Neuroprotective Effect of CR-777, a Glutathione Derivative of Withaferin A, Obtained through the Bioconversion of Withania somnifera (L.) Dunal Extract by the Fungus Beauveria bassiana
Abstract
:1. Introduction
2. Results
2.1. Production, Isolation and Structural Elucidation of the Bioconversion Products Produced through the Bioconversion of Withania somnifera Extract by Beauveria bassiana ATCC 7159
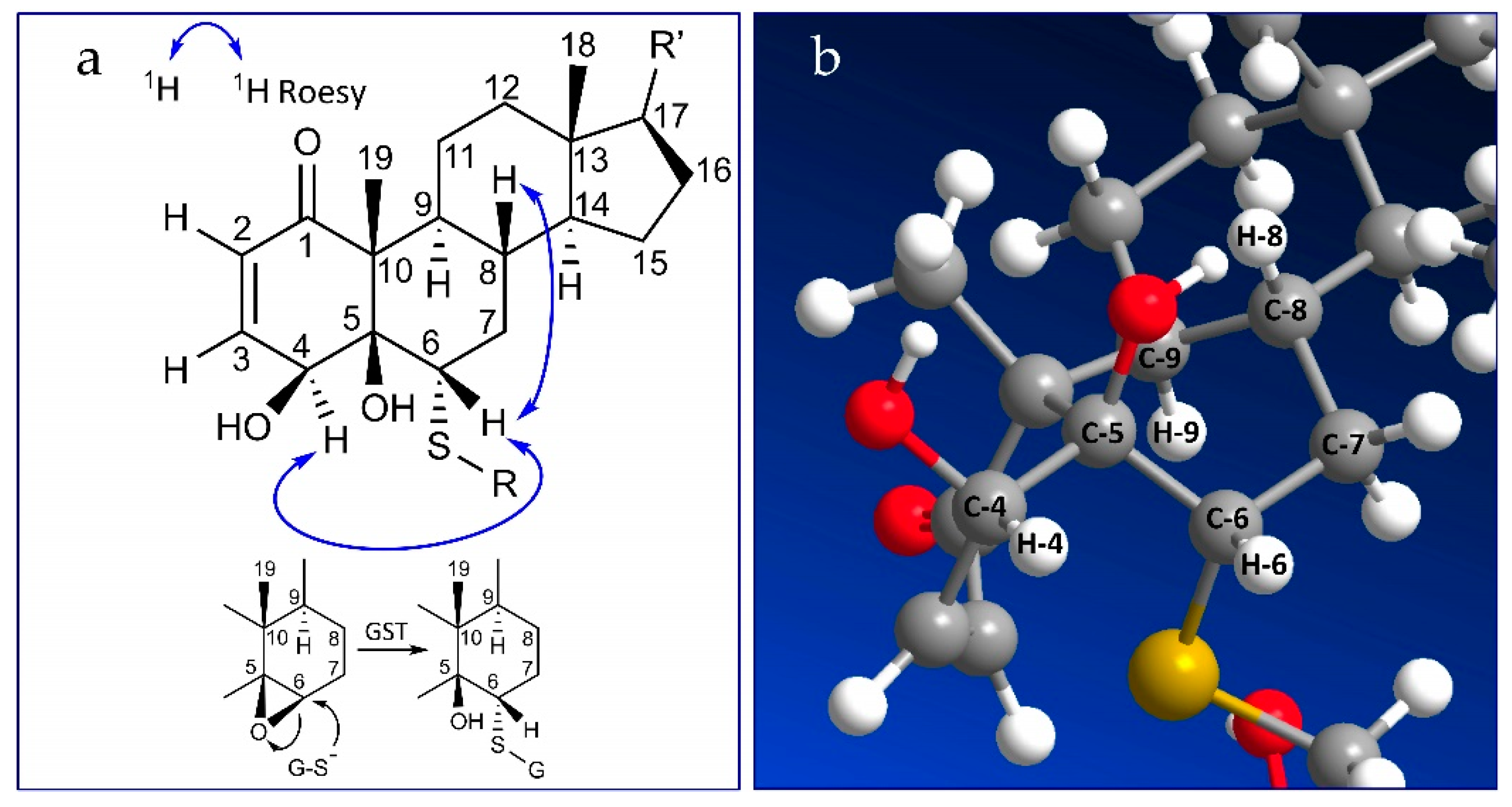
2.2. Stereochemistry of C-5 and C-6 in CR-591 (1) and CR-777 (2)
2.3. Mechanism of Formation of CR-591 (1) and CR-777 (2)
