Traceability of Geographical Origin in Gentiana straminea by UPLC-Q Exactive Mass and Multivariate Analyses
Abstract
1. Introduction
2. Results
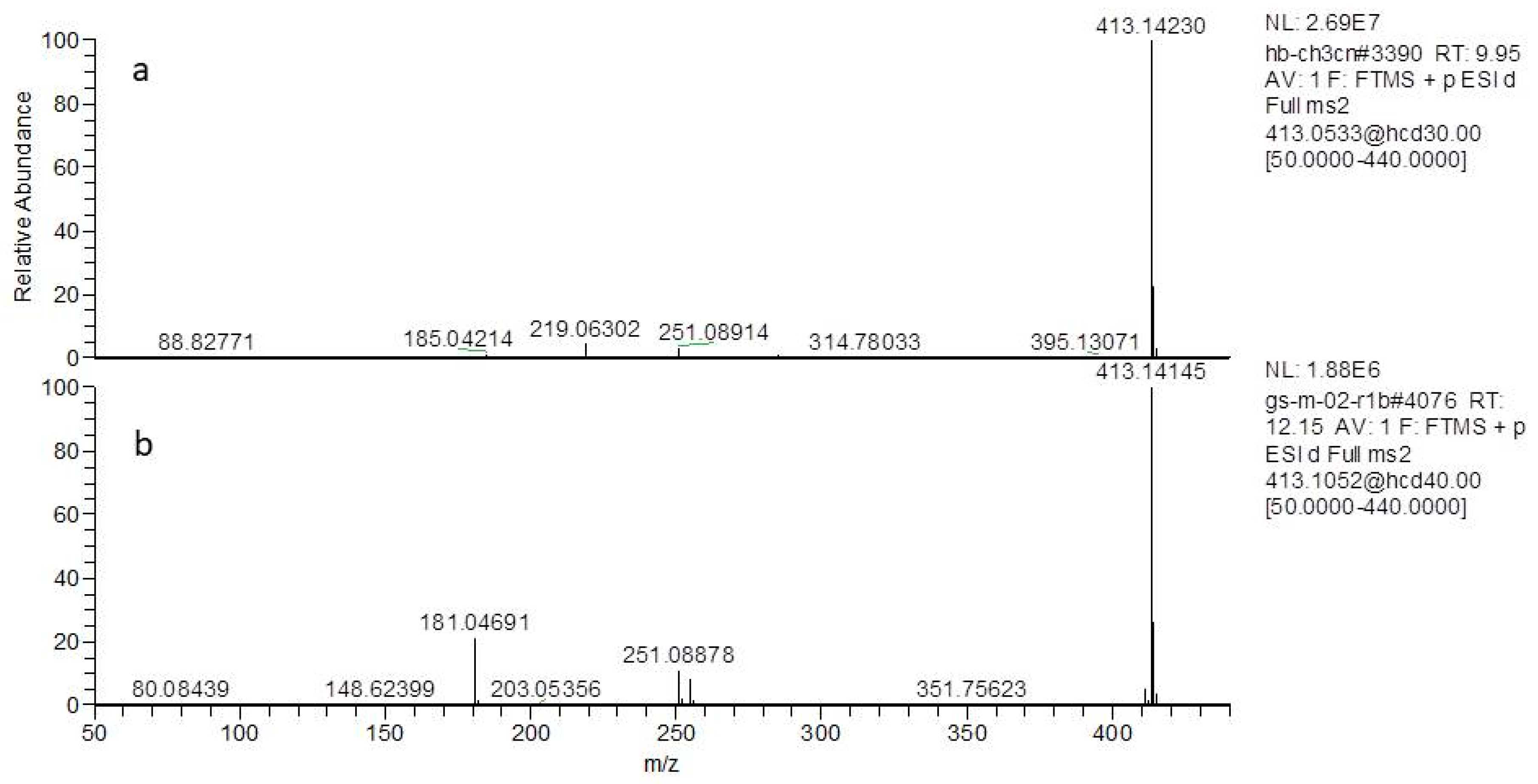
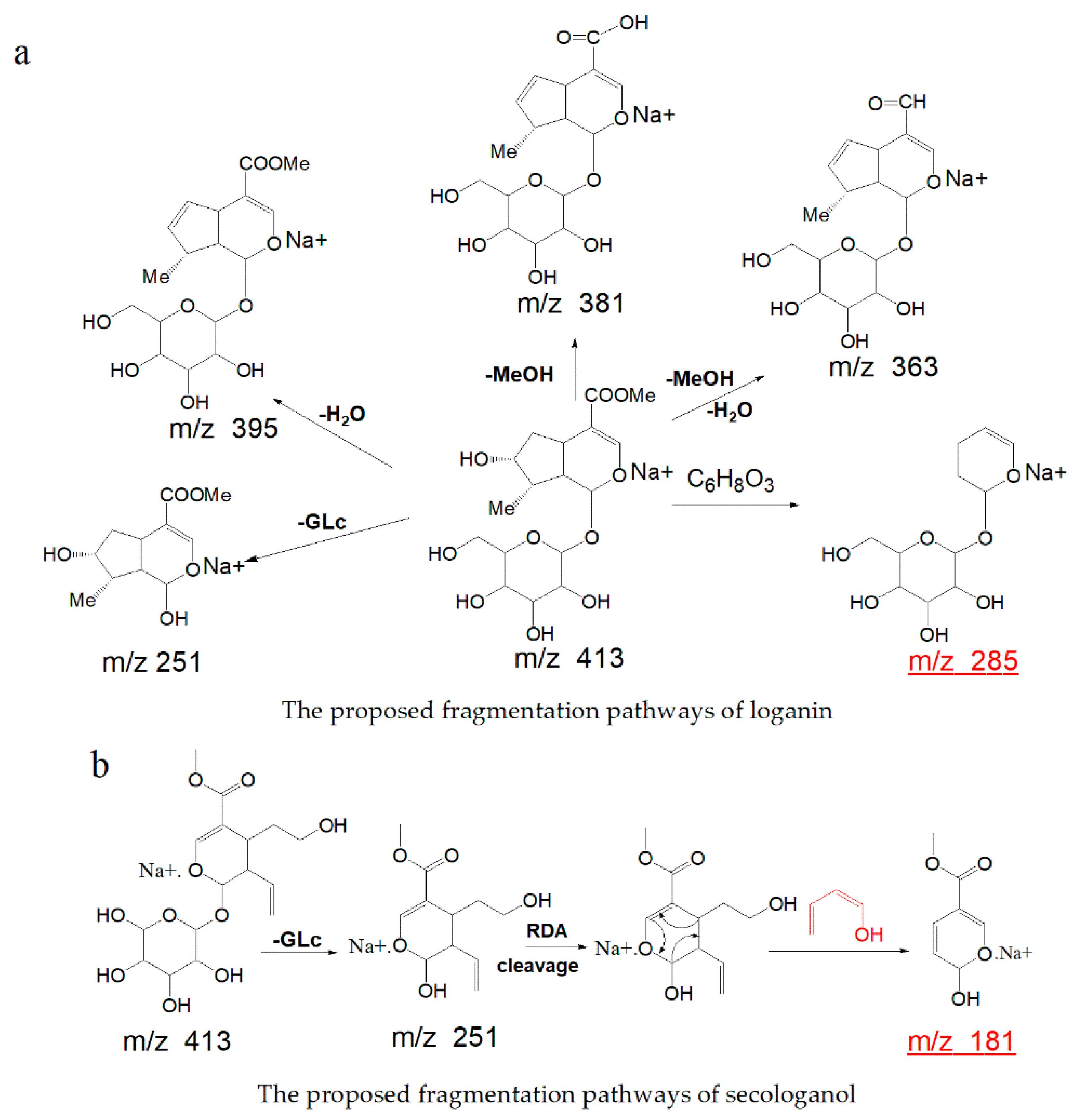
2.1. Identification of the Constituents in G. straminea by UPLC-Q Exactive Mass Spectrometer
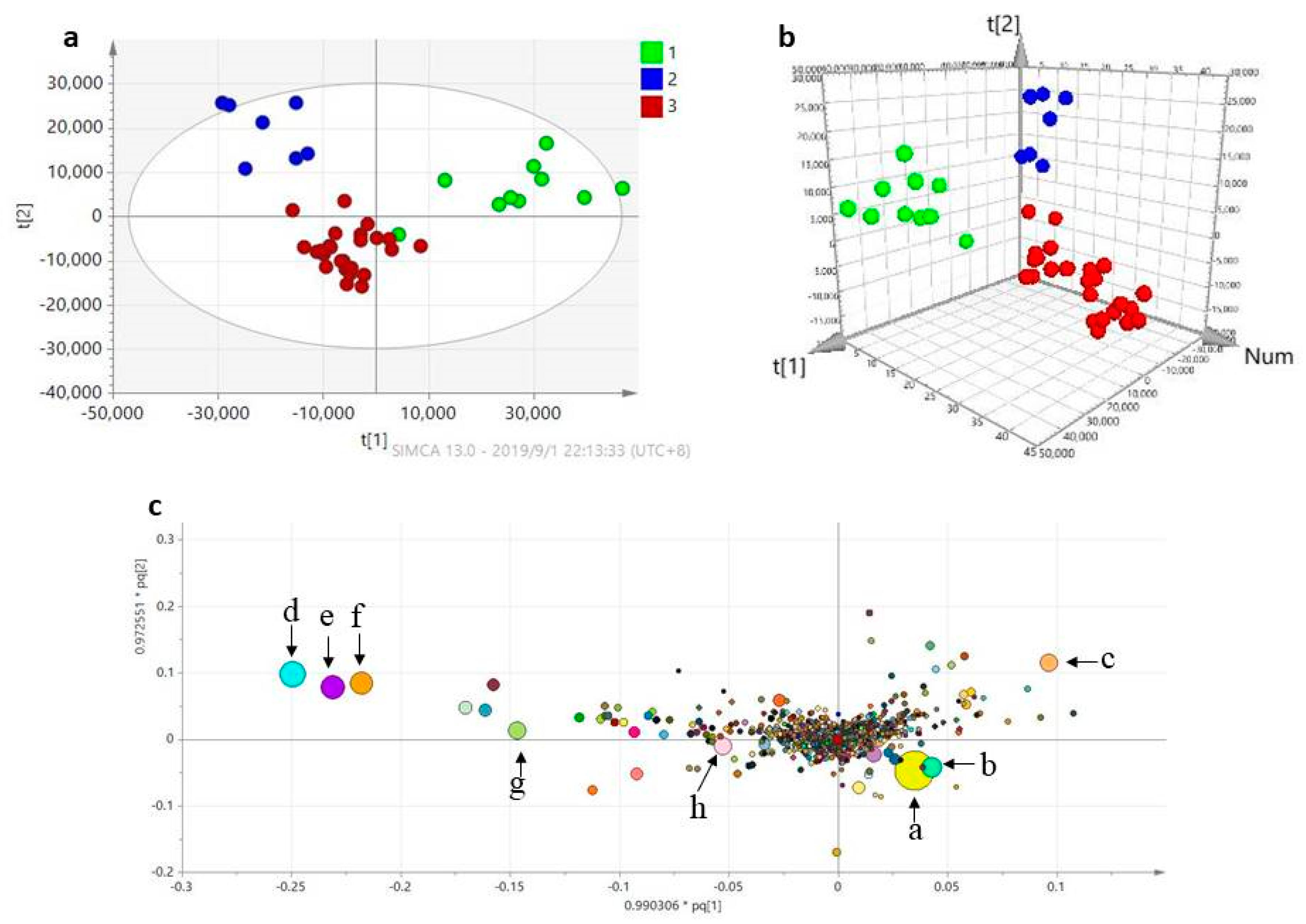
2.2. Multivariate Analysis of the Global Metabolomics Data
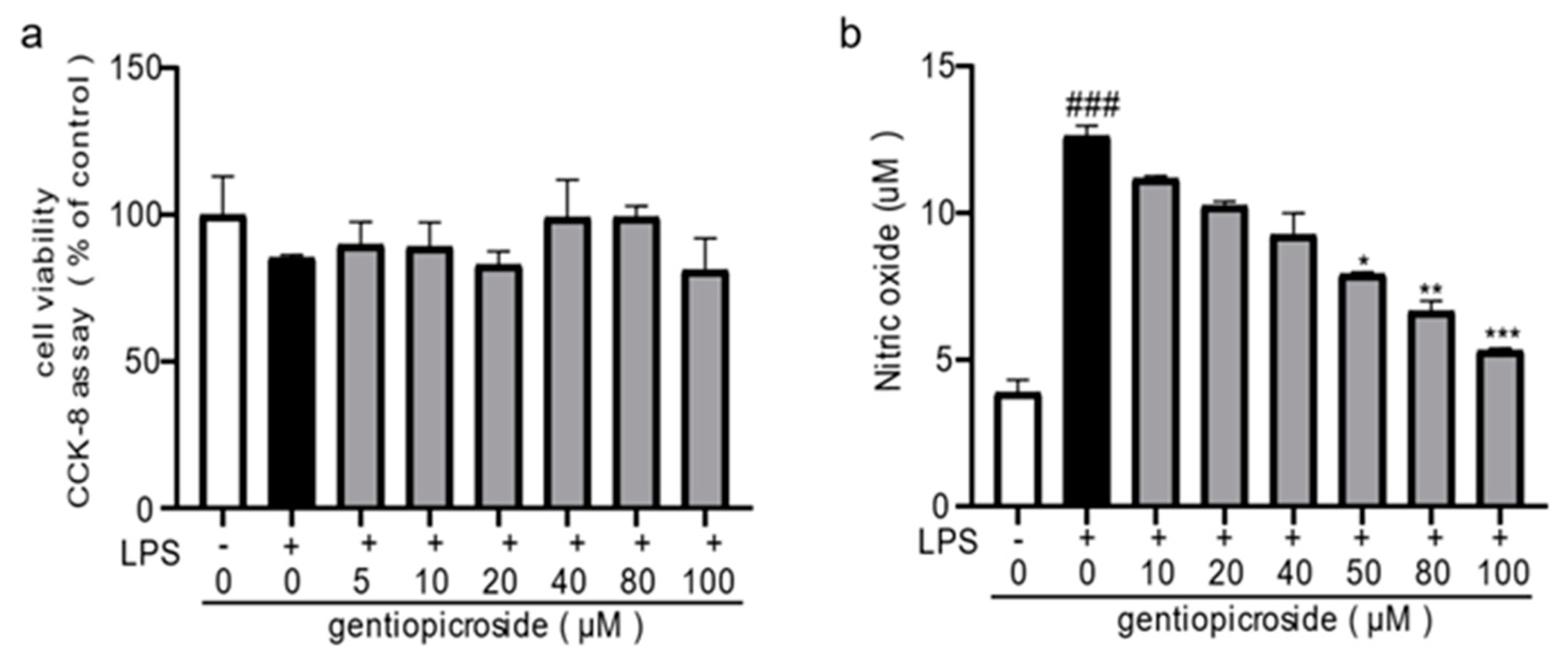
2.3. Anti-Inflammatory Effect of Characterize Components
3. Conclusions
4. Discussion
5. Materials and Methods
5.1. Plant Materials, Reagents, and Chemicals
5.2. Sample Preparation
5.3. LC-MS/MS Analysis
5.4. Data Processing and Statistical Analysis
5.5. Cell Culture and Cell Viability Measurement
5.6. Nitric Oxide (NO) Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Corvucci, F.; Nobili, L.; Melucci, D.; Grillenzoni, F.V. The discrimination of honey origin using melissopalynology and Raman spectroscopy techniques coupled with multivariate analysis. Food Chem. 2015, 169, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace solid-phase micro -extractiongas chromatographic–time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomics as a complement to phylogenetics for assessing intraspecific boundaries in the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia (Myrothamnaceae). Phytochemistry 2019, 159, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Zhan, G.Q.; Jin, M.; Zhang, H.; Dang, J.; Zhang, Y.; Guo, Z.J.; Ito, Y.C. Botany, traditional use, phytochemistry, pharmacology, quality control, and authentication of Radix Gentianae Macrophyllae-A traditional medicine: A review. Phytomedicine 2018, 46, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Xu, M.; Wang, D.; Yang, C.R.; Zeng, Y.; Zhang, Y.J. Anti-inflammatory compounds of “Qin-Jiao”, the roots of Gentiana dahurica (Gentianaceae). J. Ethnopharmacol. 2013, 147, 341–348. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Chen, S.; Hu, F.; Zhou, D. Spatial variation profiling of four phytochemical constituents in Gentiana straminea (Gentianaceae). J. Nat. Med. 2014, 68, 38–45. [Google Scholar] [CrossRef]
