Significance and Transformation of 3-Alkyl-2-Methoxypyrazines Through Grapes to Wine: Olfactory Properties, Metabolism, Biochemical Regulation, and the HP–MP Cycle
Abstract
:1. Introduction
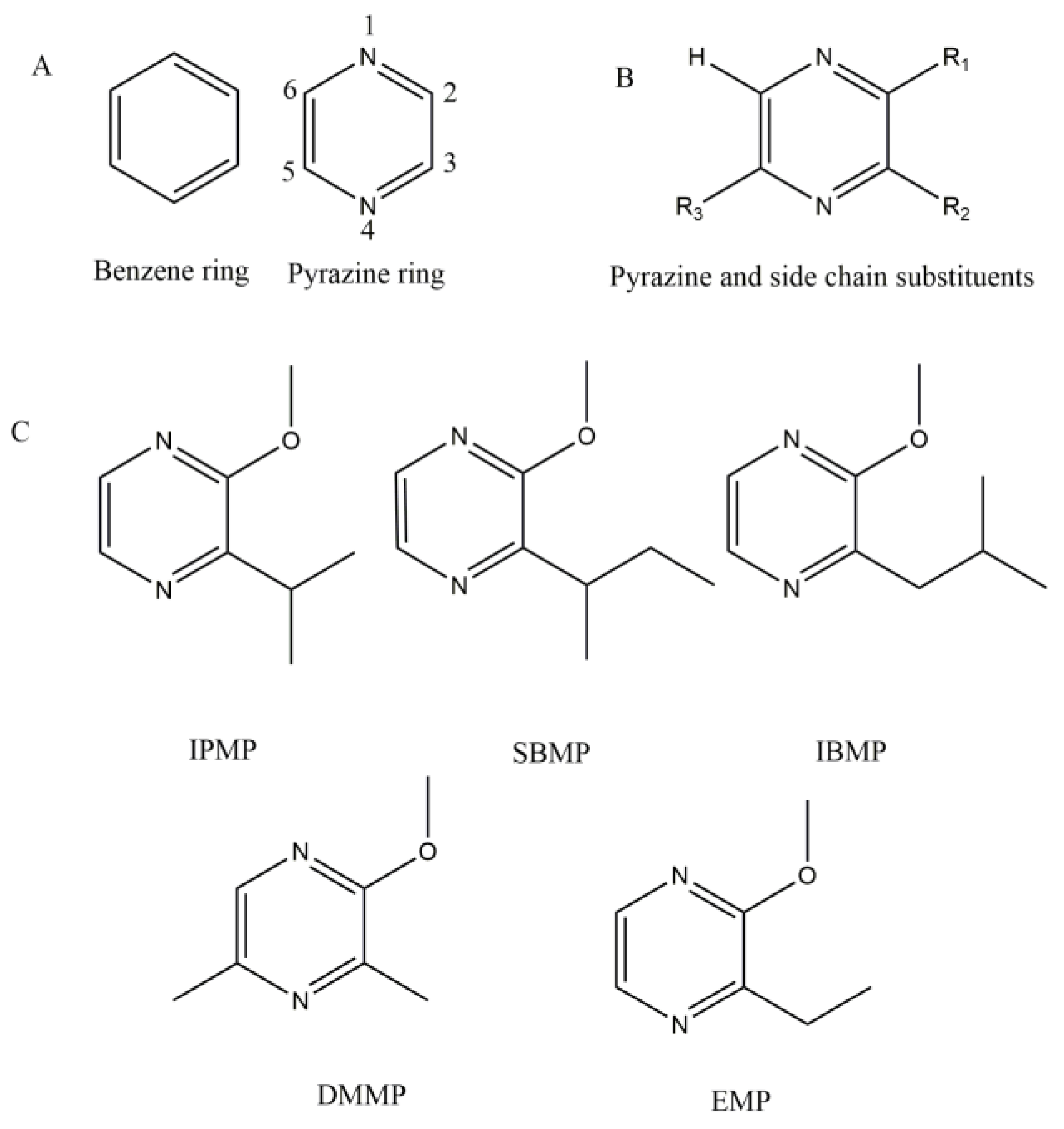
2. Molecular Structure and Sensory Properties
2.1. Structure
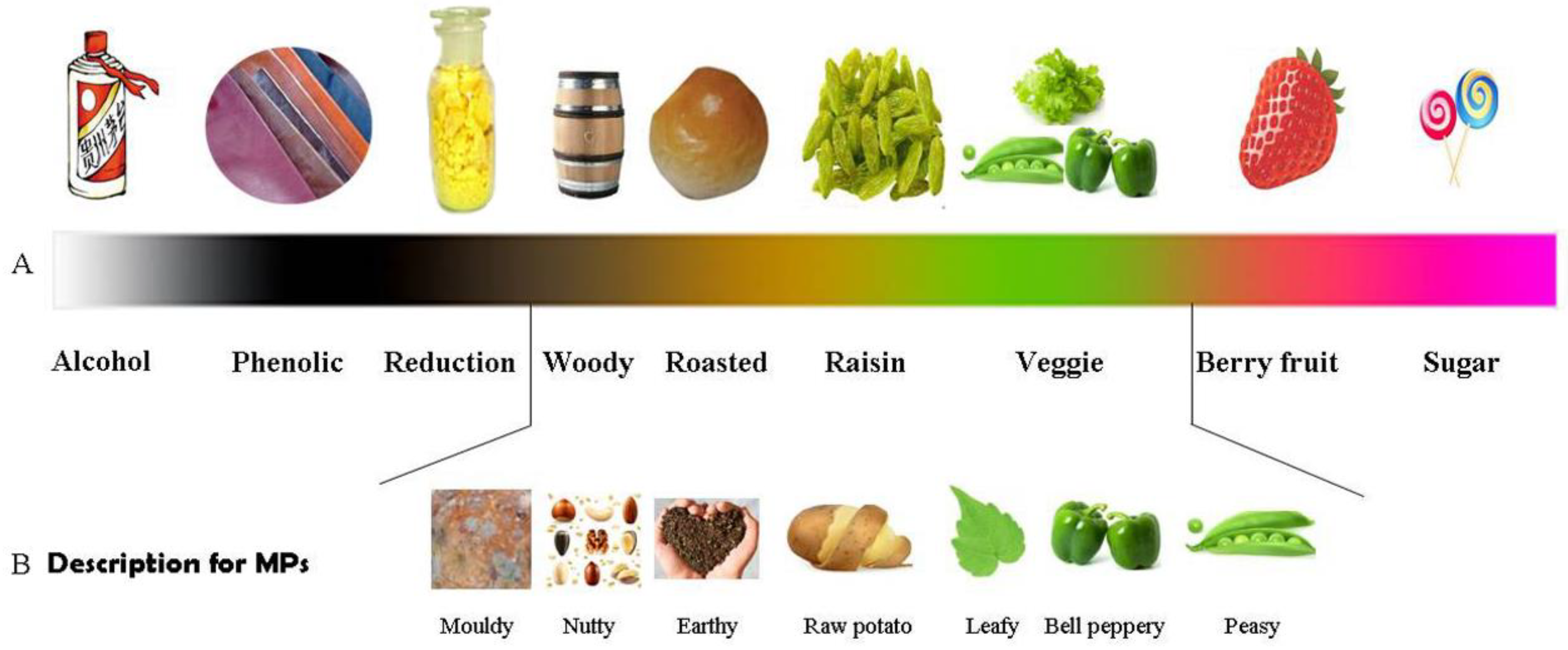
2.2. Diverse Odor Descriptions
