Valorization of Lignin as an Immobilizing Agent for Bioinoculant Production using Azospirillum brasilense as a Model Bacteria
Abstract
:1. Introduction
2. Results
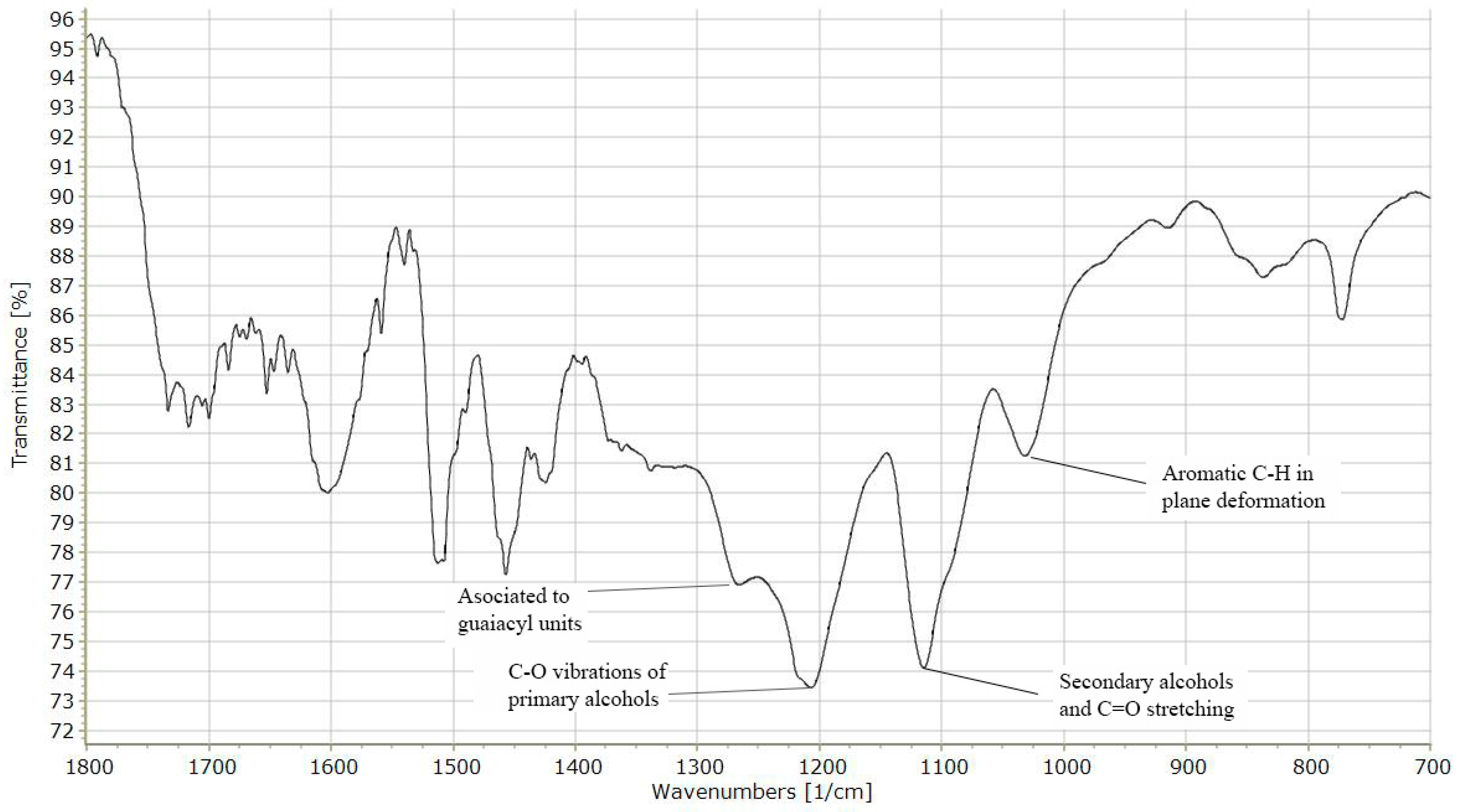
2.1. Characteristics of Lignin
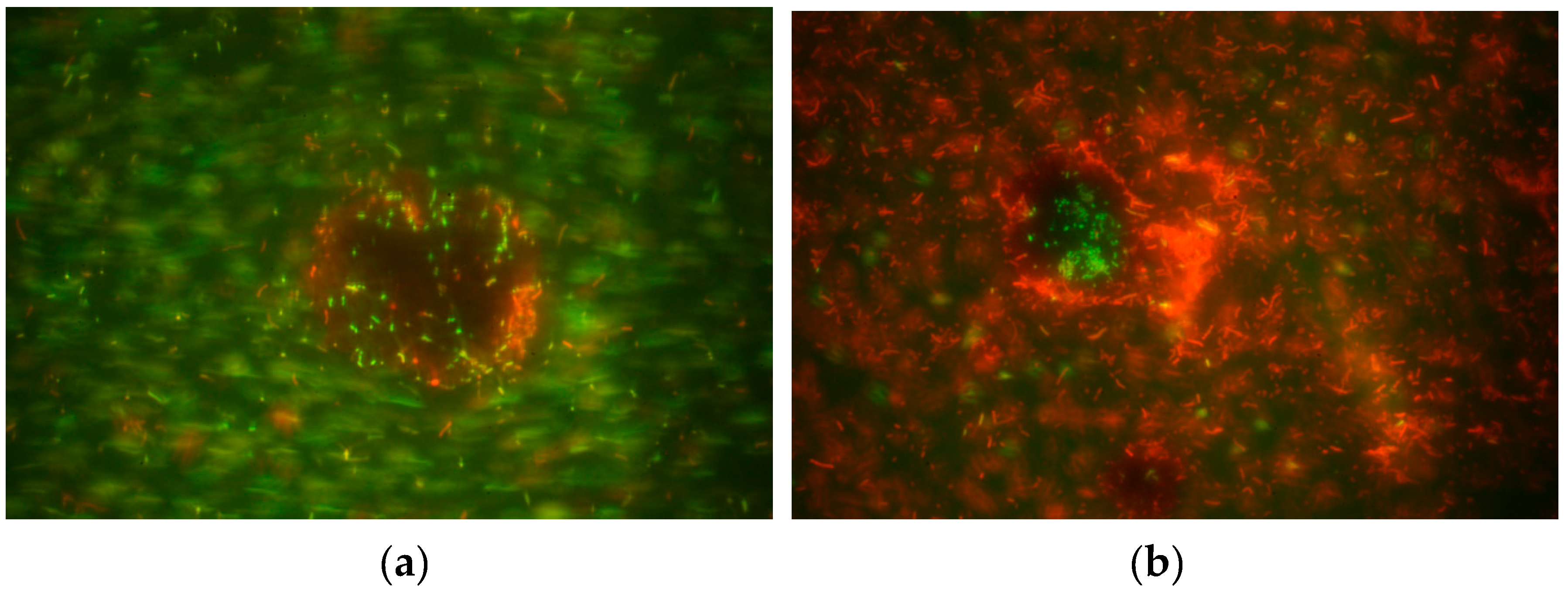
2.2. Cell Immobilization in Lignin Particles
2.3. Cell Enumeration in Lignin Particles
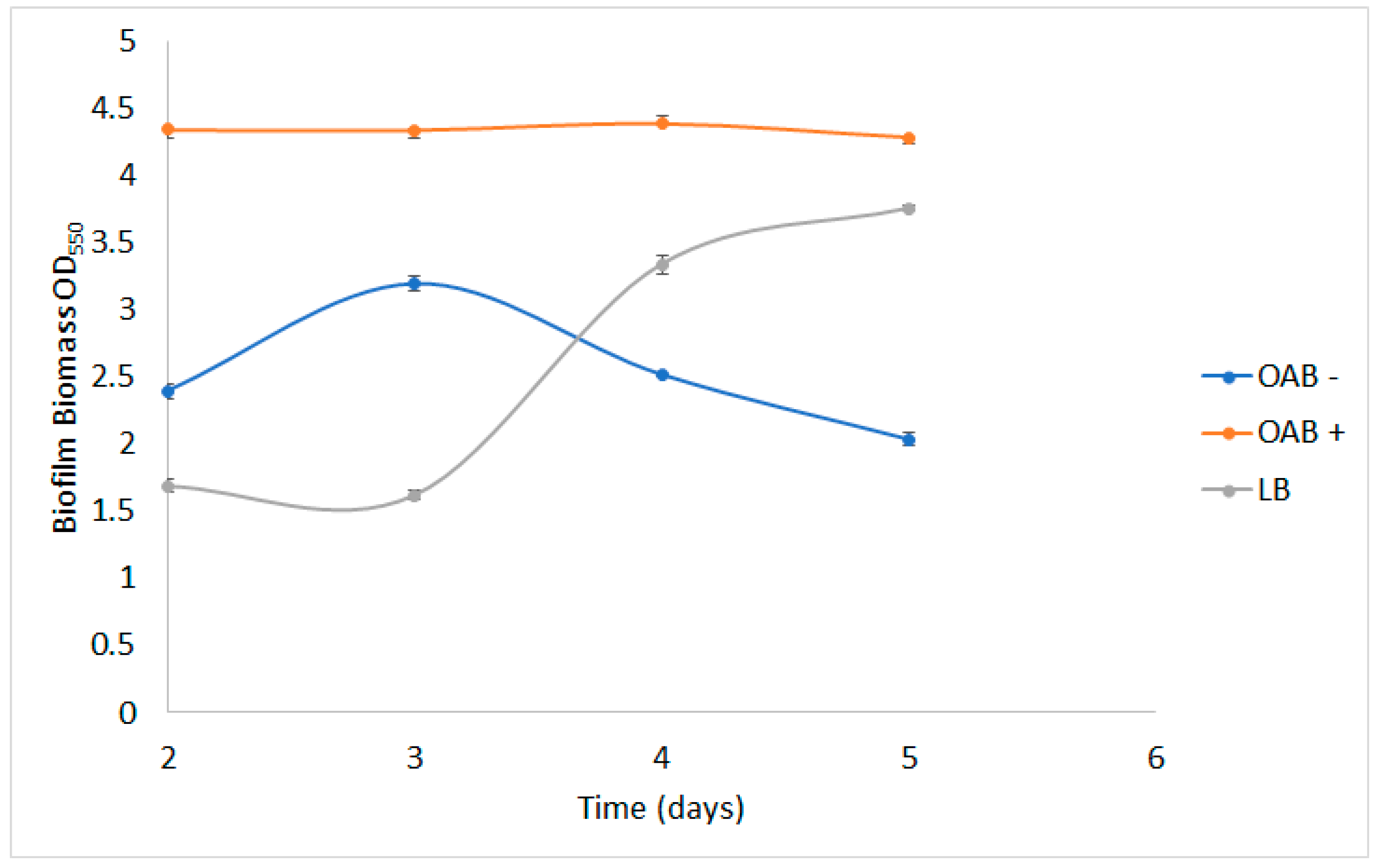
2.4. Biofilm Formation in Lignin Particles
3. Discussion
4. Materials and Methods
4.1. Lignin Extraction and Characterization
4.2. Growth and Immobilization of Azospirillum Brasilense
4.3. Cell Quantification
4.4. Biofilm Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Miransari, M. Soil microbes and plant fertilization. Appl. Microbiol. Biotechnol. 2011, 92, 875–885. [Google Scholar] [CrossRef]
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Löbermann, B.; Malusà, E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996. [Google Scholar] [CrossRef] [PubMed]
- Temprano, F.J.; Albareda, M.; Rodrı, D.N. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar]
- Singh, R.; Arora, N.K. Bacterial Formulations and Delivery Systems against Pests in Sustainable Agro-Food Production. In Reference Module in Food Science, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Jastrz, M.; Górecka, E.; Jastrzębska, M. Review article: Immobilization techniques and biopolymer carriers. Biotechnol. Food Sci. 2011, 75, 65–86. [Google Scholar]