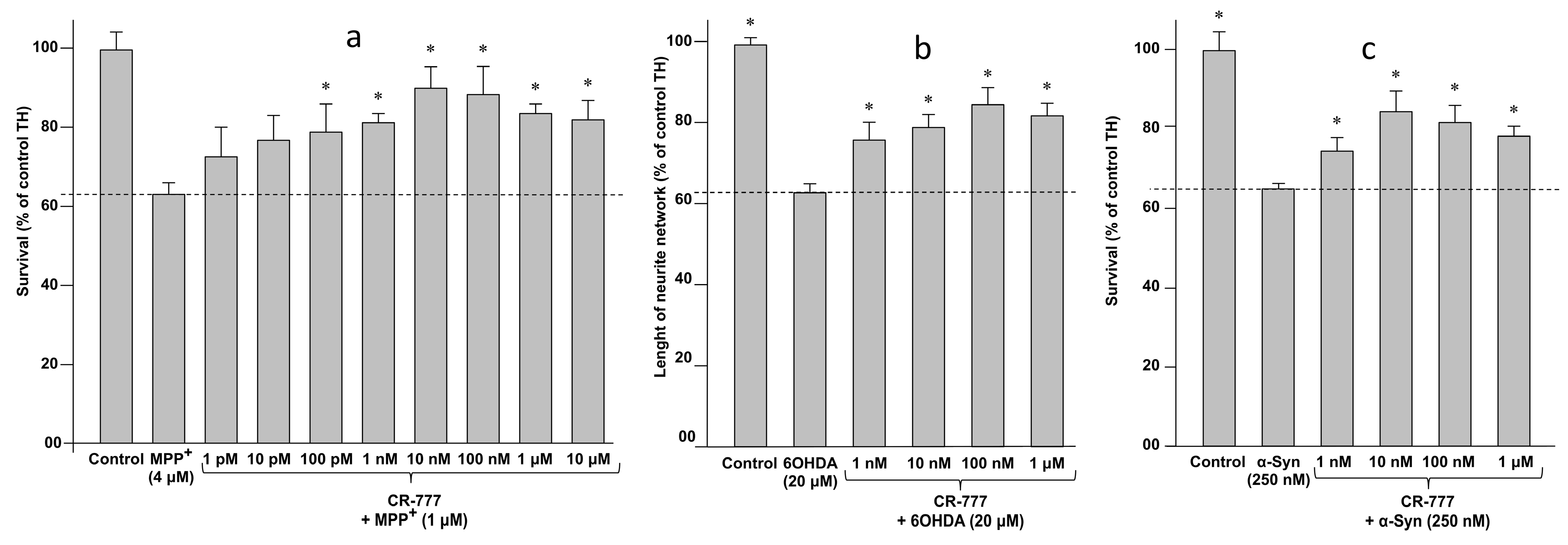
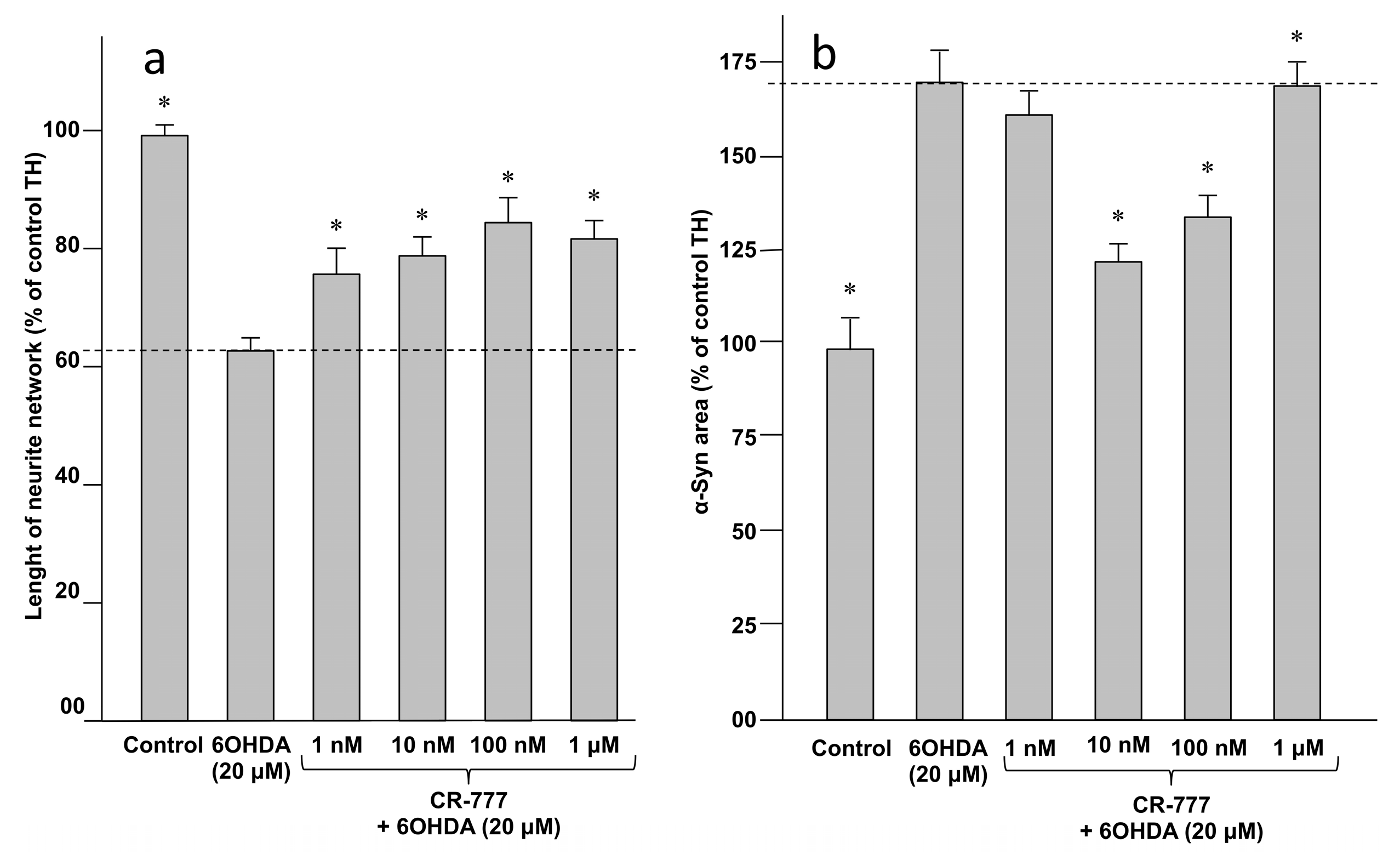
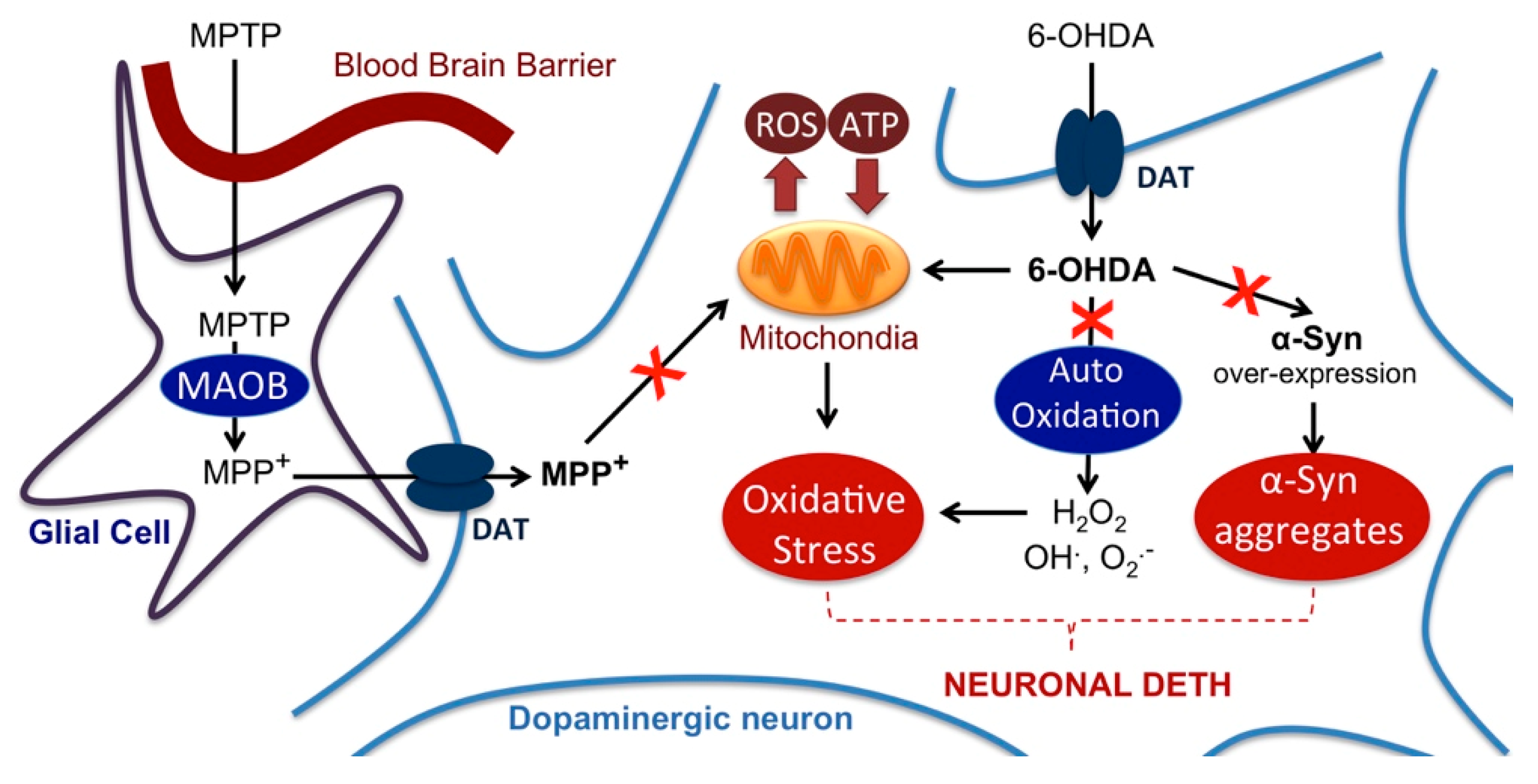
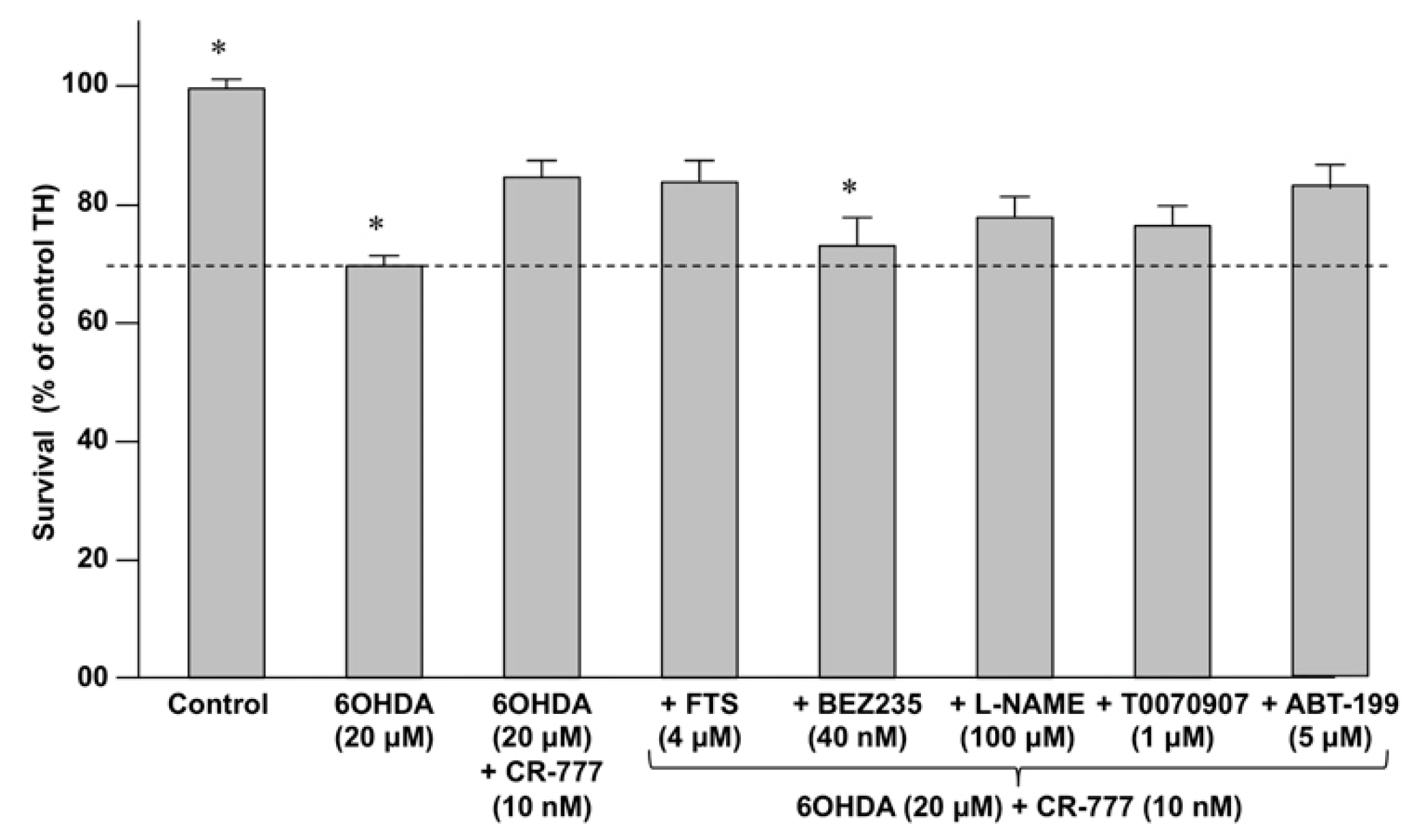
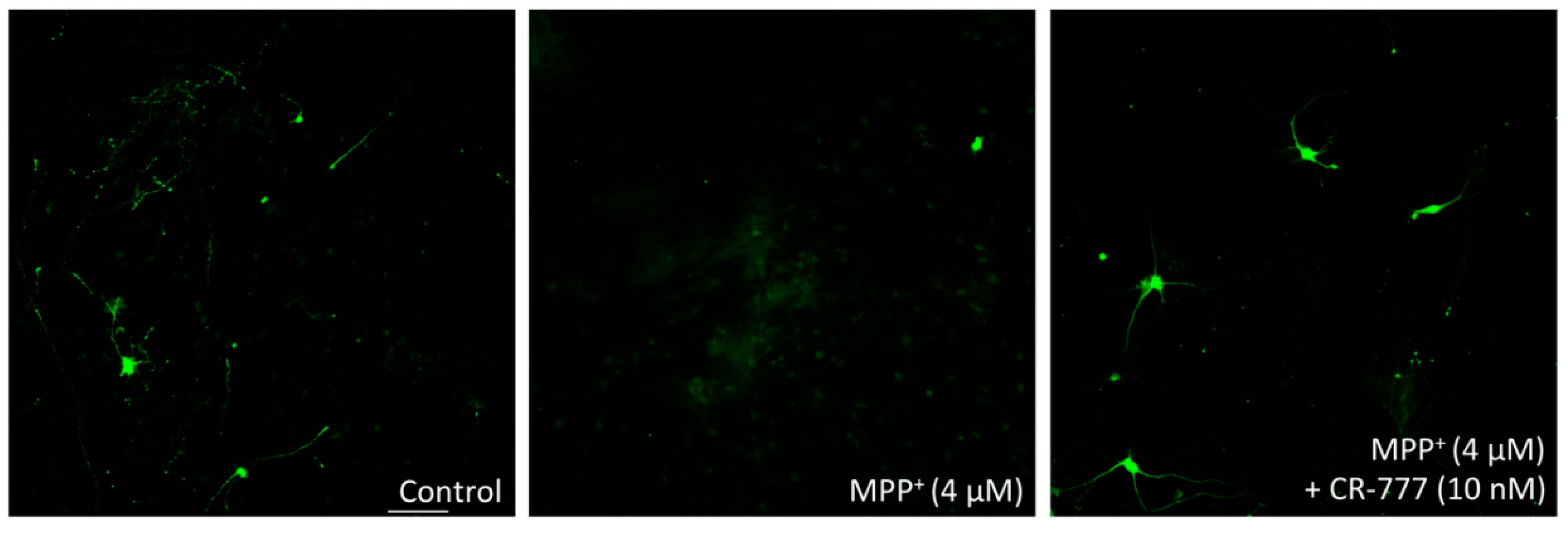
2.4. Impact on Parkinson’s Mimetics
3. Discussion
4. Materials and Methods
4.1. Analytical and Structure Elucidation Methods of Target Molecules
4.2. Plant Extraction Procedure
4.3. Cultivation of B. bassiana
4.4. Fermentation of the Plant Extract
4.5. Fractionation of Extract and Purification of Target Compounds
4.6. Isolation of Withaferin A
4.7. Compliance with the Rules for Laboratory Animal Use
4.8. Culture of Mesencephalic Neurons for MPP+ Injury
4.9. Culture of Mesencephalic Neurons for α-Synuclein Injury
4.10. CR-777 Exposure
- MPP+ injury: On day 6 of culture, the medium was removed and fresh medium was added, without or with MPP+ (Sigma Aldrich), at 4 µM diluted in control medium with or without CR-777 for 48 h.
- 6-OHDA injury: On day 6 of culture, the medium was removed and fresh medium was added, without or with 6-OHDA (Sigma Aldrich), at 20 µM diluted in control medium with or without CR-777 for 48 h.
