Advances in the Analysis of Veterinary Drug Residues in Food Matrices by Capillary Electrophoresis Techniques
Abstract
1. Introduction
2. Antibiotics
2.1. Nitroimidazoles
2.2. Fluoroquinolones
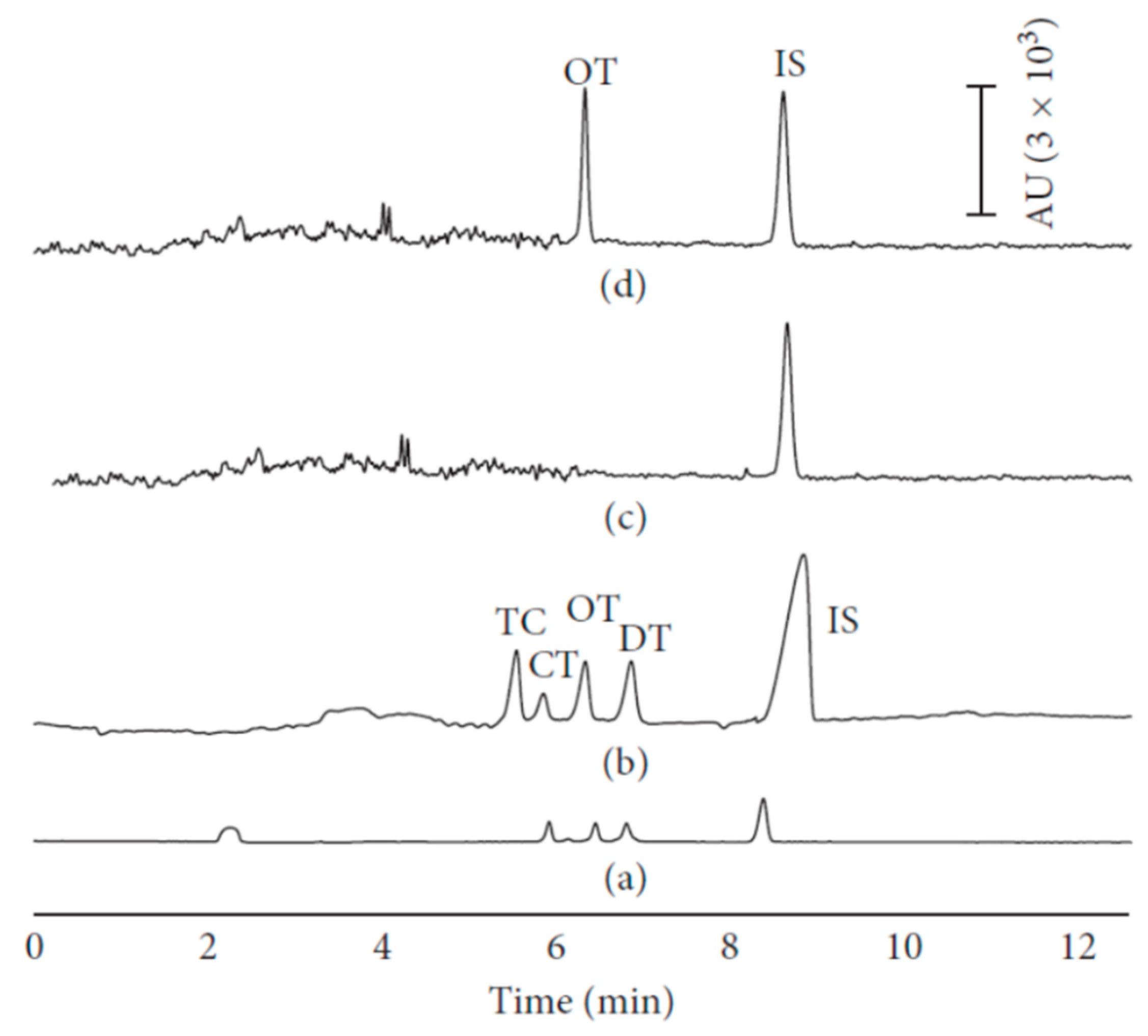
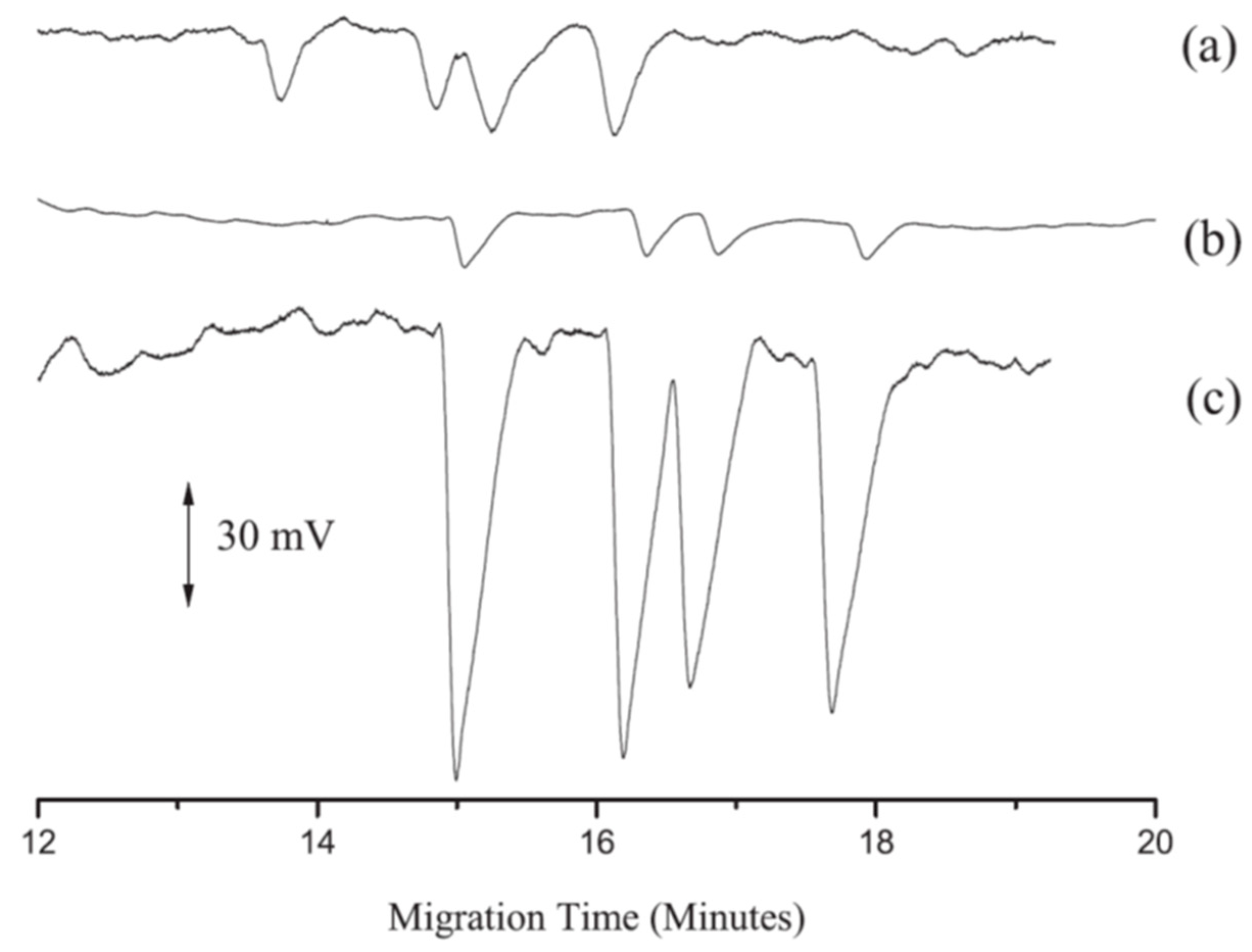
2.3. Tetracyclines
2.4. Sulfonamides
2.5. Aminoglycosides
2.6. Macrolides
2.7. β-Lactam Antibiotics
2.8. Simultaneous Analysis of Different Antibiotics
3. Other Drugs
3.1. Estrogens
3.2. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
3.3. β-Agonists
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Raza, N.; Kim, K.-H. Quantification techniques for important environmental contaminants in milk and dairy products. TrAC Trends Anal. Chem. 2018, 98, 79–94. [Google Scholar] [CrossRef]
- Commission Regulation (EU). On pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Commun. 2010, L15, 1–72. [Google Scholar]
- U.S. Food and Drug Administration. Chapter I: Food and Drugs Administration, Department of Health and Human Services, Subchapter E: Animal Drugs, Feed, and Related Products, Part 556: Tolerances for residues of New Animal Drugs in food, 2019.
- Codex Alimentarius. Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods, CX/MRL 2-2018.
- Lehotay, S.J.; Chen, Y. Hits and misses in research trends to monitor contaminants in foods. Anal. Bioanal. Chem. 2018, 410, 5331–5351. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU). Concerning the performance of analytical methods and interpretation of results. Off. J. Eur. Commun. 2002, L221, 8–36. [Google Scholar]
- Rodriguez, E.; Moreno-Bondi, M.C.; Marazuela, M.D. Multiresidue determination of fluoroquinolone antimicrobials in baby foods by liquid chromatography. Food Chem. 2011, 127, 1354–1360. [Google Scholar] [CrossRef]
- Robert, C.; Brasseur, P.-Y.; Dubois, M.; Delahaut, P.; Gillard, N. Development and validation of rapid multiresidue and multi-class analysis for antibiotics and anthelmintics in feed by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Food Addit. Contam.- Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1312–1323. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Meijer, T.; Mol, H.G.J.; van Ginkel, L.; Nielen, M.W.F. A global inter-laboratory study to assess acquisition modes for multi-compound confirmatory analysis of veterinary drugs using liquid chromatography coupled to triple quadrupole, time of flight and orbitrap mass spectrometry. Anal. Chim. Acta 2017, 962, 60–72. [Google Scholar] [CrossRef]
- Mainero Rocca, L. Veterinary drugs residues: A review of the latest analytical research on sample preparation and LC-MS based methods. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 766–784. [Google Scholar] [CrossRef]
- Marazuela, M.D. Determination of veterinary drug residues in foods by liquid chromatography-mass spectrometry: Basic and cutting-edge applications. In Liquid Chromatography: Applications, 2nd ed.; Fanali, S., Haddad, P.R., Poole, C., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 539–570. [Google Scholar]