- Kakuda, R.; Iijima, T.; Yaoita, Y.; Machida, K.; Kikuchi, M. Secoiridoid glycosides from Gentiana scabra. J. Nat. Prod. 2001, 12, 1574–1575. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.L.; Zhang, J.; Li, W.Y.; Wang, Y.Z. Phytochemistry and pharmacological activities of the Genus Gentiana (Gentianaceae). Chem. Biodivers. 2016, 13, 107–150. [Google Scholar] [CrossRef]
- Wu, J.R.; Zhao, Z.L.; Wu, L.H.; Wang, Z.T. Authentication of Gentiana straminea Maxim. and its substitutes based on chemical profiling of iridoids using liquid chromatography with mass spectrometry. Biomed. Chromatogr. 2016, 30, 2061–2066. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, P.; Feng, X.; Kodama, H.; Yu, C.; Chen, G. Qualitative and quantitative determination of ten iridoids and secoiridoids in Gentiana straminea Maxim. by LC-UV-ESI-MS. J. Nat. Med. 2012, 66, 102–108. [Google Scholar] [CrossRef]
- Pan, Z.; Fan, G.; Yang, R.P.; Luo, W.Z.; Zhou, X.D.; Zhang, Y. Discriminating Lamiophlomis rotata according to geographical origin by 1H-NMR spectroscopy and multivariate analysis. Phytochem. Anal. 2015, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, Y.L.; Jiang, S.; Chen, Y.W.; Zhang, Y.; Pan, Z. The similarity and variability of iridoid glycosides profile and antioxidant capacity of aerial and underground parts from Lamiophlomis rotata using UPLC-TOF-MS and multivariate analyses. RSC Adv. 2018, 8, 2459–2468. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.L.; Chen, Y.L.; Chen, Y.W.; Zhang, Y.; Xiang, L.; Pan, Z. Lamiophlomis rotata Identification via ITS2 Barcode and Quality Evaluation by UPLC- QTOF-MS Couple with Multivariate Analyses. Molecules 2018, 23, 3289. [Google Scholar]
