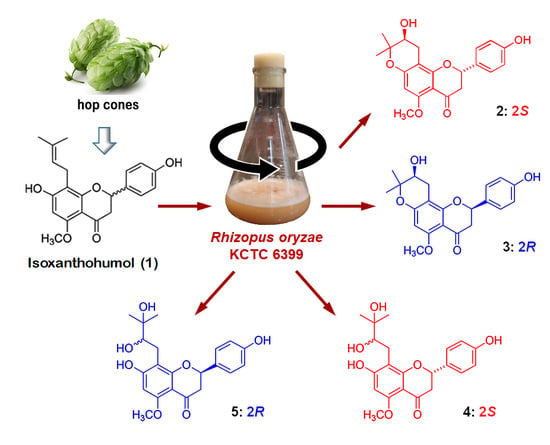
Biotransformed Metabolites of the Hop Prenylflavanone Isoxanthohumol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Microbial Biotransformation of Isoxanthohumol
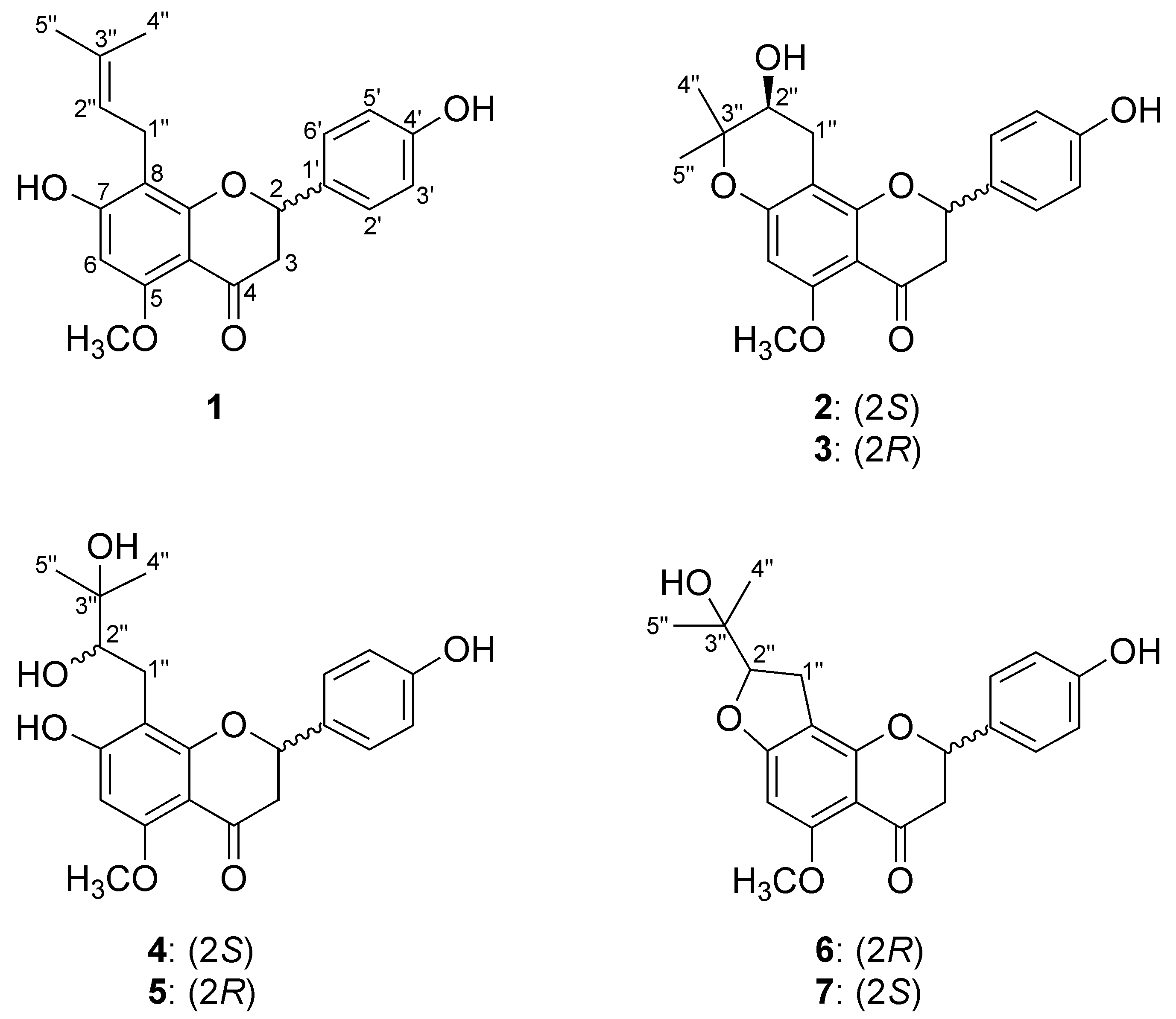
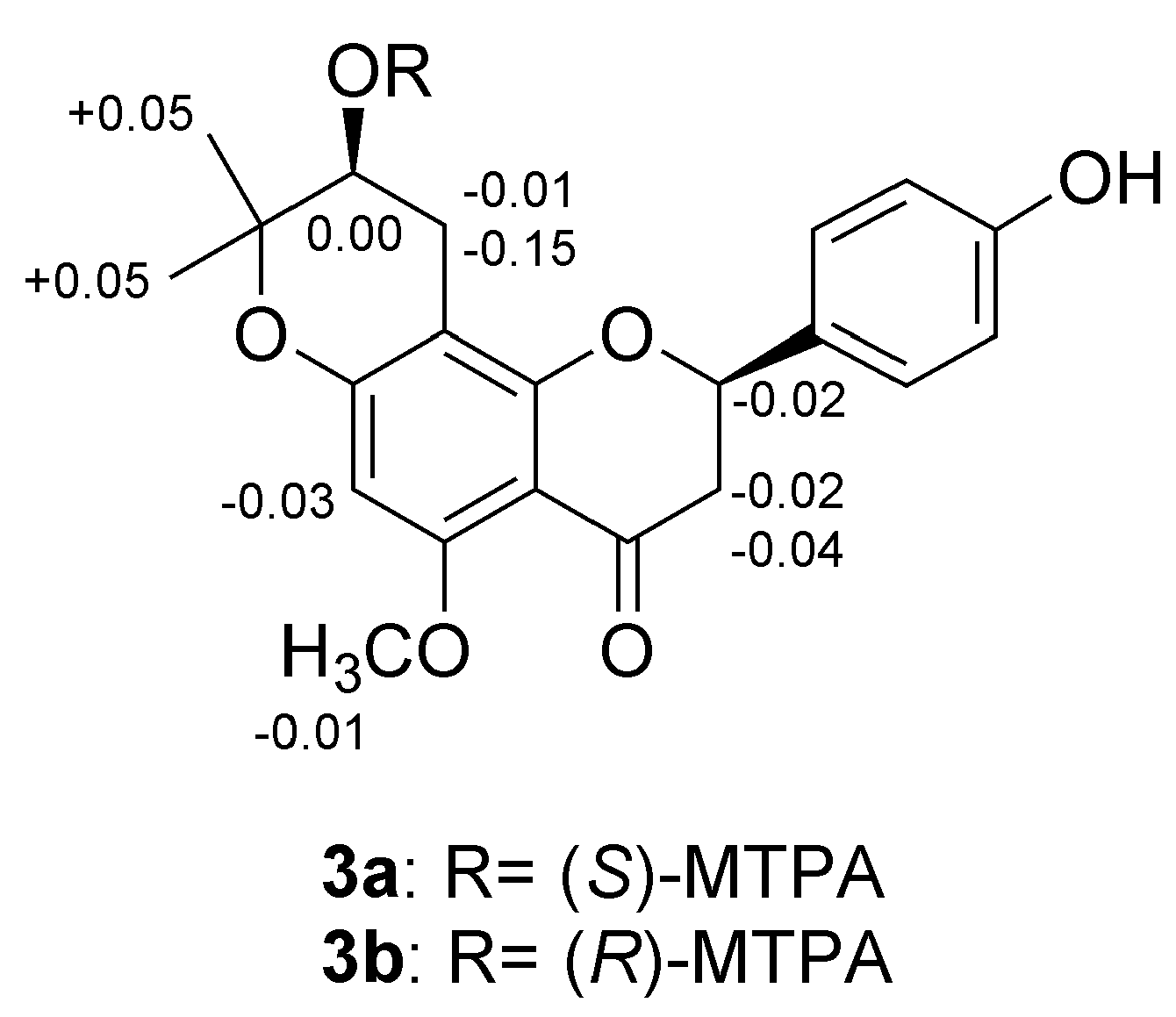
2.2. Structure Elucidation of Isoxanthohumol Metabolites
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemicals and Ingredients
3.3. Microorganisms and Fermentation
3.4. Biotransformation Screening Procedure
3.5. Biotransformation of Isoxanthohumol (1) by R. oryzae KCTC 6399
3.6. Biotransformation of 1 by F. oxysporum f.sp. lini KCTC 16325
3.7. Determination of Absolute Configuration by Modified Mosher’s Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, J.F.; Ivancic, M.; Hsu, V.L.; Deinzer, M.L. Prenylflavonoids from Humulus lupulus. Phytochemistry 1997, 44, 1575–1585. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Clawson, J.E.; Deinzer, M.L. Fate of xanthohumol and related prenylflavonoids from hops to beer. J. Agric. Food Chem. 1999, 47, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef]
- Delmulle, L.; Bellahcène, A.; Dhooge, W.; Comhaire, F.; Roelenes, F.; Huvaere, K.; Heyerick, A.; Castronovo, V.; DeKeukeleire, D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine 2006, 13, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol-Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Alt, A.P.; Klimo, K.; Knauft, J.; Frank, N.; Becker, H. Isolation and potential cancer chemopreventive activities of phenolic compounds of beer. Phytochem. Rev. 2002, 1, 369–377. [Google Scholar] [CrossRef]
- Izzo, G.; Söder, O.; Svechnikov, K. The prenylflavonoid phytoestrogens 8-prenylnaringenin and isoxanthohumol differentially suppress steroidogenesis in rat Leydig cells in ontogenesis. J. Appl. Toxicol. 2011, 31, 589–594. [Google Scholar] [CrossRef]