- Baglai, A. Enhancing detectability of anabolic-steroid residues in bovine urine by actively modulated online comprehensive two-dimensional liquid chromatography – high-resolution mass spectrometry. Anal. Chim. Acta 2018, 1013, 87–97. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Escourrou, A.; Monteau, F.; Le Bizec, B.; Dervilly-Pinel, G. Current applications and perspectives of ion mobility spectrometry to answer chemical food safety issues. TrAC Trends Anal. Chem. 2017, 94, 39–53. [Google Scholar]
- Piňero, M.-Y.; Bauza, R.; Arce, L. Thirty years of capillary electrophoresis in food analysis laboratories: Potential applications. Electrophoresis 2011, 32, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Montero, L.; Llorens, L.; Castro-Puyana, M.; Cifuentes, A. Recent advances in the application of capillary electromigration methods for food analysis and Foodomics. Electrophoresis 2018, 39, 136–159. [Google Scholar] [CrossRef] [PubMed]
- Papetti, A.; Colombo, R. High-performance capillary electrophoresis for food quality evaluation. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Elsevier: Cambridge, UK, 2019; pp. 301–377. [Google Scholar]
- Stavrou, I.J.; Agathokleous, E.A.; Kapnissi-Christodoulou, C.P. Chiral selectors in CE: Recent development and applications (mid-2014 to mid-2016). Electrophoresis 2017, 38, 786–819. [Google Scholar] [CrossRef] [PubMed]
- Iacob, B.C.; Bodoki, E.; Oprean, R. Recent advances in capillary electrochromatography using molecularly imprinted polymers. Electrophoresis 2014, 35, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Tarongoy, F.M., Jr.; Haddad, P.R.; Quirino, J.P. Recent developments in open tubular capillary electrochromatography from 2016 to 2017. Electrophoresis 2018, 39, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Týčová, A.; Ledvina, V.; Klepárník, K. Recent advances in CE-MS coupling: Instrumentation, methodology, and applications. Electrophoresis 2017, 38, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Klepárník, K. Recent advances in combination of capillary electrophoresis with mass spectrometry: methodology and theory. Electrophoresis 2015, 36, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, A.; Schmitz, O.J.; Aboul-Enein, H.Y. Application of capillary electrophoresis with capacitively coupled contactless conductivity detection (CE-C4D): An update. Biomed. Chromatogr. 2017, 31, e3945. [Google Scholar] [CrossRef]
- Long, C.; Deng, B.; Sun, S.; Meng, S. Simultaneous determination of chlortetracycline, ampicillin and sarafloxacin in milk using capillary electrophoresis with electrochemiluminescence detection. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 24–31. [Google Scholar] [CrossRef]
- Ferey, L.; Delaunay, N. Food Analysis on Electrophoretic Microchips. Sep. Purif. Rev. 2016, 45, 193–226. [Google Scholar] [CrossRef]