- Chen, G.; Wei, S.H.; Yu, C.Y. Secoiridoids from the roots of Gentiana straminea. Biochem. Syst. Ecol. 2009, 37, 766–771. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, M.; Zhang, Y.J.; Yang, C.R. New Acylated Secoiridoid Glucosides from Gentiana straminea (Gentianaceae). Helv. Chim. Acta 2009, 92, 321–327. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Liu, H.L.; Liu, X.Q.; Shang, J.N.; Zhao, J.; Yuan, C.S. Chemical constituents of Gentiana macrophylla Pall. Nat. Prod. Res. 2010, 24, 1365–1369. [Google Scholar] [CrossRef]
- He, Y.M.; Zhu, S.; Ge, Y.W.; Cai, S.Q.; Komatsu, K. Secoiridoid glycosides from the root of Gentiana crassicaulis with inhibitory effects against LPS-induced NO and IL-6 production in RAW264 macrophages. J. Nat. Med. 2015, 69, 366–374. [Google Scholar] [CrossRef]
- Tan, R.X.; Kong, L.D.; Wei, H.X. Secoiridoid glycosides and an antifungal anthranilate derivative from Gentiana tibetica. Phytochemistry 1998, 47, 1223–1226. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, J.; Mei, L.J.; Shao, Y.; Wang, Q.L.; Liu, X.J. Chemical constituents from roots of Gentiana straminea of Tibetan Medicine. J. Chin. Med. Mater. 2016, 39, 103–106. [Google Scholar] [CrossRef]
- Fan, H.; Zang, Y.; Zhang, Y.; Zhang, H.F.; Zhao, Z.; Hu, J.F. Triterpenoids and Iridoid Glycosides from Gentiana dahurica. Helv. Chim. Acta 2010, 93, 2439–2447. [Google Scholar] [CrossRef]
- Mandova, T.; Audo, G.; Michel, S.; Grougnet, R. Off-line coupling of new generation centrifugal partition chromatography device with preparative high pressure liquid chromatography-mass spectrometry triggering fraction collection applied to the recovery of secoiridoid glycosides from Centaurium erythraea Rafn. (Gentianaceae). J. Chromatogr. A 2017, 1513, 149–156. [Google Scholar] [PubMed]