- α-Synuclein injury: On day 7 of culture, the medium was removed and fresh medium was added, without or with alpha synuclein (rPeptide), at 250 nM diluted in control medium with or without CR-777 for 24 h.
4.11. Immunostaining: TH Positive Neron (Dopaminergic Neurons)
4.12. Immunostaining: Overepression of α-Syuclein (Dopaminergic Neurons)
- Monoclonal anti-tyrosine hydroxylase (TH, Sigma) antibody produced in mice at a dilution of 1/10,000 in PBS containing 1% FCS and 0.1% saponin for 2 h at room temperature. This antibody was revealed with Alexa Fluor 488 goat anti-mouse IgG (Molecular Probe) at the dilution of 1/800 in PBS containing 1% FCS and 0.1% saponin for 1 h at room temperature.
- Monoclonal anti-alpha-synuclein (Cell Signaling) antibody produced in rabbit at a dilution of 1/200 in PBS containing 1% FCS and 0.1% saponin for 2 h at room temperature. This antibody was revealed with Alexa Fluor 568 goat anti-rabbit IgG (Molecular Probe) at the dilution of 1/800 in PBS containing 1% FCS and 0.1% saponin for 1 h at room temperature.
- Immunostaining: MAP-2 tau phosphor Ser214, Thr212 (cortical neurons)
- Mouse monoclonal antibody anti phosphoT (phospho Thr212/Ser214; AT100) at a dilution of 1/400 in PBS containing 1% fetal calf serum and 0.1% saponin.
- Chicken polyclonal antibody anti microtubule-associated-protein 2 (MAP-2) at a dilution of 1/1000 in PBS containing 1% fetal calf serum and 0.1% saponin (this antibody stains specifically cell bodies and neurites, allowing study of neuronal cell survival and neurite network).
4.13. Analysis
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dey, A.; Bhattacharya, R.; Mukherjee, A.; Pandey, D.K. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnol. Adv. 2017, 35, 178–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, L.; He, J.; Zhong, L.; Duan, X.; Ouyang, L.; Zhu, Y.; Wang, T.; Zhang, Y.; Shi, J. Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur. J. Med. Chem. 2017, 141, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Fatima, M.; Mondal, A.C. Important medicinal herbs in Parkinson’s disease pharmacotherapy. Biomed. Pharmacother. 2017, 92, 856–863. [Google Scholar] [CrossRef]
- Wadhwa, R.; Konar, A.; Kaul, S.C. Nootropic potential of Ashwagandha leaves: Beyond traditional root extracts. Neurochem Int. 2016, 95, 109–118. [Google Scholar] [CrossRef]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef]
- Kurapati, K.R.V.; Atluri, V.S.R.; Samikkannu, T.; Nair, M.P.N. Ashwagandha (Withania somnifera) Reverses β-Amyloid1-42 Induced Toxicity in Human Neuronal Cells: Implications in HIV-Associated Neurocognitive Disorders (HAND). PLoS ONE 2013, 8, e77624. [Google Scholar] [CrossRef]
- Nagashayana, N.; Sankarankutty, P.; Nampoothiri, M.R.; Mohan, P.K.; Mohanakumar, K.P. Association of L-DOPA with recovery following Ayurveda medication in Parkinson’s disease. J. Neurol. Sci. 2000, 176, 124–127. [Google Scholar] [CrossRef]
- Tripathi, N.; Shrivastava, D.; Mir, B.A.; Kumar, S.; Govil, S.; Vahedi, M.; Bisen, P.S. Metabolomic and Biotechnological approaches to determine therapeutic potential of Withania somnifera (L.) Dunal: A Review. Phytomedicine 2017, 50, 127–136. [Google Scholar] [CrossRef]
- Shruti, S.B.; Rao, N.J.; Hingorani, L.L. Safety assessment of Withania somnifera extract standardized for Withaferin A: Acute and sub-acute toxicity study. J. Ayurveda Integr. Med. 2016, 7, 30–37. [Google Scholar]
- Prabu, P.C.; Panchapakesan, S. Prenatal developmental toxicity evaluation of Withania somnifera root extract in Wistar rats. Drug Chem. Toxicol. 2015, 38, 50–56. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef]
- Falkenberg, K.D.; Jakobs, A.; Matern, J.C.; Dörner, W.; Uttarkar, S.; Trentmann, A.; Steinmann, S.; Coulibaly, A.; Schomburg, C.; Mootz, H.D.; et al. Withaferin A, a natural compound with anti-tumor activity, is a potent inhibitor of transcription factor C/EBPβ. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1349–1358. [Google Scholar] [CrossRef]
- Tekula, S.; Khurana, A.; Anchi, P.; Godugu, C. Withaferin-A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed. Pharmacother. 2018, 106, 1428–1440. [Google Scholar] [CrossRef]
- Kotagale, N.R.; Kedia, A.; Gite, R.; Rahmatkar, S.N.; Gawande, D.Y.; Umekar, M.J.; Taksande, B.G. Withaferin A attenuates Alcohol Abstinence Signs in Rats. Pharmacogn. J. 2018, 10, 1190–1195. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, S.K.; Majeed, R.; Ahmad, M.; Sangwan, P.I.; Purnima, B.; Saxsena, A.K.; Suri, K.A.; Mukherjee, D.; Taneja, S.C. Ring A structural modified derivatives of withaferin A and the evaluation of their cytotoxic potential. Steroids 2011, 76, 1213–1222. [Google Scholar] [CrossRef]
- Joshi, P.; Misra, L.; Siddique, A.A.; Srivastava, M.; Kumar, S.; Darokar, M.P. Epoxide group relationship with cytotoxicity in withanolide derivatives from Withania somnifera. Steroids 2014, 79, 19–27. [Google Scholar] [CrossRef]
- Llanos, G.G.; Araujo, L.M.; Jimenez, I.A.; Moujir, L.M.; Rodríguez, J.; Jimenez, C.; Bazzocchi, I.L. Structure-based design, synthesis, and biological evaluation of withaferin A-analogues as potent apoptotic inducers. Eur. J. Med. Chem. 2017, 140, 52–64. [Google Scholar] [CrossRef]
- Bommarius, A.S. Biocatalysis: A Status Report. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 319–345. [Google Scholar] [CrossRef]
- Reetz, M.T. Biocatalysis in Organic Chemistry and Biotechnology: Past, Present, and Future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef]
- Chaudhary, A.; Singh, N.; Dalvi, M.; Wele, A. A progressive review of Sandhana kalpana (Biomedical fermentation): An advanced innovative dosage form of Ayurveda. Ayu 2011, 32, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Singh, S.; Ur Rahman, L. Biotransformation studies using hairy root cultures—A review. Biotechnol. Adv. 2011, 30, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.S.; Won, T.J.; Nam, S.Y.; Kim, Y.B.; Lee, Y.C.; Park, S.Y.; Park, H.Y.; Hwang, K.W.; Lee, D.I. Therapeutic advantages of medicinal herbs fermented with Lactobacillus plantarum, in topical application and its activities on atopic dermatitis. Phytother. Res. 2009, 23, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Manwar, J.; Mahadik, K.; Sathiyanarayanan, L.; Paradkar, A.; Patil, S. Comparative antioxidant potential of Withania somnifera based herbal formulation prepared by traditional and non-traditional fermentation processes. Integr. Med. Res. 2013, 2, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria bassiana as an endophyte: A critical review on associated methodology and biocontrol potential. BioControl 2017, 62, 1–17. [Google Scholar] [CrossRef]
- Grogan, G.J.; Holland, H.L. The biocatalytic reactions of Beauveria spp. J. Mol. Catal. B Enzym. 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Rabhi, C.; Arcile, G.; Cariel, L.; Lenoir, C.; Bignon, J.; Wdzieczak-Bakala, J.; Ouazzani, J. Antiangiogenic-Like Properties of Fermented Extracts of Ayurvedic Medicinal Plants. J. Med. Food 2015, 18, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, C.; Cariel, L.; Ouazzani, J.; Arcile, G. Method for Producing a Plant Extract and Associated Compositions. WO/2014/202469. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014202469 (accessed on 24 December 2014).