- Breadmore, M.C.; Wuethrich, A.; Li, F.; Phung, S.C.; Kalsoom, U.; Cabot, J.M.; Tehranirokh, M.; Shallan, A.I.; Abdul Keyon, A.S.; See, H.H.; et al. Recent advances in enhancing the sensitivity of electrophoresis and electrochromatography in capillaries and microchips (2014–2016). Electrophoresis 2017, 38, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gan, N.; Shen, Z.; Cao, J.; Hu, F.; Li, T. Microchip electrophoresis based aptasensor for multiplexed detection of antibiotics in foods via a stir-bar assisted multi-arm junctions recycling for signal amplification. Biosens. Bioelectron. 2019, 130, 139–146. [Google Scholar]
- Šlampová, A.; Malá, Z.; Gebauer, P.E.; Boček, P. Recent progress of sample stacking in capillary electrophoresis (2014–2016). Electrophoresis 2017, 38, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Springer, V.; Jacksén, J.; Ek, P.; Lista, A.G.; Emmer, A. Determination of fluoroquinolones in bovine milk samples using a pipette-tip SPE step based on multiwalled carbon nanotubes prior to CE separation. J. Sep. Sci. 2014, 37, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Wei, S.; Yao, S.; Zhang, J.; Huang, H. Selective extraction and determination of fluoroquinolones in bovine milk samples with montmorillonite magnetic molecularly imprinted polymer and capillary electrophoresis. Anal. Bioanal. Chem. 2016, 408, 589–598. [Google Scholar] [CrossRef]
- Ramautar, R.; Somsen, G.W.; de Jong, G.J. Developments in coupled solid-phase extraction–capillary electrophoresis 2013–2015. Electrophoresis 2016, 37, 35–44. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Pellerano, R.G.; Pezza, L.; Redigolo Pezza, H. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 2018, 182, 1–21. [Google Scholar] [CrossRef]
- D’Orazio, G.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á.; Fanali, S. Evaluation of the combination of a dispersive liquid-liquid microextraction method with micellar electrokinetic chromatography coupled to mass spectrometry for the determination of estrogenic compounds in milk and yogurt. Electrophoresis 2015, 36, 615–625. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Liu, H.; Wu, X.; Li, J.; Chen, L. Dispersive liquid-liquid microextraction for four phenolic environmental estrogens in water samples followed by determination using capillary electrophoresis. Electrophoresis 2016, 37, 2502–2508. [Google Scholar] [CrossRef]
- Kitagawa, F.; Otsuka, K. Recent applications of on-line sample preconcentration techniques in capillary electrophoresis. J. Chromatogr. A 2014, 1335, 43–60. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sánchez-Brunete, C.; González, L. Pesticides: classification and properties. In Analysis of Pesticides in Food and Environmental Samples; Tadeo, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–34. [Google Scholar]
- U.S. Food and Drug Administration. Chapter I: Food and Drugs Administration, Department of Health and Human Services, Subchapter E: Animal Drugs, Feed, and Related Products, Part 530: Extralabel drugs use in animals, 2019.