- Wang, S.F.; Xu, Y.M.; Chen, P.H.; Zhang, Y.F. Structural characterization of secoiridoid glycosides by high performance liquid chromatography/electrospray ionization. Rapid Commun. Mass Spectrom. 2014, 28, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Mehrotra, N.; Asthana, R.K.; Gupta, R.C. Liquid chromatography/tandem mass spectrometric study and analysis of xanthone and secoiridoid glycoside composition of Swertia chirata, a potent antidiabetic. Rapid Commun. Mass Spectrom. 2006, 20, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zeng, W.L.; He, C.Y.; Annie Bligh, S.W.; Liu, Q.; Yang, L.; Wang, Z.T. Characterization of metabolites of sweroside in rat urine using ultra-high-perfor mance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry and NMR spectroscopy. J. Mass Spectrom. 2014, 49, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Wang, X.S.; Zhao, M.G.; Huang, X.X.; Xu, X.L. Gentiopicroside protects neurons from astrocyte-mediated inflammatory injuries by inhibition of nuclear factor-κB and mitogen-activated protein kinase signaling pathways. Neuroreport 2018, 29, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, Z.; Lan, X.; Liao, Z.; Chen, M. Sweroside Alleviated LPS-Induced Inflammation via SIRT1 Mediating NF-κB and FOXO1 Signaling Pathways in RAW264.7 Cells. Molecules 2019, 24, 872. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission. Chinese Pharmacopoeia; Chemical and Technologic Press: Beijing, China, 2015; pp. 270–271. ISBN 9787506744393. [Google Scholar]
- Patel, N.; Tyagi, R.K.; Tandel, N.; Garg, N.; Soni, N. The Molecular Targets of Swertiamarin and its Derivatives Confer Anti-Diabetic and Anti-Hyperlipidemic Effects. Curr. Drug Targets 2018, 19, 1958–1967. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Tao, Y.; Liu, C. Swertiamarin Attenuates Experimental Rat Hepatic Fibrosis by Suppressing Angiotensin II-Angiotensin Type 1 Receptor-ERK Signaling. J. Pharmacol. Exp. Ther. 2016, 395, 247–255. [Google Scholar] [CrossRef]