- Rabhi, C.; Cariel, L.; Ouazzani, J.; Arcile, G. Use of a withania Extract for the Treatment of Amyloidrelated Diseases. WO/2016/150481. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016150481 (accessed on 29 September 2016).
- Rabhi, C.; Cariel, L.; Ouazzani, J.; Arcile, G. Use of a withania Extract for the Treatment of Alpha Synucleinopathies. WO/2016/166565. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016166565 (accessed on 20 October 2016).
- Maurya, R. Withanolides: A Prospective Drug for Infectious and Tropical Diseases. In Science of Ashwagandha: Preventive and Therapeutic Potentials; Kaul, S., Wadhwa, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 105–120. [Google Scholar]
- Singh, G.; Kumar, P. Evaluation of antimicrobial efficacy of flavonoids of Withania somnifera L. Indian J. Pharm. Sci. 2011, 73, 473–478. [Google Scholar]
- Shanazbanu; Shashidara, S.; Babu, V.L.A.; Dhanapal, R. Isolation of withaferin-A from Withania somnifera Dun. leaves and its antibacterial activity. Asian J. Chem. 2006, 18, 1243–1247. [Google Scholar]
- Choudhary, M.I.; Dur-e-Shanhwar; Parveen, Z.; Jabbar, A.; Ali, I.; Atta-ur-Rahman. Antifungal steroidal lactones from Withania coagulance. Phytochemistry 1995, 40, 1243–1246. [Google Scholar] [CrossRef]
- Bansod, S.D.; Rai, M. Antifungal activity of essential oils from Indian medicinal plants against human pathogenic Aspergillus fumigatus and A. niger. World J. Med. Sci. 2008, 3, 81–88. [Google Scholar]
- Punetha, H.; Singh, S.; Gaur, A.K. Antifungal and antibacterial activities of crude withanolides extract from the roots of Withania somnifera (L.) Dunal (Ashwagandha). Environ. Conserv. J. 2010, 11, 65–69. [Google Scholar]
- Javadian, F.; Sepehri, Z.; Saeidi, S.; Hassanshahian, M. Antifungal effects of the extract of the Withania somnifera on Candida albicans. Adv. Herb. Med. 2017, 3, 31–37. [Google Scholar]
- Forlani, L.; Juárez, M.P.; Lavarías, S.; Pedrini, N. Toxicological and biochemical response of the entomopathogenic fungus Beauveria bassiana after exposure to deltamethrin. Pest. Manag. Sci. 2014, 70, 751–756. [Google Scholar] [CrossRef]
- Bryant, D. Glutathione Conjugation of Herbicides and Fungicides in Plants and Fungi: Functional Characterization of Glutathione Transferases from Phytopathogens. Ph.D. Thesis, Durham University, Durham, UK, 2004. [Google Scholar]