- Mudry, M.D.; Martinez, R.A.; Nieves, M.; Carballo, M.A. Biomarkers of geno- toxicity and genomicin stability in a non-human primate, Cebus libidinosus (Cebidae, Platyrrhini), exposed to nitroimidazole derivatives. Mutat. Res. Genet. Toxicol. Environ. 2011, 721, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mesa, M.; Lara, F.J.; Cruces-Blanco, C.; García-Campaña, A.M. Determination of 5-nitroimidazole residues in milk by capillary electrochromatography with packed C18 silica beds. Talanta 2015, 144, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, F.; Ai, L. Simultaneous determination of seven nitroimidazole residues in meat byusing HPLC-UV detection with solid-phase extraction. J. Chromatogr. B 2007, 857, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Su, Y.; Liao, X.; Yang, N.; Yang, X.; Choi, M.M.F. Determination of five nitroimidazole residues in artificial porcine muscle tissue samples by capillary electrophoresis. Talanta 2012, 88, 646–652. [Google Scholar] [CrossRef]
- Tejada-Casado, C.; Hernández-Mesa, M.; del Olmo-Iruela, M.; García-Campaña, A.M. Capillary electrochromatography coupled with dispersive liquid-liquid microextraction for the analysis of benzimidazole residues in water samples. Talanta 2016, 161, 8–14. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Kim, K.-H.; Shamsipur, M.; Deep, A.; Hong, J. Recent advances in liquid-phase microextraction techniques for the analysis of environmental pollutants. TrAC Trends Anal. Chem. 2017, 97, 83–95. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Airado-Rodríguez, D.; Cruces-Blanco, C.; García-Campaña, A.M. Novel cation selective exhaustive injection-sweeping procedure for 5-nitroimidazole determination in waters by micellar electrokinetic chromatography using dispersive liquid-liquid microextraction. J. Chromatogr. A 2014, 9, 65–72. [Google Scholar] [CrossRef]
- Tejada-Casado, C.; Moreno-González, D.; Lara, F.J.; García-Campana, A.M. Monsalud del Olmo-Iruela, Determination of benzimidazoles in meat samples by capillary zoneelectrophoresis tandem mass spectrometry following dispersiveliquid–liquid microextraction. J. Chromatogr. A 2017, 1490, 212–219. [Google Scholar] [CrossRef]
- Airado-Rodríguez, D.; Hernández-Mesa, M.; García-Campaña, A.M.; Cruces-Blanco, C. Evaluation of the combination of micellar electrokinetic capillary chromatography with sweeping and cation selective exhaustive injection for the determination of 5-nitroimidazoles in egg samples. Food Chem. 2016, 213, 215–222. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Ahmed Siddique, M.B.; Kamal, A.; Coyne, M.S. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 704–720. [Google Scholar] [CrossRef]
- Golomb, B.A.; Koslik, H.J.; Redd, A.J. Fluoroquinolone-induced serious, persistent, multisymptom adverse effects. BMJ Case Rep 2015, 2015, bcr2015209821. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.C.; Taylor, M.J.; Smith, H.L. Prevalence and characteristics of hospital inpatients with reported fuoroquinolone allergy. Int. J. Clin. Pharm. 2018, 40, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Czyrski, A. Analytical Methods for Determining Third and Fourth Generation Fluoroquinolones: A Review. Chromatographia 2017, 80, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Piňero, M.-Y.; Garrido-Delgado, R.; Bauza, R.; Arce, L.; Valcárcel, M. Easy sample treatment for the determination of enrofloxacin and ciprofloxacin residues in raw bovine milk by capillary electrophoresis. Electrophoresis 2012, 33, 2978–2986. [Google Scholar] [CrossRef] [PubMed]
- De Quirós, A.R.-B.; Sendón, R. Molecularly imprinted polymers: Applications in food science. In Molecularly Imprinted Polymers (MIPs): Challenges, Uses and Prospects; Queen, T., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 1–42. [Google Scholar]
- Lombardo-Agüí, M.; García-Campaña, A.M.; Gámiz-Gracia, L.; Cruces Blanco, C. Laser induced fluorescence coupled to capillary electrophoresis for the determination of fluoroquinolones in foods of animal origin using molecularly imprinted polymers. J. Chromatogr. A 2010, 1217, 2237–2242. [Google Scholar] [CrossRef]
- Moreno-González, D.; Lara, F.J.; Gámiz-Gracia, L.; García-Campaña, A.M. Molecularly imprinted polymer as in-line concentrator in capillary electrophoresis coupled with mass spectrometry for the determination of quinolones in bovine milk samples. J. Chromatogr. A 2014, 1360, 1–8. [Google Scholar] [CrossRef]
- Lara, F.J.; García-Capaña, A.M.; Alés-Barrero, F.; Bosque-Sendra, J.M. In-line solid-phase extraction preconcentration in capillary electrophoresis-tandem mass spectrometry for the multiresidue detection íof quinolones in meat by pressurized liquid extraction. Electrophoresis 2008, 29, 2117–2125. [Google Scholar] [CrossRef]