- Ma, L.Q.; Yu, Y.; Chen, H.; Li, M.; Ihsan, A.; Tong, H.Y.; Huang, X.J.; Gao, Y. Sweroside Alleviated Aconitine-Induced Cardiac Toxicity in H9c2 Cardiomyoblast Cell Line. Front. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, H.; Meng, B.; Zhu, B.; Yu, X.; Xiang, G. Sweroside-mediated mTORC1 hyperactivation in bone marrow mesenchymal stem cells promotes osteogenic differentiation. Cell. Biochem. 2019. [Google Scholar] [CrossRef]
- Mihailović, V.; Mihailović, M.; Uskoković, A.; Arambašić, J.; Mišić, D.; Stanković, V.; Katanić, J.; Mladenović, M.; Solujić, S.; Matić, S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2013, 52, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.X.; Liang, M.L.; Fang, Y.; Zhao, F.; Tian, J.S.; Zhang, X.; Qin, X.M. Deciphering the Differential Effective and Toxic Responses of Bupleuri Radix following the Induction of Chronic Unpredictable Mild Stress and in Healthy Rats Based on Serum Metabolic Profiles. Front. Pharmacol. 2018, 8, 995. [Google Scholar] [CrossRef] [PubMed]
- He, H.Q.; Wu, Y.X.; Nie, Y.J.; Wang, J.; Ge, M.; Qian, F. LYRM03, an ubenimex derivative, attenuates LPS-induced acute lung injury in mice by suppressing the TLR4 signaling pathway. Acta Pharmacol. Sin. 2017, 38, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Gentiana straminea are available from the authors. |
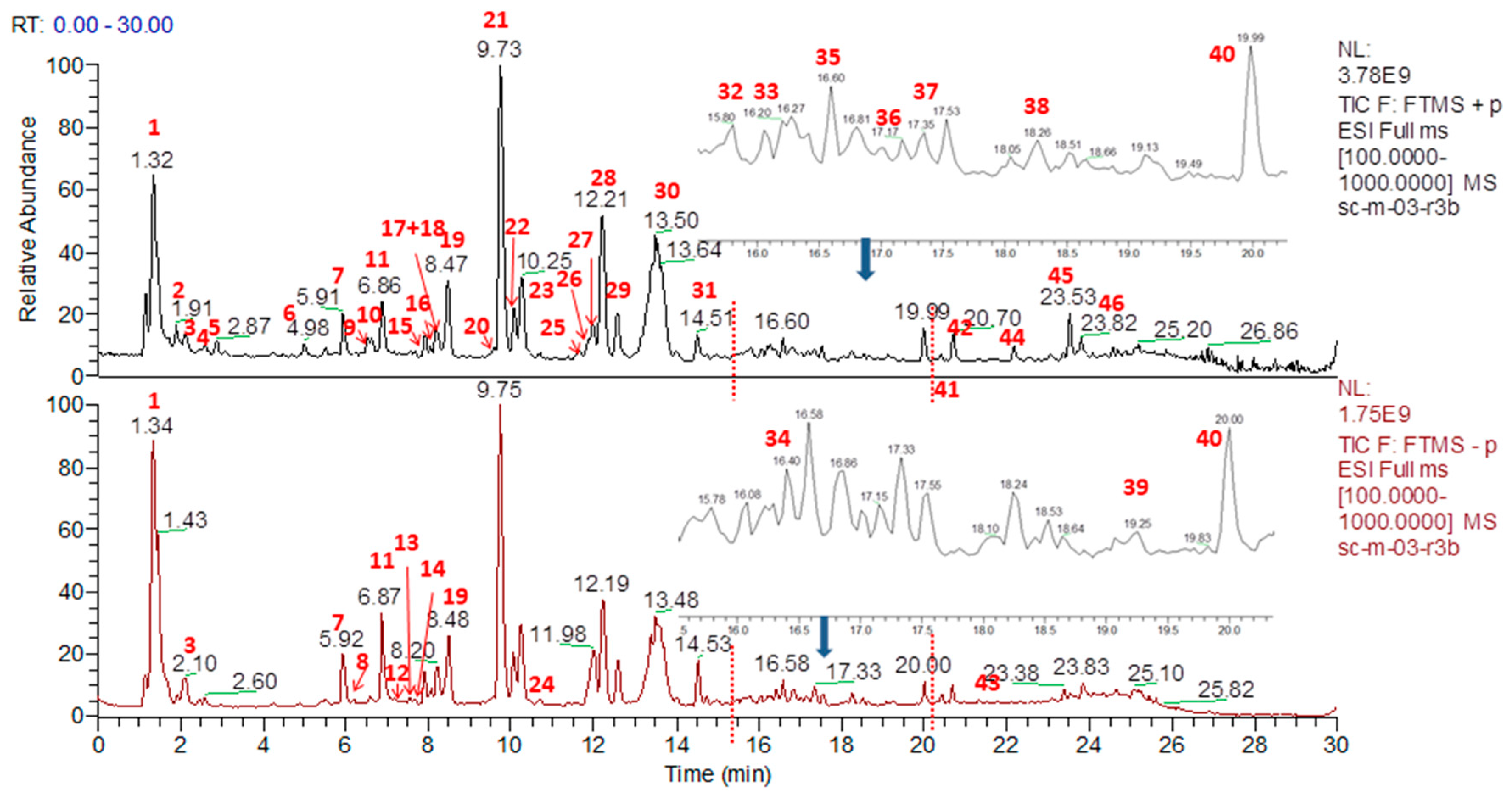
| Peak No. | RT (min) | Compound | Formula | Calculated (Da) | Selected Ion | Precursor Ion (Da) | Mass Accuracy (ppm) | Class |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.33 | gentiobiose | C12H22O11 | 342.11622 | [M + Na]+ | 365.10510 | −0.25 | sugars |
| 2 | 1.91 | gentianose | C18H32O16 | 504.16904 | [M + Na]+ | 527.15784 | −0.19 | sugars |
| 3 | 2.12 | morroniside | C17H26O11 | 406.14752 | [M + H]+ | 407.13815 | −4.22 | iridoids |
| 4 | 2.41 | eustomorusside | C17H24O11 | 408.12678 | [M + Na]+ | 431.11603 | −0.13 | secoiridoids |