- Nikolic, D.; Li, Y.; Chadwick, L.R.; Pauli, G.F.; van Breeman, R.B. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J. Mass Spectrom. 2005, 40, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Huszcza, E.; Tronina, T. Transformation of isoxanthohumol by fungi. J. Mol. Catal. B Enzym. 2009, 61, 221–224. [Google Scholar] [CrossRef]
- Possemiers, S.; Bolca, S.; Grootaert, C.; Heyerick, A.; Decroos, K.; Dhooge, W.; De Keukeleire, D.; Rabot, S.; Verstraete, W.; Van de Wiele, T. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J. Nutr. 2006, 136, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6288. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.L.; Wang, W.; Chen, F.; Dong, Y.C.; Liu, X.J.; Ni, H.; Chen, Q.H. Production of 8-prenylnaringenin from isoxanthohumol through biotransformation by fungi cells. J. Agric. Food Chem. 2011, 59, 7419–7426. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; McChesney, J.D.; Hufford, C.D. The use of microorganisms for the study of drug metabolism. Med. Res. Rev. 1985, 5, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Hufford, C.D. Use of microorganisms for the study of drug metabolism: An update. Med. Res. Rev. 1991, 11, 473–501. [Google Scholar] [CrossRef]
- Venisetty, R.K.; Cidii, V. Application of microbial biotransformation for the new drug discovery using natural drugs as substrates. Curr. Pharm. Biotechnol. 2003, 4, 153–167. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, I.-S. Microbial metabolism of the prenylated chalcone xanthohumol. J. Nat. Prod. 2006, 69, 1522–1524. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.-H.; Kang, B.Y.; Lee, I.-S. Microbial metabolites of 8-prenylnaringenin, an estrogenic prenylflavanone. Arch. Pharm. Res. 2008, 31, 1241–1246. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, M.-A.; Lee, I.-S. Microbial transformation of isoxanthohumol, a hop prenylflavonoid. Nat. Prod. Sci. 2008, 14, 269–273. [Google Scholar]