- Xu, X.; Liu, L.; Jia, Z.; Shu, Y. Determination of enrofloxacin and ciprofloxacin in foods of animal origin by capillary electrophoresis with field amplified sample stacking–sweeping technique. Food Chem. 2015, 176, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Gasilova, N.; Qiao, L.; Zhou, Y.-L.; Zhang, X.-X.; Girault, H.H. Highly sensitive detection of five typical fluoroquinolones in low-fat milk byfield-enhanced sample injection-based CE in bubble cell capillary. Electrophoresis 2014, 35, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Herrera, A.V.; Hernández-Borges, J.; Borges-Miquel, T.M.; Rodríguez-Delgado, M.A. Dispersive liquid-liquid microextraction combined with nonaqueous capillary electrophoresis for the determination of fluoroquinolone antibiotics in waters. Electrophoresis 2010, 31, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Springer, V.H.; Lista, A.G. In-line coupled single drop liquid–liquid–liquid microextraction with capillary electrophoresis for determining fluoroquinolones in water samples. Electrophoresis 2015, 36, 1572–1579. [Google Scholar] [CrossRef]
- Ma, T.Y.; Vickroy, T.W.; Shien, J.H.; Chou, C.C. Improved nonaqueous capillary electrophoresis for tetracyclines at subparts per billion level. Electrophoresis 2012, 33, 1679–1682. [Google Scholar] [CrossRef]
- Deng, B.; Xu, Q.; Lu, H.; Ye, L.; Wang, Y. Pharmacokinetics and residues of tetracycline in crucian carp muscle using capillary electrophoresis on-line coupled with electrochemiluminescence detection. Food Chem. 2012, 134, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Quiroz, C.A.; Hernández-Chávez, J.F.; Ulloa-Mercado, G.; Gortáres-Moroyoqui, P.; Martínez-Macías, R.; Meza-Escalante, E.; Serrano-Palacios, D. Simultaneous quantification of antibiotics in wastewater from pig farms by capillary electrophoresis. J. Chromatogr. B 2018, 1092, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Islas, G.; Rodriguez, J.A.; Perez-Silva, I.; Miranda, J.M.; Ibarra, I.S. Solid-Phase Extraction and Large-Volume Sample Stacking-Capillary Electrophoresis for Determination of Tetracycline Residues in Milk. J. Anal. Methods Chem. 2018, 2018, 5394527. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, D.; Lupión-Enríquez, I.; Garcia-Campaňa, A.M. Trace determination of tetracyclines in water samples by capillary zone electrophoresis combining off-line and on-line sample preconcentratio. Electrophoresis 2016, 37, 1212–1219. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Z.; Huang, Z.; Shao, C. Large volume sample stacking of cationic tetracycline antibiotics toward 10 ppb level analysis by capillary electrophoresis with UV detection. Electrophoresis 2016, 37, 2963–2969. [Google Scholar] [CrossRef]
- Mu, G.; Liu, H.; Xu, L.; Tian, L.; Luan, F. Matrix Solid-Phase Dispersion Extraction and Capillary Electrophoresis Determination of Tetracycline Residues in Milk. Food Anal. Methods 2012, 5, 148–153. [Google Scholar] [CrossRef]
- Zhou, C.; Deng, J.; Shi, G.; Zhou, T. β-cyclodextrin-ionic liquid polymer based dynamically coating for simultaneous determination of tetracyclines by capillary electrophoresis. Electrophoresis 2017, 38, 1060–1067. [Google Scholar] [CrossRef]
- Casado-Terrones, S.; Segura-Carretero, A.; Busi, S.; Dinelli, G.; Fernández-Gutiérrez, A. Determination of tetracycline residues in honey by CZE with ultraviolet absorbance detection. Electrophoresis 2007, 28, 2882–2887. [Google Scholar] [CrossRef]
- Amin, N.C.; Blanchin, M.D.; Aké, M.; Fabre, H. Capillary electrophoresis methods for the analysis of antimalarials. Part II. Achiral separative methods. J. Chromatogr. A 2013, 1276, 1–11. [Google Scholar] [CrossRef]
- Mikus, P.; Maráková, K.; Veizerová, L.; Piešt’anský, J. Determination of quinine in beverages by online coupling capillary isotachophoresis to capillary zone electrophoresis with UV spectrophotometric detection. J. Sep. Sci. 2011, 34, 3392–3398. [Google Scholar] [CrossRef]
- Kluska, M.; Marciniuk-Kluska, A.; Prukala, D.; Prukala, W. Analytics of quinine and its derivatives. Crit. Rev. Anal. Chem. 2016, 46, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Huang, S.H.; Singco, B.; Huang, H.-Y. Analyses of sulfonamide antibiotics in meat samples by on-line concentration capillary electrochromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 7640–7647. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Wang, Q.; He, C.; Zhou, L.; Wang, J.; Pu, Q. Rapid and sensitive determination of sulfonamide residues in milk and chicken muscle by microfluidic chip electrophoresis. J. Agric. Food Chem. 2012, 60, 1613–1618. [Google Scholar] [CrossRef]