- Bryant, D.; Cummins, I.; Dixon, D.P.; Edwards, R. Cloning and characterization of a theta class glutathione transferase from the potato pathogen Phytophthora infestans. Phytochemistry 2006, 67, 1427–1434. [Google Scholar] [CrossRef]
- Visanji, N.P.; Orsi, A.; Johnston, T.H.; Howson, P.A.; Dixon, K.; Callizot, N.; Brotchie, J.M.; Daryl, D.; Rees, D.D. PYM50028, a novel, orally active, nonpeptide neurotrophic factor inducer, prevents and reverses neuronal damage induced by MPP+ in mesencephalic neurons and by MPTP in a mouse model of Parkinson’s disease. FASEB J. 2008, 22, 2488–2497. [Google Scholar] [CrossRef]
- Hernandez-Baltazar, D.; Zavala-Flores, L.M.; Villanueva-Olivo, A. The 6-hydroxydopamine model and parkinsonian pathophysiology: Novel findings in an older model. Neurología 2017, 32, 533–539. [Google Scholar] [CrossRef]
- Rocha, E.M.; DeMiranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Emrah Er, E.; Blenis, J. The Ras-ERK and PI3K-mTOR Pathways: Cross-talk and Compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Bockaert, J.; Marin, P. mTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015, 95, 1157–1187. [Google Scholar] [CrossRef]
- Schinelli, S.; Zuddas, A.; Kopin, I.J.; Barker, J.L.; Di Porzio, U. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine metabolism and 1-methyl-4-phenylpyridinium uptake in dissociated cell cultures from the embryonic mesencephalon. J. Neurochem. 1988, 50, 1900–1907. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, D.; Zarnowska, E.D.; Du, Z.; Werbel, B.; Valliere, C.; Pearce, R.A.; Thomson, J.A.; Zhang, S.C. Directed Differentiation of Dopaminergic Neuronal Subtypes fromHuman Embryonic Stem Cells. Stem Cells 2005, 23, 781–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callizot, N.; Combes, M.; Steinschneider, R.; Poindron, P. Operational dissection of β-amyloid cytopathic effects on cultured neurons. J. Neurosci. Res. 2013, 91, 706–716. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |

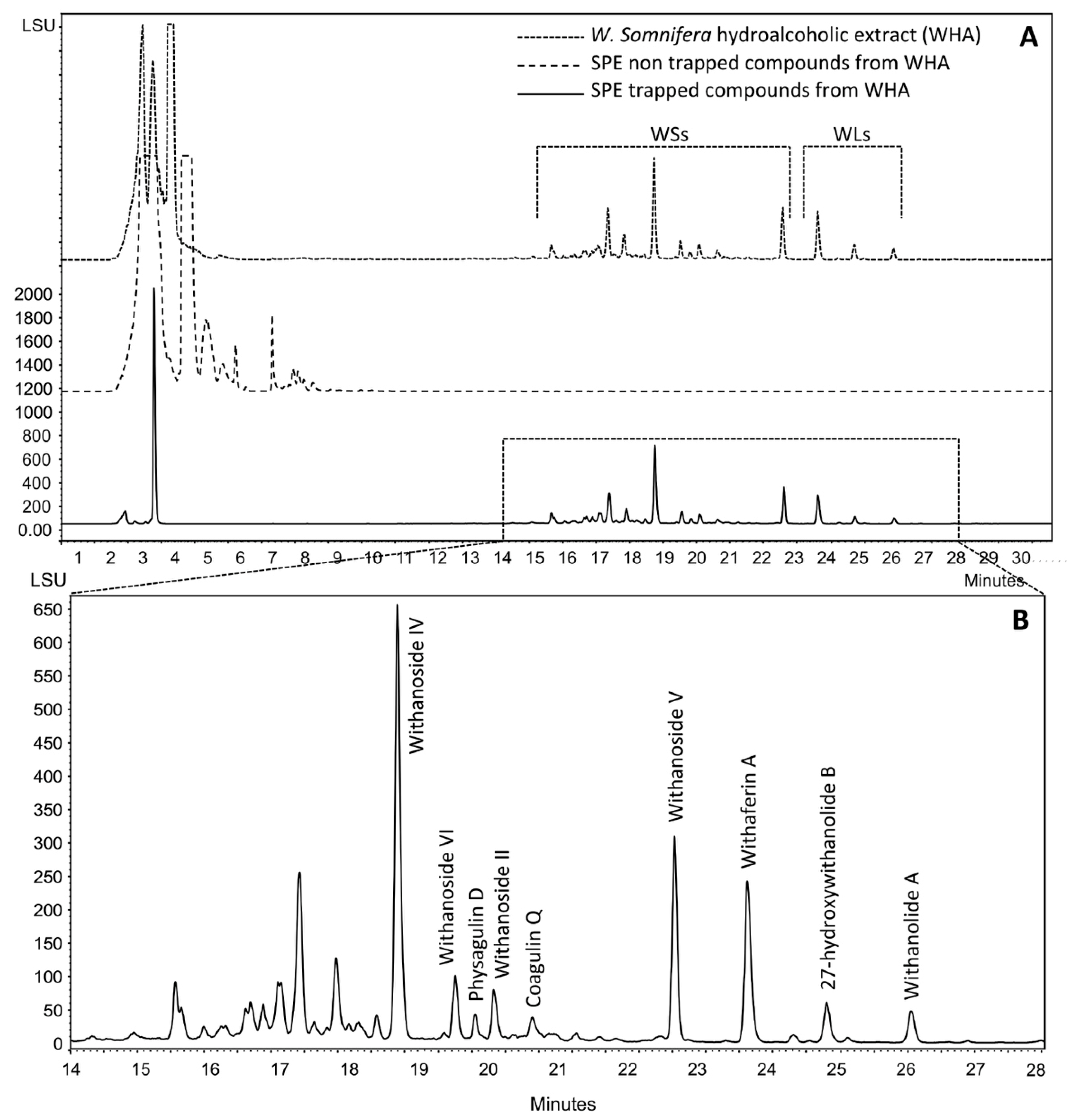
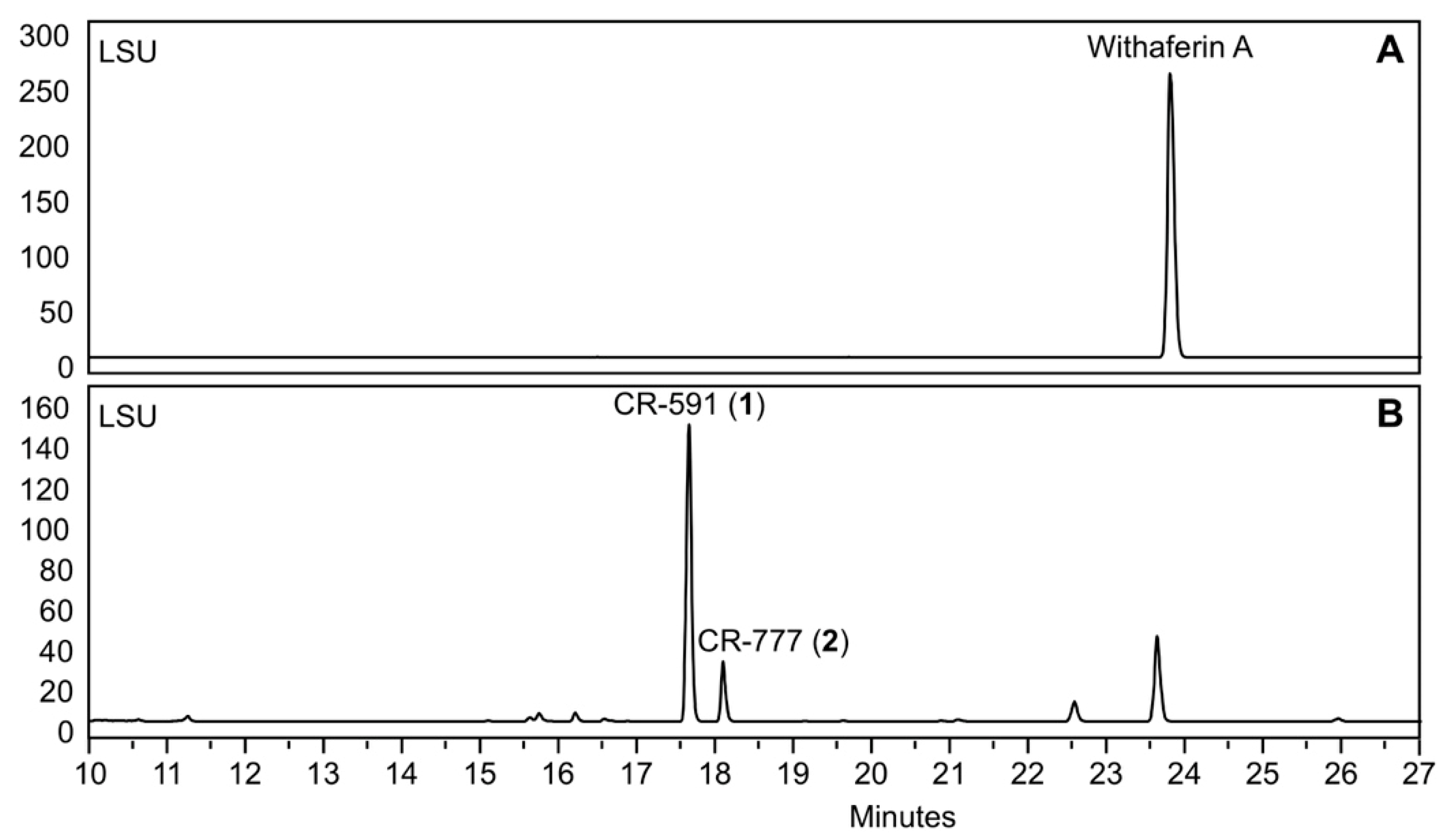
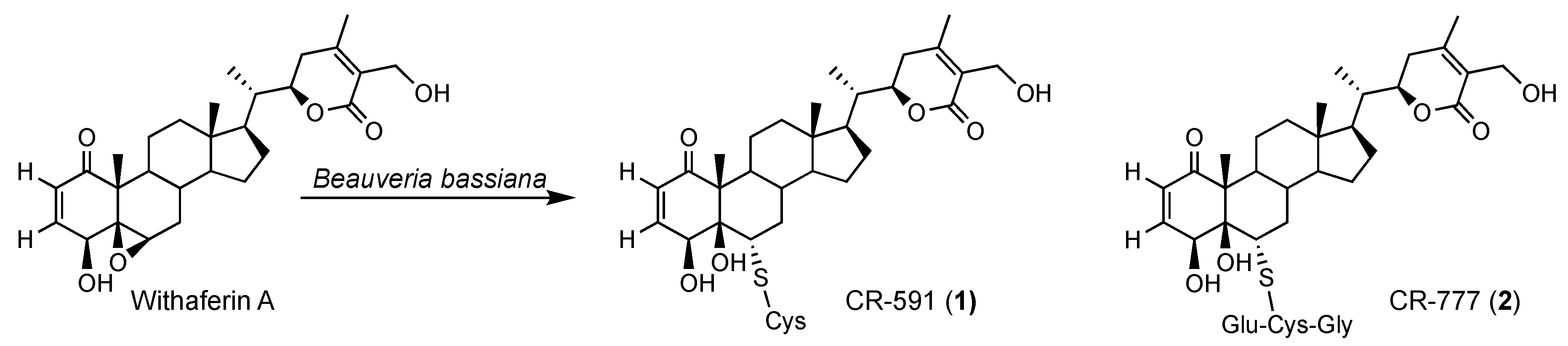
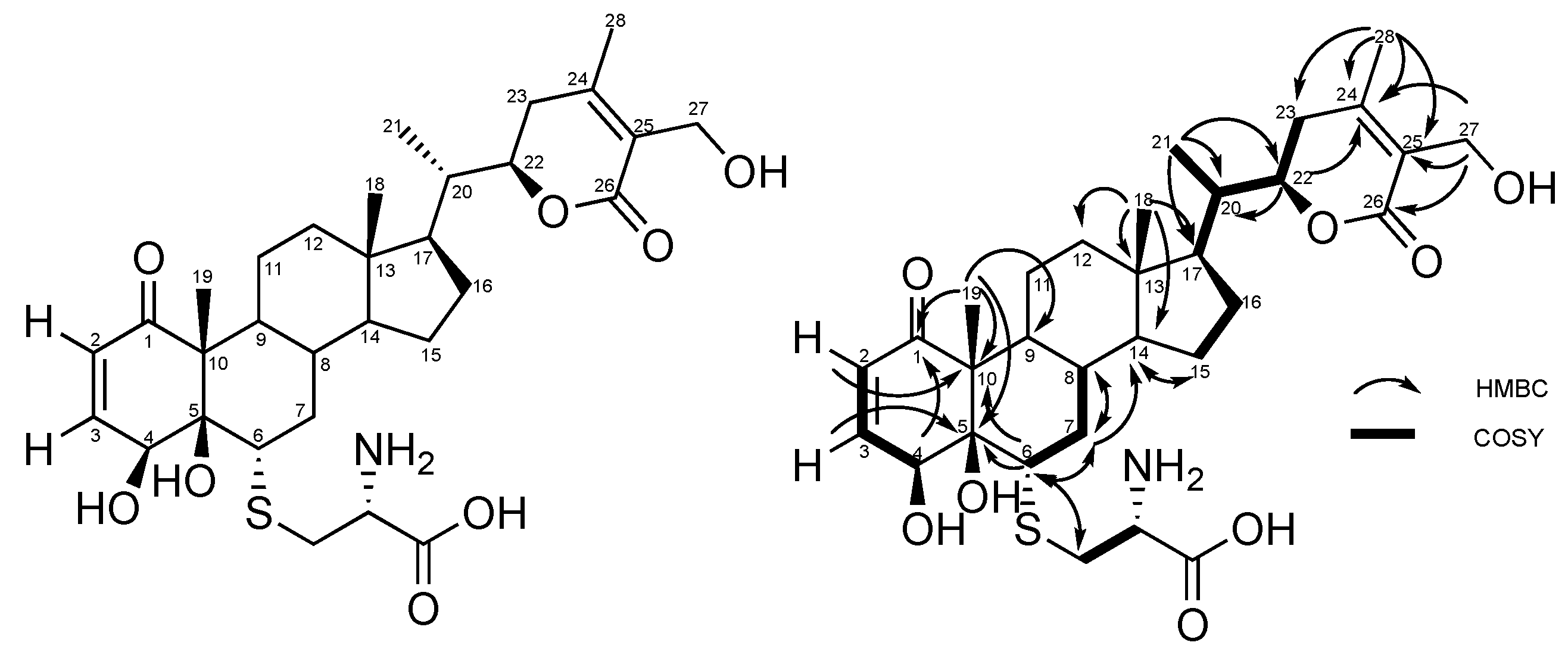
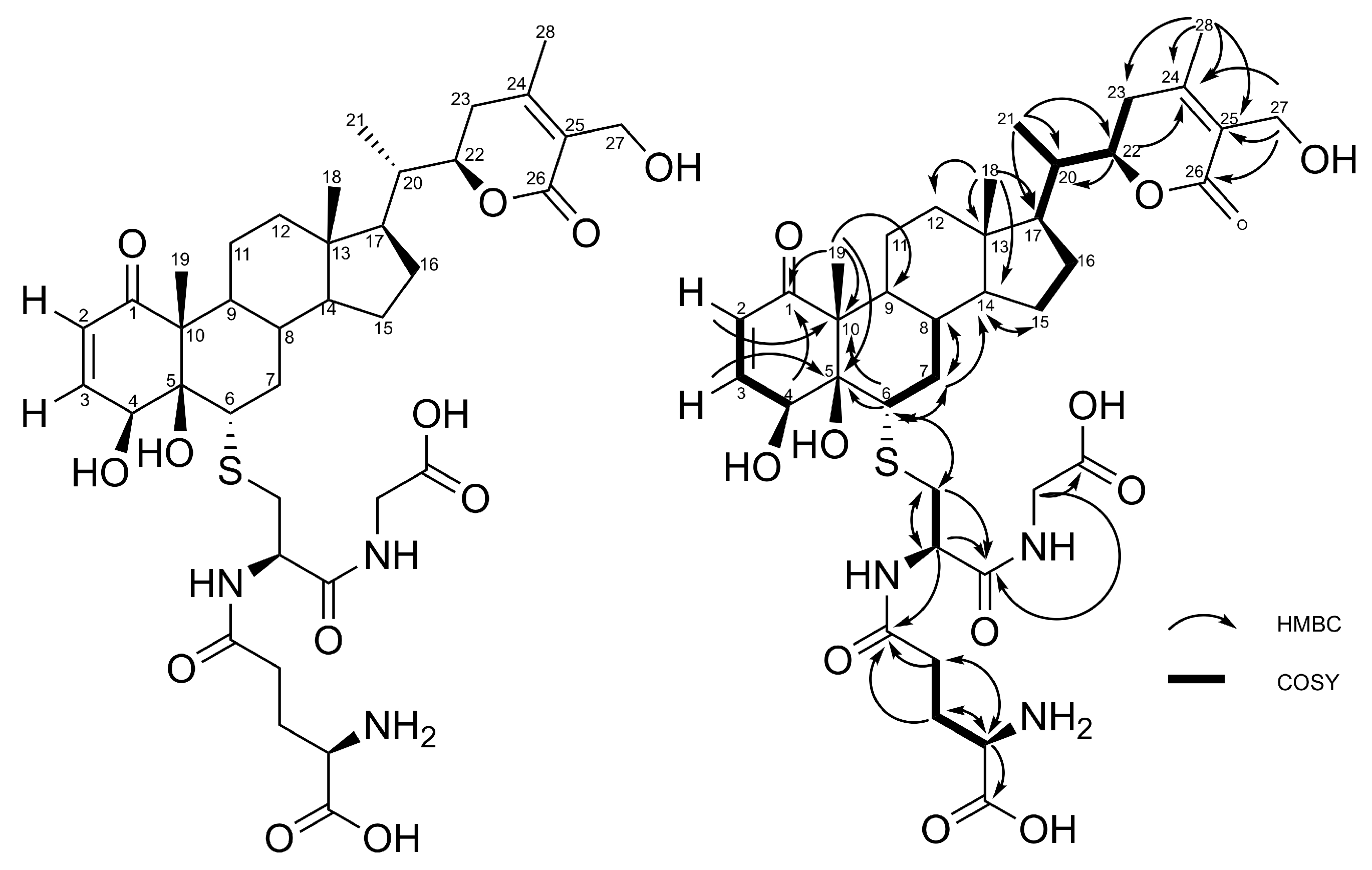

| Position | δC | δH (mult; J in Hz) | HMBC |
|---|---|---|---|
| 1 | 204.1 | - | |