| 5 | 2.66 | miserotoxin | C9H17O8N | 267.09542 | [M + Na]+ | 290.08566 | 0.15 | alkaloids |
| 6 | 4.88 | gladiatoside C1 | C29H26O12 | 566.14243 | [M + Na]+ | 589.13708 | 0.82 | flavanoids |
| 7 | 5.85 | kingsidic acide | C16H22O11 | 390.11622 | [M + Na]+ | 413.10522 | −0.19 | iridoids |
| 8 | 6.20 | secologanic acide | C16H24O10 | 374.12130 | [M + Na]+ | 397.11047 | −0.16 | secoiridoids |
| 9 | 6.49 | 6′-O-β-d-glucopyranosyl loganic acide | C22H34O15 | 538.18978 | [M + Na]+ | 561.17889 | −0.12 | iridoids |
| 10 | 6.59 | 6′-O-acetylgentiopicroside | C16H24O12 | 398.12130 | [M + Na]+ | 431.15219 | −0.76 | iridoids |
| 11 | 6.87 | loganic acide | C18H22O10 | 376.13695 | [M + Na]+ | 399.12595 | −0.20 | iridoids |
| 12 | 7.10 | kushenol I | C16H24O10 | 454.19916 | [M + Na]+ | 477.17078 | −3.81 | flavanoids |
| 13 | 7.29 | 1-O-β-d-gulcopyranosyl-4-epiamplexin | C15H22O10 | 362.12130 | [M + Na]+ | 385.11685 | 1.49 | secoiridoids |
| 14 | 7.63 | macrophylloside A | C27H24O9 | 492.14204 | [M + H]+ | 493.14243 | −1.50 | secoiridoids |
| 15 | 7.91 | 8-epi-kingsidic acide | C16H22O11 | 390.11622 | [M + Na]+ | 413.10532 | −0.17 | iridoids |
| 16 | 8.02 | paederotoside | C28H34O15 | 633.18252 | [M + Na]+ | 633.1796 | 0.46 | iridoids |
| 17 | 8.16 | 6′-O-β-d-glucopyranosyl gentiopicroside | C22H30O14 | 518.16356 | [M + Na]+ | 541.15302 | −0.06 | secoiridoids |
| 18 | 8.29 | isovitexin-7-O-β-d-glucopyranoside | C27H30O15 | 594.15848 | [M + Na]+ | 617.14746 | −0.13 | flavanoids |
| 19 | 8.51 | swertiamarin | C16H20O10 | 374.12130 | [M + Na]+ | 397.11060 | −0.13 | secoiridoids |
| 20 | 9.58 | 2′-acetylswertiamarin | C18H24O11 | 416.13187 | [M + Na]+ | 439.12115 | −0.12 | secoiridoids |
| 21 | 9.78 | gentiopicroside | C16H20O9 | 356.11074 | [M + Na]+ | 379.09982 | −0.19 | secoiridoids |
| 22 | 9.95 | loganin | C17H26O10 | 390.15260 | [M + Na]+ | 413.14166 | −0.18 | iridoids |
| 23 | 10.28 | sweroside | C16H22O9 | 358.12639 | [M + Na]+ | 381.11551 | −0.18 | secoiridoids |