- Dai, T.; Duan, J.; Li, X.; Xu, X.; Shi, H.; Kang, W. Determination of Sulfonamide Residues in Food by Capillary Zone Electrophoresis with On-Line Chemiluminescence Detection Based on an Ag(III) Complex. Int. J. Mol. Sci. 2017, 18, 1286. [Google Scholar] [CrossRef] [PubMed]
- Farouk, F.; Azzazy, H.M.E.; Niessen, W.M.A. Challenges in the determination of aminoglycoside antibiotics, a review. Anal. Chim. Acta 2015, 890, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, D.; Lara, F.J.; Jurgovská, N.; Gámiz-Gracia, L.; García-Campana, A.M. Determination of aminoglycosides in honey by capillary electrophoresis tandem mass spectrometry and extraction with molecularly imprinted polymers. Anal. Chim. Acta 2015, 891, 321–328. [Google Scholar] [CrossRef]
- Liu, H.; Li, N.; Liu, X.; Qian, Y.; Qiu, J.; Wang, X. Poly(N-acryloyl-glucosamine-co-methylenebisacrylamide)-based hydrophilic magnetic nanoparticles for the extraction of aminoglycosides in meat samples. J. Chromatogr. A 2019, in press. [Google Scholar] [CrossRef]
- Zhu, G.; Bao, C.; Liu, W.; Yan, X.; Liu, L.; Xiao, J.; Chen, C. Rapid detection of ags using microchip capillary electrophoresis contactless conductivity detection. Curr. Pharm. Anal. 2019, 15, 9–16. [Google Scholar] [CrossRef]
- Commission Regulation (EU). On measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. Off. J. Eur. Commun. 1996, 125, 10–32. [Google Scholar]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Greko, C.; Moreno, M.A.; Pomba, M.C.; Rantala, M.; Ružauskas, M.; Sanders, P.; et al. Macrolides and lincosamides in cattle and pigs: Use and development of antimicrobial resistance. Vet. J. 2014, 200, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Şanli, S.; Palabiyik, I.M.; Şanli, N.; Guzel-Seydim, Z.B.; Alsancak, G. Optimization of the experimental conditions for macrolide antibiotics in high performance liquid chromatography by using response surface methodology and determination of tylosin in milk samples. J. Anal. Chem. 2011, 66, 838–847. [Google Scholar] [CrossRef]
- Dickson, L.C.; O’Byrne, C.; Chan, W. A quantitative method for residues of macrolide antibiotics in porcine kidney by liquid chromatography/ tandem mass spectrometry. J. AOAC Int. 2012, 9, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-Q.; Guo, X.; Chen, G.-H.; Zhou, J.-W.; Zou, X.-M.; Liao, X.; Hou, T. Determination of five macrolide antibiotic residues in milk by micellar electrokinetic capillary chromatography with field amplified sample stacking. J. Food Saf. 2018, 38, e12382. [Google Scholar] [CrossRef]
- Lorenzetti, A.S.; Lista, A.G.; Domini, C.E. Reverse ultrasound-assisted emulsification-microextraction of macrolides from chicken fat followed by electrophoretic determination. LWT-Food Sci. Technol. 2019, 113, 108334. [Google Scholar] [CrossRef]
- Cameron-Veas, K.; Solà-Ginés, M.; Moreno, M.A.; Fraile, L.; Migura-Garcia, L. Impact of the use of β-lactam antimicrobials on the emergence of Escherichia coli isolates resistant to cephalosporins under standard pig-rearing conditions. Appl. Environ. Microbiol. 2015, 81, 1782–1787. [Google Scholar] [CrossRef]
- Cameron-Veas, K.; Moreno, M.A.; Fraile, L.; Migura-Garcia, L. Shedding of cephalosporin resistant Escherichia coli in pigs from conventional farms after early treatment with antimicrobials. Vet. J. 2016, 211, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Piñero, M.-Y.; Bauza, R.; Arce, L.; Valcárcel, M. Determination of penicillins in milk of animal origin by capillary electrophoresis: Is sample treatment the bottleneck for routine laboratories. Talanta 2014, 119, 75–82. [Google Scholar] [CrossRef]
- Hancu, G.; Sasebeşi, A.; Rusu, A.; Kelemen, H.; Ciurba, A. Study of the electrophoretic behavior of cephalosporins by Capillary Zone Electrophoresis. Adv. Pharm. Bull. 2015, 5, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; He, W.; Zhang, L.; Duan, C. Analysis of penicillin and its β-lactamase hydrolysis products in milk using capillary zone electrophoresis. Anal. Methods 2015, 7, 4602–4607. [Google Scholar] [CrossRef]
- Liu, W.-L.; Wu, C.-Y.; Li, Y.-T.; Huang, H.-Y. Penicillin analyses by capillary electrochromatography-mass spectrometry with different charged poly(stearylmethacrylate–divinylbenzene) monoliths as stationary phases. Talanta 2012, 101, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Rao, Q.; Zhu, K.; Jang, Z.; Ding, S. Simultaneous determination of five tetracycline and macrolide antiobiotics in feeds using HPCE. J. Sep. Sci. 2009, 32, 4254–4260. [Google Scholar] [CrossRef]
- Regulation (EC) No 1831/2003, Annex I: list of additives, Edition 11/2019 (277).