| 2 | 127.5 | 5.89 (1H; dd; 10.2; 2.0) | C-4, C-10 |
| 3 | 148.8 | 6.58 (1H; dd; 10.2, 1.9) | C-1, C-5 |
| 4 | 67.3 | 4.89 (1H; m) | C-1, C-2, C-3, C-6 |
| 5 | 81.4 | - | |
| 6 | 52.4 | 3.02 (1H; d; 12.3) | C-4, C-5, C-7, C-10, Cys (35,3) |
| 7 | 38.7 | 2.19 (1H; m), 1.55 (1H; m) | C-6, C-8, C-9, C-14 |
| 8 | 36.7 | 1.67 (1H; m) | |
| 9 | 47.0 | 1.39 (1H; m) | |
| 10 | 59.3 | - | |
| 11 | 28.4 | 1.81 (1H; m), 1.42 (1H; m) | |
| 12 | 40.5 | 1.14 (2H; m) | |
| 13 | 44.4 | - | |
| 14 | 56.3 | 1.18 (1H; m) | C-15 |
| 15 | 25.2 | 1.70 (1H; m), 1.27 (1H; m) | C-14 |
| 16 | 24.3 | 1.39 (1H; m), 0.90 (1H; m) | C-13 |
| 17 | 53.1 | 1.23 (1H; m) | |
| 18 | 12.4 | 0.75 (3H; s) | C-12, C-13, C14, C-17 |
| 19 | 10.5 | 1.25 (3H; s) | C-1, C-5, C-9, C-10 |
| 20 | 40.3 | 1.94 (1H; m) | |
| 21 | 13.6 | 0.99 (3H; d; 6.8) | C-17, C-20, C-22 |
| 22 | 80.1 | 4.44 (1H; d; 13.4) | C-20, C-24 |
| 23 | 30.8 | 2.52 (1H; d; 17.3), 2.16 (1H; m) | C-22, C-24, C-25 |
| 24 | 157.8 | - | |
| 25 | 126.4 | - | |
| 26 | 168.5 | - | |
| 27 | 56.5 | 4.34 (2H; m) | C-24, C-25, C-26 |
| 28 | 20.3 | 2.09 (3H; s) | C-23, C-24, C-25 |
| L-Cys | 35.3 | 3.13 (1H; dd; 14.3, 4.7), 2.95 (1H; dd; 14.3, 6.6) | C-6, Cys (55,7) |
| 55.7 | 3.70 (1H; m) | Cys (35,3), Cys (172,8) |
| Position | δC | δH (mult; J in Hz) | HMBC |
|---|---|---|---|
| 1 | 204.2 | - | |
| 2 | 127.5 | 5.89 (1H; dd; 10.2; 2.0) | C-4, C-10 |
| 3 | 148.6 | 6.57 (1H; dd; 10.2, 1.9) | C-1, C-5 |
| 4 | 67.3 | 4.90 (1H; m) | C-1, C-2, C-3, C-6 |
| 5 | 81.3 | - | |
| 6 | 52.7 | 3.16 (1H; d; 12.3) | C-4, C-5, C-7, Cys (36.3) |
| 7 | 38.5 | 2.15 (1H; m), 1.51 (1H; m) | C-6, C-8, C-9, C-14 |
| 8 | 36.6 | 1.66 (1H; m) | |
| 9 | 47.0 | 1.39 (1H; m) | |
| 10 | 59.2 | - | |
| 11 | 28.5 | 1.81 (1H; m), 1.40 (1H; m) | |
| 12 | 40.3 | 1.95 (1H; m) | |
| 13 | 44.3 | - | |
| 14 | 56.3 | 1.18 (1H; m) | C-15 |
| 15 | 25.2 | 1.70 (1H; m), 1.29 (1H; m) | C-14 |
| 16 | 24.2 | 1.38 (1H; m), 0.89 (1H; m) | C-13 |
| 17 | 53.1 | 1.23 (1H; m) | |
| 18 | 12.3 | 0.75 (3H; s) | C-12, C-13, C14, C-17 |
| 19 | 10.3 | 1.25 (3H; s) | C-1, C-5, C-9, C-10 |
| 20 | 40.3 | 1.94 (1H; m) | |
| 21 | 13.6 | 0.99 (1H; d; 6.8) | C-17, C-20, C-22 |
| 22 | 80.1 | 4.44 (1H; d; 13.4) | |
| 23 | 30.8 | 2.52 (1H; d; 17.3), 2.14 (1H; m) | C-22, C-24, C-25 |
| 24 | 157.8 | - | |
| 25 | 126.4 | - | |
| 26 | 168.5 | - | |