| 24 | 10.67 | coniferin | C16H22O8 | 342.13147 | [M + Na]+ | 365.11405 | −1.98 | lignin |
| 25 | 10.85 | Unknown | C15H24ON2 | 248.18886 | [M + Na]+ | 271.17780 | −0.32 | alkaloids |
| 26 | 11.85 | Unknown | C18H18O10 | 394.09000 | [M + Na]+ | 417.07639 | −0.82 | -- |
| 27 | 12.03 | Homoorientin | C21H20O11 | 448.10057 | [M + Na]+ | 471.08981 | −0.12 | flavanoids |
| 28 | 12.35 | Secologanol | C17H26O10 | 390.15260 | [M + Na]+ | 413.14172 | −0.16 | secoiridoids |
| 29 | 12.61 | qinjiaoside A | C17H24O11 | 404.13187 | [M + Na]+ | 427.12112 | −0.13 | secoiridoids |
| 30 | 13.36 | 8-epikingisde/7-ketologanin | C17H24O10 | 388.13695 | [M + Na]+ | 411.12610 | −0.16 | iridoids |
| 31 | 14.54 | vitexin/isovitexin | C21H20O10 | 432.10565 | [M + Na]+ | 455.09482 | −0.14 | flavanoids |
| 32 | 15.80 | olivieroside A | C25H26O11 | 502.14752 | [M + H]+ | 503.13646 | −3.75 | secoiridoids |
| 33 | 16.20 | pneumonanthoside | C19H30O7 | 370.19916 | [M + Na]+ | 393.18814 | −0.21 | lignin |
| 34 | 16.40 | isoorientin-4′-O-glucoside | C27H30O16 | 610.15339 | [M + Na]+ | 633.12128 | −3.46 | flavanoids |
| 35 | 16.60 | saprosmoside H | C34H42O21S | 818.19394 | [M + Na]+ | 841.21564 | 3.79 | secoiridoids |
| 36 | 17.17 | 6-p-coumaroy barlerin | C28H34O14 | 594.19486 | [M + Na]+ | 617.16246 | −3.60 | iridoids |
| 37 | 17.36 | 7-O-feruloylorientin | C31H28O14 | 624.14791 | [M + Na]+ | 647.13690 | −0.13 | flavanoids |
| 38 | 18.26 | flavoconomelin | C28H32O15 | 608.17413 | [M + Na]+ | 631.14191 | −3.49 | flavanoids |
| 39 | 19.27 | alboside III | C22H32O15 | 536.17413 | [M + Na]+ | 559.1639 | 2.36 | secoiridoids |
| 40 | 19.99 | rindoside | C35H42O21 | 798.22187 | [M + Na]+ | 821.21045 | −0.15 | secoiridoids |
| 41 | 20.40 | unknown | C20H30O5 | 350.20933 | [M + Na]+ | 373.19589 | −0.87 | -- |
| 42 | 20.70 | triforoside | C35H42O20 | 782.22695 | [M + Na]+ | 805.21564 | −0.14 | secoiridoids |