- Gaffield, W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides: Determination of aglycone chirality in flavanone glycosides. Tetrahedron 1970, 26, 4093–4108. [Google Scholar] [CrossRef]
- Slade, D.; Ferreira, D.; Marais, J.P.J. Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 2005, 66, 2177–2215. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configuration of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Shi, G.; Gu, Z.; He, K.; Wood, K.V.; Zeng, L.; Ye, Q.; MacDougal, J.M.; McLaughlin, J.L. Applying Mosher’s method to acetogenins bearing vicinal diols. The absolute configurations of muricatetrocin C and rollidecins A and B, new bioactive acetogenins from Rollinia mucosa. Bioorg. Med. Chem. 1996, 4, 1281–1286. [Google Scholar] [CrossRef]
- Julaeha, E.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Ng, S.W. Meranzin hydrate from Muraya paniculata. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o620. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.T. Features of electronic circular dichroism and tips for its use in determining absolute configuration. Tetrahedron Asymmetry 2017, 28, 1199–1211. [Google Scholar] [CrossRef]
- Herath, W.H.M.W.; Ferreira, D.; Khan, I.A. Microbial transformation of xanthohumol. Phytochemistry 2003, 62, 673–677. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Huszcza, E. Biotransformations of prenylated hop flavonoids for drug discovery and production. Curr. Drug Metab. 2013, 14, 1083–1097. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products, A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; pp. 161–178. ISBN 978-0-470-74168-9. [Google Scholar]