| 27 | 56.5 | 4.34 (2H; m) | C-24, C-25, C-26 |
| 28 | 20.2 | 2.09 (3H; s) | C-23, C-24, C-25 |
| L-Cys | 36.3 | 3.08 (1H; dd; 13.7, 5.7), 2.81 (1H; dd; 13.7, 7.6) | C-6, Cys (55.0), Cys (172.9) |
| 55.0 | 4.59 (1H; t; 6.) | Cys (36.3), Cys (172.9), Glu (175.2) | |
| 172.9 | - | ||
| L-Glu | 27.9 | 2.13 (2H; m) | Glu (55.5), Glu (175.2) |
| 33.2 | 2.54 (2H; m) | Glu (55.5), Glu (175.2) | |
| 55.5 | 3.67 (1H; m) | Glu (27.9), Glu (33.2), Glu (173.9) | |
| 173.9 | - | ||
| 175.2 | - | ||
| L-Gly | 42.9 | 3.87 (2H, m) | Cys (172.9), Gly (173.9) |
| 172.9 | - | ||
| 173.9 | - |
| Inhibitor | Activity |
|---|---|
| Farnesylthiosalicylic acid (FTS) | Inhibits the Ras/Raf pathway by disrupting Ras at the membrane. |
| BEZ-235 | Dual PI3K/mTOR inhibitor |
| T0070907 | Selective PPAR-γ inhibitor |
| Nitro-L-arginine methyl ester (L-NAME) | Inhibitor of nitric oxide synthase (NOS). |
| ABT-199 | Inhibitor of BLC2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabhi, C.; Arcile, G.; Le Goff, G.; Da Costa Noble, C.; Ouazzani, J. Neuroprotective Effect of CR-777, a Glutathione Derivative of Withaferin A, Obtained through the Bioconversion of Withania somnifera (L.) Dunal Extract by the Fungus Beauveria bassiana. Molecules 2019, 24, 4599. https://doi.org/10.3390/molecules24244599
Rabhi C, Arcile G, Le Goff G, Da Costa Noble C, Ouazzani J. Neuroprotective Effect of CR-777, a Glutathione Derivative of Withaferin A, Obtained through the Bioconversion of Withania somnifera (L.) Dunal Extract by the Fungus Beauveria bassiana. Molecules. 2019; 24(24):4599. https://doi.org/10.3390/molecules24244599
Chicago/Turabian StyleRabhi, Chérif, Guillaume Arcile, Géraldine Le Goff, Christian Da Costa Noble, and Jamal Ouazzani. 2019. "Neuroprotective Effect of CR-777, a Glutathione Derivative of Withaferin A, Obtained through the Bioconversion of Withania somnifera (L.) Dunal Extract by the Fungus Beauveria bassiana" Molecules 24, no. 24: 4599. https://doi.org/10.3390/molecules24244599
APA StyleRabhi, C., Arcile, G., Le Goff, G., Da Costa Noble, C., & Ouazzani, J. (2019). Neuroprotective Effect of CR-777, a Glutathione Derivative of Withaferin A, Obtained through the Bioconversion of Withania somnifera (L.) Dunal Extract by the Fungus Beauveria bassiana. Molecules, 24(24), 4599. https://doi.org/10.3390/molecules24244599