- Gotovtsev, P.M.; Yuzbasheva, E.Y.; Gorin, K.V.; Butylin, V.V.; Badranova, G.U.; Perkovskaya, N.I.; Mostova, E.B.; Namsaraev, Z.B.; Rudneva, N.I.; Komova, A.V.; et al. Immobilization of microbial cells for biotechnological production: Modern solutions and promising technologies. Appl. Biochem. Microbiol. 2015, 51, 792–803. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Kuhn, E.; Tucker, M.P.; Yang, B. Production of Jet Fuel-Range Hydrocarbons from Hydrodeoxygenation of Lignin over Super Lewis Acid Combined with Metal Catalysts. ChemSusChem 2018, 11, 285–291. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Zhai, R.; Wen, Z.; Jin, M. Recent advances in lignin valorization with bacterial cultures: Microorganisms, metabolic pathways, and bio-products. Biotechnol. Biofuels 2019, 12, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation - A comprehensive evaluation. Appl. Soil Ecol. 2012, 61, 171–189. [Google Scholar] [CrossRef]
- Jahan, M.S.; Chowdhury, D.A.N.; Islam, M.K.; Moeiz, S.M.I. Characterization of lignin isolated from some nonwood available in Bangladesh. Bioresour. Technol. 2007, 98, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Savy, D.; Piccolo, A. Physical-chemical characteristics of lignins separated from biomasses for second-generation ethanol. Biomass and Bioenergy 2014, 62, 58–67. [Google Scholar] [CrossRef]
- Valdespino-Rodríguez, Á.; Pérez-Martín, D.J.A.; González-Barajas, M.F.; Villicaña-Ambriz, H.; Campos-Castellanos, D.; Escalante, F.M.E.; Sánchez, A.; Aguilar-Garnica, E. Optimization of ethyl lactate-based organosolv process for lignin recovery from the solid waste of a wheat straw biorefinery. J. Chem. Technol. Biotechnol. 2019, 32220. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A. Cell immobilization on lignin-polyvinylpyrrolidone material for anaerobic digestion. Environ. Eng. Sci. 2019, 36, 478–490. [Google Scholar] [CrossRef]
