NH4+ and NO3− fluxes in tea (Camellia sinensis) roots measured by scanning ion-selective electrode technique. Sci. Rep. 2016, 6, 38370. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.M.; Engel, R.E.; Chen, C.C.; Wallander, R. Microbial immobilization of nitrogen-15 labelled ammonium and nitrate in an agricultural soil. Soil Sci. Soc. Am. J. 2015, 79, 595–602. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Y.; Gerendas, J.; Härdter, R.; Sattelmacher, B. Effect of root zone pH and form and concentration of nitrogen on accumulation of quality-related components in green tea. J. Sci. Food Agric. 2007, 87, 1505–1516. [Google Scholar] [CrossRef]
- Ding, G.Z.; Hou, J.; Chen, L.; Ma, F.M.; Chen, L.J. Cloning of nia Gene and Differential Gene Expressions Induced by Nitrate and Ammonium Nitrogen in Sugar Beet (Beta vulgaris L.). Acta Agron. Sin. 2011, 37, 1949–1955. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Wang, F.; Wan, Q.; Ruan, J.Y. Transcriptome analysis using RNA-Seq revealed the effects of nitrogen form on major secondary metabolite biosynthesis in tea (Camellia sinensis) plants. Acta Physiol. Plant 2018, 40, 127. [Google Scholar] [CrossRef]
- Fan, K.; Fan, D.; Ding, Z.; Su, Y.; Wang, X. Cs-miR156 is involved in the nitrogen form regulation of catechins accumulation in tea plant (Camellia sinensis L.). Plant Physiol. Biochem. 2015, 97, 350–360. [Google Scholar] [CrossRef]
- Tan, F.; Tan, C.; Zhao, A.; Li, M.L. Simultaneous determination of free amino acid content in tea infusions by using high-performance liquid chromatography with fluorescence detection coupled with alternating penalty trilinear decomposition algorithm. J. Agric. Food Chem. 2011, 59, 10839–10847. [Google Scholar] [CrossRef]
- Horanni, R.; Engelhardt, U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Compos. Anal. 2013, 31, 94–100. [Google Scholar] [CrossRef]
- Yan, J.; Cai, Y.; Wang, Y.; Lin, X.; Li, H. Simultaneous determination of amino acids in tea leaves by micellar electrokinetic chromatography with laser-induced fluorescence detection. Food Chem. 2014, 143, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Song, W.; Zhao, B.; Dou, Y.L. Rapid and selective quantification of l-theanine in ready-to-drink teas from Chinese market using SPE and UPLC-UV. Food Chem. 2012, 135, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, Y.; Dai, L.; Zhang, D.; Li, J.; Yuan, W.; Li, Y.; Zhou, H. A High-performance liquid chromatographic method for simultaneous determination of 21 free amino acids in tea. Food Anal. Method 2013, 6, 69–75. [Google Scholar] [CrossRef]
- Hung, Y.T.; Chen, P.C.; Chen, R.L.C.; Chen, T.J. Sequential determination of tannin and total amino acid contents in tea for taste assessment by a fluorescent flow-injection analytical system. Food Chem. 2010, 118, 876–881. [Google Scholar] [CrossRef]
- Feldheim, W.; Yongvanit, P.; Cummings, P.H. Investigation of the presence and significance of theanine in the tea plant. J. Sci. Food Agric. 2010, 37, 527–534. [Google Scholar] [CrossRef]
- Baptista, J.; Lima, E.; Paiva, L.; Andradeb, A.L.; Alvesb, M.G. Comparison of Azorean tea theanine to teas from other origins by HPLC/DAD/FD. Effect of fermentation, drying temperature, drying time and shoot maturity. Food Chem. 2012, 132, 2181–2187. [Google Scholar] [CrossRef]
- Feng, L.; Gao, M.J.; Hou, R.Y.; Hu, X.Y.; Zhang, L.; Wan, X.C.; Wei, S. Determination of quality constituents in the young leaves of albino tea cultivars. Food Chem. 2014, 155, 98–104. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Cai, H.; Zhou, W.; Xu, F. H2O2 mediates nitrate-induced iron chlorosis by regulating iron homeostasis in rice. Plant Cell Environ. 2018, 41, 767–781. [Google Scholar] [CrossRef]