- Vera-Candioti, L.; Olivieri, A.C.; Goicoechea, H.C. Development of a novel strategy for preconcentration of antibiotic residues in milk and their quantitation by capillary electrophoresis. Talanta 2010, 82, 213–221. [Google Scholar] [CrossRef]
- Kowalski, P.; Plenis, A.; Oledzka, I.; Konieczna, L. Optimization and validation of the micellar electrokinetic capillary chromatographic method for simultaneous determination of sulfonamide and amphenicol-type drugs in poultry tissue. J. Pharm. Biomed. Anal. 2011, 54, 160–170. [Google Scholar] [CrossRef]
- Liu, L.; Wan, Q.; Xu, X.; Duan, S.; Yang, C. Combination of micelle collapse and field-amplified sample stacking in capillary electrophoresis for determination of trimethoprim and sulfamethoxazole in animal-originated foodstuffs. Food Chem. 2017, 219, 7–12. [Google Scholar] [CrossRef]
- He, T.; Xu, Z.; Ren, J. Pressure-assisted electrokinetic injection stacking for seven typical antibiotics in waters to achieve μg/L level analysis by capillary electrophoresis with UV detection. Microchem. J. 2019, 146, 1295–1300. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Wu, W.; Yuan, X.; Wu, X.; Lin, X.; Xie, Z. Analysis of phenolic xenoestrogens by pressurized CEC with amperometric detection. Electrophoresis 2010, 31, 1011–1018. [Google Scholar] [CrossRef]
- European Commission. Council Regulation 324/2004/EC.2005. Off. J. Eur. Commun. 2004, L58, 16. [Google Scholar]
- Alshana, U.; Göğer, N.G.; Ertaş, N. Dispersive liquid–liquid microextraction combined with field-amplified sample stacking in capillary electrophoresis for the determination of non-steroidal anti-inflammatory drugs in milk and dairy products. Food Chem. 2013, 138, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Nechaeva, D.; Bulatov, A. HPLC-MS/MS determination of non-steroidal anti-inflammatory drugs in bovine milk based on simultaneous deep eutectic solvents formation and its solidification. Microchem. J. 2019, 150, 104080. [Google Scholar] [CrossRef]
- Espina-Benitez, M.; Araujo, L.; Prieto, A.; Navalón, A.; Vílchez, J.L.; Valera, P.; Zambrano, A.; Dugas, V. Development of a new microextraction fiber combined to on-line sample stacking capillary electrophoresis UV detection for acidic drugs determination in real water samples. Int. J. Environ. Res. Public Health 2017, 14, 739. [Google Scholar] [CrossRef] [PubMed]
- Anurukvorakun, O.; Buchberger, W.; Himmelsbach, M.; Klampel, C.W.; Suntornsuk, L. A sensitive non-aqueous capillary electrophoresis-mass spectrometric method for multiresidue analyses of beta-agonists in pork. Biomed. Chromatogr. 2010, 24, 588–599. [Google Scholar] [PubMed]
- Wang, C.-C.; Lu, C.-C.; Chen, Y.-L.; Cheng, H.-L.; Wu, S.-M. Chemometric optimization of cation-selective exhaustive injection sweeping micellar electrokinetic chromatography for quantification of ractopamine in porcine meat. J. Agric. Food Chem. 2013, 61, 5914–5920. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-Y.; Wang, C.-C.; Kou, H.-S.; Wu, S.M. Dialkyl anionic surfactant in field-amplified sample injection andsweeping-micellar electrokinetic chromatography for determinationof eight leanness-promoting β-agonists in animal feeds. J. Pharm. Biomed. Anal. 2017, 141, 222–228. [Google Scholar] [CrossRef]
- Gao, F.; Wu, M.; Zhang, Y.; Wang, G.; Wang, Q.; He, P.; Fang, Y. Sensitive determination of four β2-agonists in pig feed by capillary electrophoresis using on-line sample preconcentration with contactless conductivity detection. J. Chromatogr. B 2014, 973, 29–32. [Google Scholar] [CrossRef]

| CE-Technique | Food Matrix | Sensitivity | Ref. |
|---|---|---|---|
| Nitroimidazoles | |||
| LLE-SPE-CEC-UV | bovine milk | LOQ: 19–96 (μg/L) | [38] |
| DLLME-CEC-UV | water | LOQ: 5.7–9.3 (μg/L) | [41] |