- Zur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic responses of bacterial cells to immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xu, A.; Elmerich, C.; Ma, L.Z. Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions. ISME J. 2017, 11, 1602–1613. [Google Scholar] [CrossRef] [Green Version]
- Cassán, F.D.; Okon, Y.; Creus, C.M. Handbook for Azospirillum; Cassán, F.D., Okon, Y., Creus, C.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-06541-0. [Google Scholar]
- Zhang, X.; Zhao, H.; Zhang, J.; Li, Z. Growth of Azotobacter vinelandii in a solid-state fermentation of technical lignin. Bioresour. Technol. 2004, 95, 31–33. [Google Scholar] [CrossRef]
- Bashan, Y.; Levanony, H. An improved selection technique and medium for the isolation and enumeration of Azospirillum brasilense. Can. J. Microbiol. 1985, 31, 947–952. [Google Scholar] [CrossRef]
- Piccinin, G.G.; Braccini, A.L.; Dan, L.G.M.; Scapim, C.A.; Ricci, T.T.; Bazo, G.L. Efficiency of seed inoculation with Azospirillum brasilense on agronomic characteristics and yield of wheat. Ind. Crops Prod. 2013, 43, 393–397. [Google Scholar] [CrossRef]
- Bashan, Y.; Trejo, A.; de-Bashan, L.E. Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol. Fertil. Soils 2011, 47, 963–969. [Google Scholar] [CrossRef]
- Schoebitz, M.; Simonin, H.; Poncelet, D. Starch filler and osmoprotectants improve the survival of rhizobacteria in dried alginate beads. J. Microencapsul. 2012, 29, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Andreou, L.V. Preparation of genomic DNA from bacteria. Methods Enzymol. 2013, 529, 143–151. [Google Scholar]