- Cartwright, R.A.; Roberts, E.A.H.; Wood, D.J. Theanine, an amino-acid N-ethylamide present in tea. J. Sci. Food Agric. 2010, 5, 597–599. [Google Scholar] [CrossRef]
- Oh, K.; Kato, T.; Xu, H.L. Transport of nitrogen assimilation in xylem vessels of green tea plants fed with NH4−N and NO3−N. Pedosphere 2008, 18, 222–226. [Google Scholar] [CrossRef]
- Hachiya, T.; Terashima, I.; Noguchi, K. Increase in respiratory cost at high growth temperature is attributed to high protein turnover cost in Petunia × hybrida petals. Plant Cell Environ. 2007, 30, 1269–1283. [Google Scholar] [CrossRef]
- Miller, A.; Cramer, M. Root nitrogen acquisition and assimilation. Plant Soil 2004, 274, 1–36. [Google Scholar] [CrossRef]
- Sari, F.; Velioglu, Y.S. Changes in theanine and caffeine contents of black tea with different rolling methods and processing stages. Eur. Food Res. Technol. 2013, 237, 229–236. [Google Scholar] [CrossRef]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wirén, N. Ammonium triggers lateral root branching in Arabidopsis in an ammonium transporter1;3-dependent manner. Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, D.R.; Barrios-Masias, F.H.; Hausmann, N.T.; Jackson, L.E.; Schachtman, D.P. Tomato root transcriptome response to a nitrogen-enriched soil patch. BMC Plant Biol. 2010, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zang, U.; Lamersdorf, N.; Borken, W. Response of the fine root system in a Norway spruce stand to 13 years of reduced atmospheric nitrogen and acidity input. Plant Soil. 2011, 339, 435–445. [Google Scholar] [CrossRef]
- Hachiya, T.; Noguchi, K. Integrative response of plant mitochondrial electron transport chain to nitrogen source. Plant Cell Rep. 2011, 30, 195–204. [Google Scholar] [CrossRef]
- Ruffel, S.; Krouk, G.; Ristova, D.; Shasha, D.; Birnbaum, K.D.; Coruzzi, G.M. Nitrogen economics of root foraging: Transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for n supply vs. demand. Proc. Natl. Acad. Sci. USA 2012, 108, 18524–18529. [Google Scholar] [CrossRef]
- Joshi, R.; Rana, A.; Gulati, A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chem. 2015, 176, 357–366. [Google Scholar] [CrossRef]
- Yu, P.; Yeo, A.S.L.; Low, M.Y.; Zhou, W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, L.; Wei, K.; Wang, L.; Cheng, H.; Wu, L.; Li, H. Characteristics of Free Amino Acids (the Quality Chemical Components of Tea) under Spatial Heterogeneity of Different Nitrogen Forms in Tea (Camellia sinensis) Plants. Molecules 2019, 24, 415. https://doi.org/10.3390/molecules24030415
Ruan L, Wei K, Wang L, Cheng H, Wu L, Li H. Characteristics of Free Amino Acids (the Quality Chemical Components of Tea) under Spatial Heterogeneity of Different Nitrogen Forms in Tea (Camellia sinensis) Plants. Molecules. 2019; 24(3):415. https://doi.org/10.3390/molecules24030415
Chicago/Turabian StyleRuan, Li, Kang Wei, Liyuan Wang, Hao Cheng, Liyun Wu, and Hailin Li. 2019. "Characteristics of Free Amino Acids (the Quality Chemical Components of Tea) under Spatial Heterogeneity of Different Nitrogen Forms in Tea (Camellia sinensis) Plants" Molecules 24, no. 3: 415. https://doi.org/10.3390/molecules24030415
APA StyleRuan, L., Wei, K., Wang, L., Cheng, H., Wu, L., & Li, H. (2019). Characteristics of Free Amino Acids (the Quality Chemical Components of Tea) under Spatial Heterogeneity of Different Nitrogen Forms in Tea (Camellia sinensis) Plants. Molecules, 24(3), 415. https://doi.org/10.3390/molecules24030415