| DLLME-CZE-MS/MS | poultry and porcine meat | LOQ: 4–16 (μg/kg) | [44] |
| DLLME-CSEI-sweep-MEKC-UV | water | LOQ: 2.05–8.14 (ng/mL) | [43] |
| SPE-CSEI-sweep-MEKC-UV | egg | LOQ: 6.99–16.8 (ng/g) | [45] |
| Fluoroquinolones | |||
| SPE-CZE-UV | bovine milk | LOD: 7.5–11.6 (μg/L) | [28] |
| MMMIPs-CZE-UV | bovine milk | LOD: 12.9–18.8 (μg/L) | [29] |
| PPT/SPE-CZE-UV | bovine milk | LOQ: 0.06–0.1 (mg/kg) | [50] |
| MISPE-CZE-LIF | bovine milk, pig kidney | LOQ: 0.55–35 (μg/kg) | [52] |
| MISPE-CZE-MS/MS | bovine milk | LOQ: 3.2–4.7 (μg/kg) | [53] |
| PLE-SPE-CZE-MS/MS | meat | LOQ: 130–470 (ng/kg) | [54] |
| FASS-sweep-CZE-UV | milk, meat | LOD: 5.70, 7.39 (ng/mL) | [55] |
| FESI-CZE-UV and CZE-MS | bovine milk | LOQ: 2.3–8.3 (μg/kg) | [56] |
| DLLME-NACE-UV | water | LOQ: 5.43–461 (μg/L) | [57] |
| SD-LLLME-NACE-UV | water | LOD: 10.1, 55.3 (μg/L) | [58] |
| Tetracyclines | |||
| CZE-ECL | fish | LOD: 1.8 ng/mL | [60] |
| FASI-CZE-UV | water | LOQ: 23–59 μg/L | [61] |
| SPE-LVSS-PS-CZE-UV | milk | LOD: 18.60–23.83 (μg/L) | [62] |
| MSPD-CZE-UV | milk | LOD: 0.0745–0.0808 (μg/mL) | [65] |
| SPE-LVSS-PS-CZE-UV | water | LOQ: 67–167 (ng/L) | [63] |
| LVSS-CZE-UV | water | LOD: 8.1–14.5 (μg/L) | [64] |
| CZE-AD | water | LOD: 0.33–0.67 (μM) | [66] |
| SPE- CZE-UV | honey | LOD: 23.9–49.3 (μg/kg) | [67] |
| Sulfonamides | |||
| SPE-CEC-MS | meat | LOD: 0.01–0.14 (μg/L) | [71] |
| microchip-CE-LIF | milk, meat | LOQ: 0.6–7.7 (μg/L) | [72] |
| SPE-CZE-ECL | milk, meat | LOD: 0.62–3.14 (μg/mL) | [73] |
| CZE-UV and CZE-MS | meat, water | LOD: 0.33–180 (μg/L) | [68] |
| CITP-CZE-UV | beverages, water | LOD: 2.29 (ng/mL) | [69] |
| Aminoglycosides | |||
| MISPE-FASS-CZE-MS/MS | honey | LOQ: 1.4–94.8 (μg/kg) | [75] |
| microchip-CE-CCD | standard solutions | LODs: 0.89–3.1 (μg/mL) | [77] |
| Macrolides | |||
| FASS-MEKC-UV | milk | LOD: 0.002–0.004 (mg/kg) | [83] |
| R-USAEME-CZE-DAD | chicken fat | LOQ: 22.1–47.0 (μg/kg) | [84] |
| β-lactam antibiotics (penicillins) | |||
| PPT/CZE-UV | milk | LOQ: 0.04–1.7 (μg/mL) | [89] |
| ASEI-CEC-MS | milk | LOD: 0.05–0.2 (μg/L) | [90] |
| β-lactam antibiotics (cephalosporins) | |||
| CZE-UV | complex matrices | LOQ: 4.33–8.00 (μg/mL) | [88] |
| Estrogens | |||
| SPE-p-CEC-AD | bovine milk, diary products | LOD: 2–50 (ng/mL) | [98] |
| DLLME-MEKC-ESI-MS/MS | bovine and goat milk, diary products | LOD: 1–61 (μg/L) | [32] |
| DLLME-MEKC-UV | water | LOD: 0.3–0.6 (μg/L) | [33] |
| NSAIDs | |||
| DLLME-FASS-CZE-UV | bovine milk, dairy products | LOQ: 10–43.7 (μg/kg) | [99] |
| SPME-CZE-UV | water | LOQ: 2.91–3.86 (μg/L) | [102] |
| β-agonists | |||
| SPE-NACE-MS | meat | LOD: 0.3 (ppb) | [103] |
| CSEI-sweep-MEKC-UV | meat | LOD: 3–5 (ng/g) | [104] |
| FASI-sweep-MEKC-UV | commercial animal feeds | LOD: 5–20 (ng/mL) | [105] |
| FESI-CE-C4D | pig feed | LOD: 0.02 (mg/L) | [106] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, R.; Papetti, A. Advances in the Analysis of Veterinary Drug Residues in Food Matrices by Capillary Electrophoresis Techniques. Molecules 2019, 24, 4617. https://doi.org/10.3390/molecules24244617
Colombo R, Papetti A. Advances in the Analysis of Veterinary Drug Residues in Food Matrices by Capillary Electrophoresis Techniques. Molecules. 2019; 24(24):4617. https://doi.org/10.3390/molecules24244617
Chicago/Turabian StyleColombo, Raffaella, and Adele Papetti. 2019. "Advances in the Analysis of Veterinary Drug Residues in Food Matrices by Capillary Electrophoresis Techniques" Molecules 24, no. 24: 4617. https://doi.org/10.3390/molecules24244617
APA StyleColombo, R., & Papetti, A. (2019). Advances in the Analysis of Veterinary Drug Residues in Food Matrices by Capillary Electrophoresis Techniques. Molecules, 24(24), 4617. https://doi.org/10.3390/molecules24244617