| 43 | 21.29 | oliveramine | C20H20N2O4 | 352.14231 | [M + Na]+ | 375.12127 | −2.89 | alkaloids |
| 44 | 22.15 | 2-methoxyanofinic acid | C14H16O4 | 248.10486 | [M + Na]+ | 271.08786 | −2.51 | phenolic acides |
| 45 | 23.53 | 1β,2α,3α,24-tetrahydroxyursa-12,20(30)-dien-28-oic acid | C30H46O6 | 502.32944 | [M + H]+ | 503.33640 | −0.17 | triterpenoids |
| 46 | 23.81 | 1β,2α,3α,24-tetrahydroxyurs-12-en-28-oic acid | C30H48O6 | 504.34509 | [M + H]+ | 505.35211 | −0.15 | triterpenoids |
| Location | Longitude (E) | Latitude (N) | Altitude (m) | No. of Samples |
|---|---|---|---|---|
| Gansu | 101.9308–104.7733 | 33.36501–34.526 | 2905–3572 | 10 |
| Qinghai | 96.6487–101.7353 | 33.7805–33. 9361 | 3516–3789 | 7 |
| Sichuan | 98.7732–100.5236 | 31.0219–33.2510 | 3400–3796 | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Xiong, F.; Chen, Y.-L.; Wan, G.-G.; Zhang, Y.; Chen, Z.-W.; Cao, W.-F.; Zhou, G.-Y. Traceability of Geographical Origin in Gentiana straminea by UPLC-Q Exactive Mass and Multivariate Analyses. Molecules 2019, 24, 4478. https://doi.org/10.3390/molecules24244478
Pan Z, Xiong F, Chen Y-L, Wan G-G, Zhang Y, Chen Z-W, Cao W-F, Zhou G-Y. Traceability of Geographical Origin in Gentiana straminea by UPLC-Q Exactive Mass and Multivariate Analyses. Molecules. 2019; 24(24):4478. https://doi.org/10.3390/molecules24244478
Chicago/Turabian StylePan, Zheng, Feng Xiong, Yi-Long Chen, Guo-Guo Wan, Yi Zhang, Zhi-Wei Chen, Wen-Fu Cao, and Guo-Ying Zhou. 2019. "Traceability of Geographical Origin in Gentiana straminea by UPLC-Q Exactive Mass and Multivariate Analyses" Molecules 24, no. 24: 4478. https://doi.org/10.3390/molecules24244478
APA StylePan, Z., Xiong, F., Chen, Y.-L., Wan, G.-G., Zhang, Y., Chen, Z.-W., Cao, W.-F., & Zhou, G.-Y. (2019). Traceability of Geographical Origin in Gentiana straminea by UPLC-Q Exactive Mass and Multivariate Analyses. Molecules, 24(24), 4478. https://doi.org/10.3390/molecules24244478