- Takashima, J.; Ohsaki, A. Brosimacutins A-I, nine new flavonoids from Brosimum acutifolium. J. Nat. Prod. 2002, 65, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Nikolic, D.; Chadwick, L.R.; Pauli, G.F.; van Breemen, R.B. Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). Drug Metab. Dispos. 2006, 34, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Liu, X.; Jia, X.; Chen, J.; Hu, M. Coupling of conjugating enzymes and efflux transporters: Impact on bioavailability and drug interactions. Curr. Drug Metab. 2005, 6, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Purchartová, K.; Valentová, K.; Pelantová, H.; Marhol, P.; Cvačka, J.; Havlíček, L.; Křenková, A.; Vavříková, A.; Biedermann, D.; Chambers, C.S.; et al. Prokaryotic and eukaryotic aryl sulfotransferases: Sulfation of quercetin and its derivatives. Chem. Cat. Chem. 2015, 7, 3152–3162. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are currently unavailable from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Yim, S.-H.; Han, F.; Kang, B.Y.; Choi, H.J.; Jung, D.-W.; Williams, D.R.; Gustafson, K.R.; Kennelly, E.J.; Lee, I.-S. Biotransformed Metabolites of the Hop Prenylflavanone Isoxanthohumol. Molecules 2019, 24, 394. https://doi.org/10.3390/molecules24030394
Kim HJ, Yim S-H, Han F, Kang BY, Choi HJ, Jung D-W, Williams DR, Gustafson KR, Kennelly EJ, Lee I-S. Biotransformed Metabolites of the Hop Prenylflavanone Isoxanthohumol. Molecules. 2019; 24(3):394. https://doi.org/10.3390/molecules24030394
Chicago/Turabian StyleKim, Hyun Jung, Soon-Ho Yim, Fubo Han, Bok Yun Kang, Hyun Jin Choi, Da-Woon Jung, Darren R. Williams, Kirk R. Gustafson, Edward J. Kennelly, and Ik-Soo Lee. 2019. "Biotransformed Metabolites of the Hop Prenylflavanone Isoxanthohumol" Molecules 24, no. 3: 394. https://doi.org/10.3390/molecules24030394
APA StyleKim, H. J., Yim, S.-H., Han, F., Kang, B. Y., Choi, H. J., Jung, D.-W., Williams, D. R., Gustafson, K. R., Kennelly, E. J., & Lee, I.-S. (2019). Biotransformed Metabolites of the Hop Prenylflavanone Isoxanthohumol. Molecules, 24(3), 394. https://doi.org/10.3390/molecules24030394