- O’Toole, G.A. Microtiter dish Biofilm formation assay. J. Vis. Exp. 2010, 10–11. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G.; Lifshitz, R. Isolation and Characterization of Plant Growth-Promoting Rhizobacteria. In Methods in Plant Molecular Biology and Biotechnology; Glick, B.R., Thompson, J.E., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 331–345. ISBN 0849351642. [Google Scholar]
Sample Availability: Samples of lignin are not generally available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Olivares, V.R.; Vazquez-Bello, E.A.; Aguilar-Garnica, E.; Escalante, F.M.E. Valorization of Lignin as an Immobilizing Agent for Bioinoculant Production using Azospirillum brasilense as a Model Bacteria. Molecules 2019, 24, 4613. https://doi.org/10.3390/molecules24244613
Tapia-Olivares VR, Vazquez-Bello EA, Aguilar-Garnica E, Escalante FME. Valorization of Lignin as an Immobilizing Agent for Bioinoculant Production using Azospirillum brasilense as a Model Bacteria. Molecules. 2019; 24(24):4613. https://doi.org/10.3390/molecules24244613
Chicago/Turabian StyleTapia-Olivares, Victor Rogelio, Eimy Alejandra Vazquez-Bello, Efrén Aguilar-Garnica, and Froylán M.E. Escalante. 2019. "Valorization of Lignin as an Immobilizing Agent for Bioinoculant Production using Azospirillum brasilense as a Model Bacteria" Molecules 24, no. 24: 4613. https://doi.org/10.3390/molecules24244613
APA StyleTapia-Olivares, V. R., Vazquez-Bello, E. A., Aguilar-Garnica, E., & Escalante, F. M. E. (2019). Valorization of Lignin as an Immobilizing Agent for Bioinoculant Production using Azospirillum brasilense as a Model Bacteria. Molecules, 24(24), 4613. https://doi.org/10.3390/molecules24244613