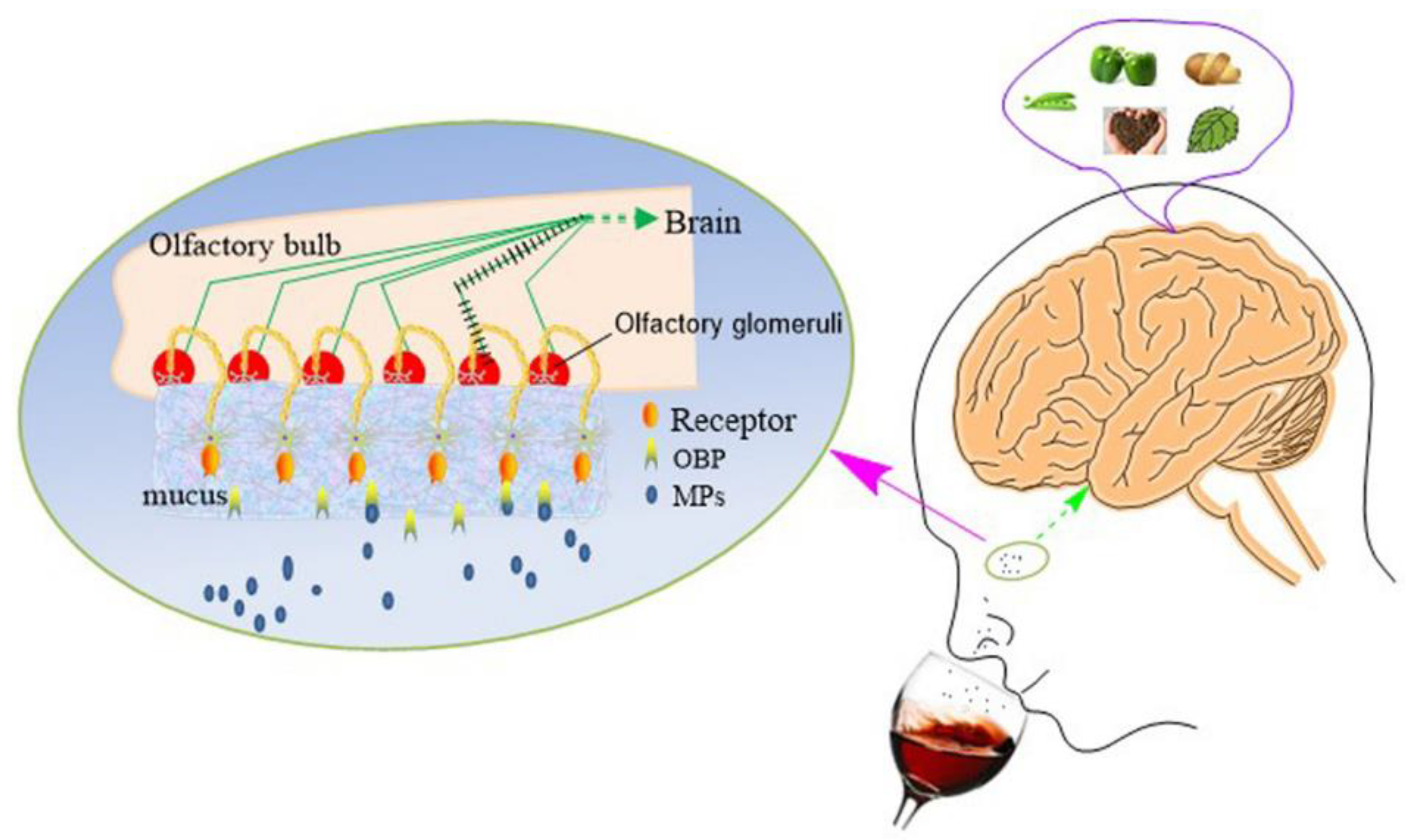
2.3. Extremely Low Odor Detection Threshold
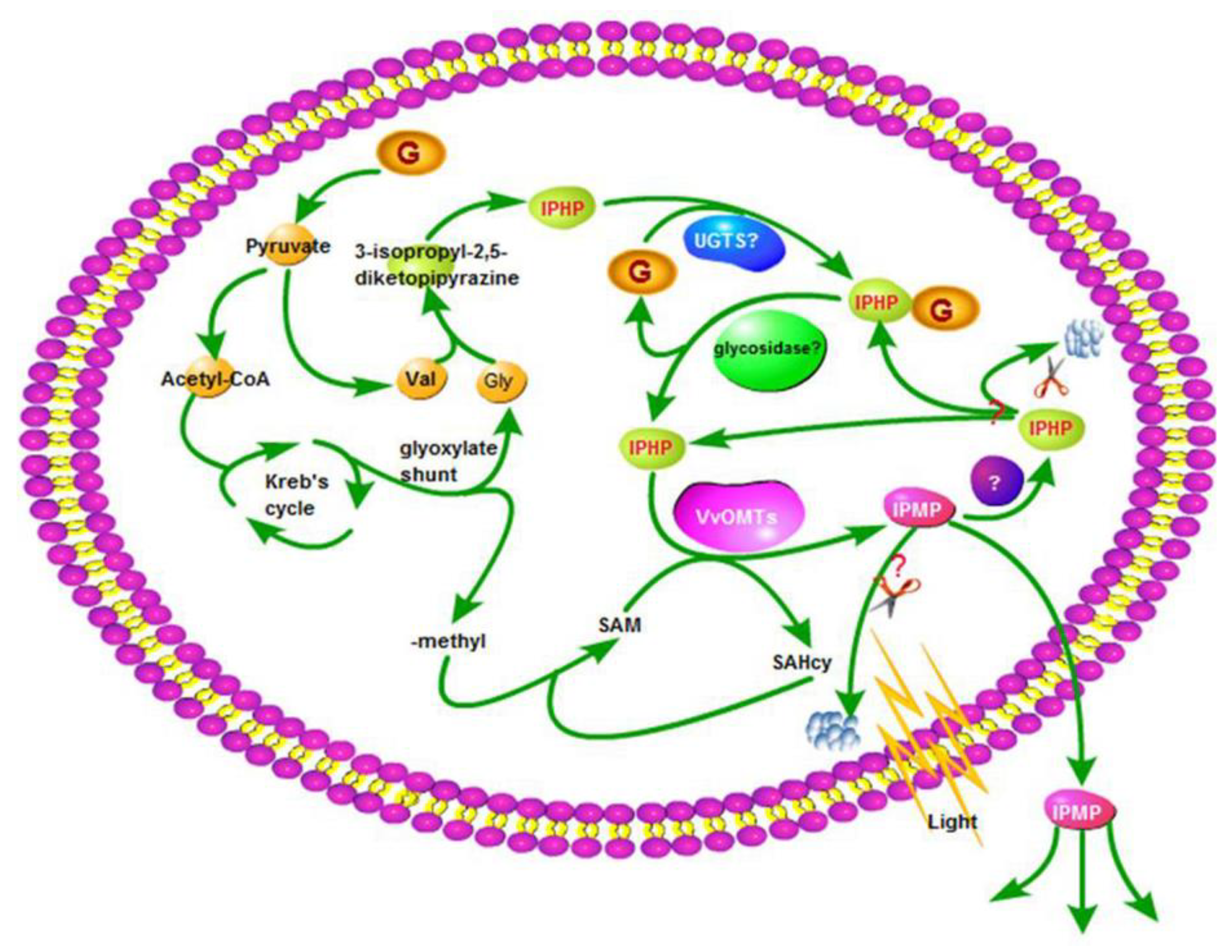
3. Metabolic Pathways for MPs in Grapes
3.1. Biosynthesis
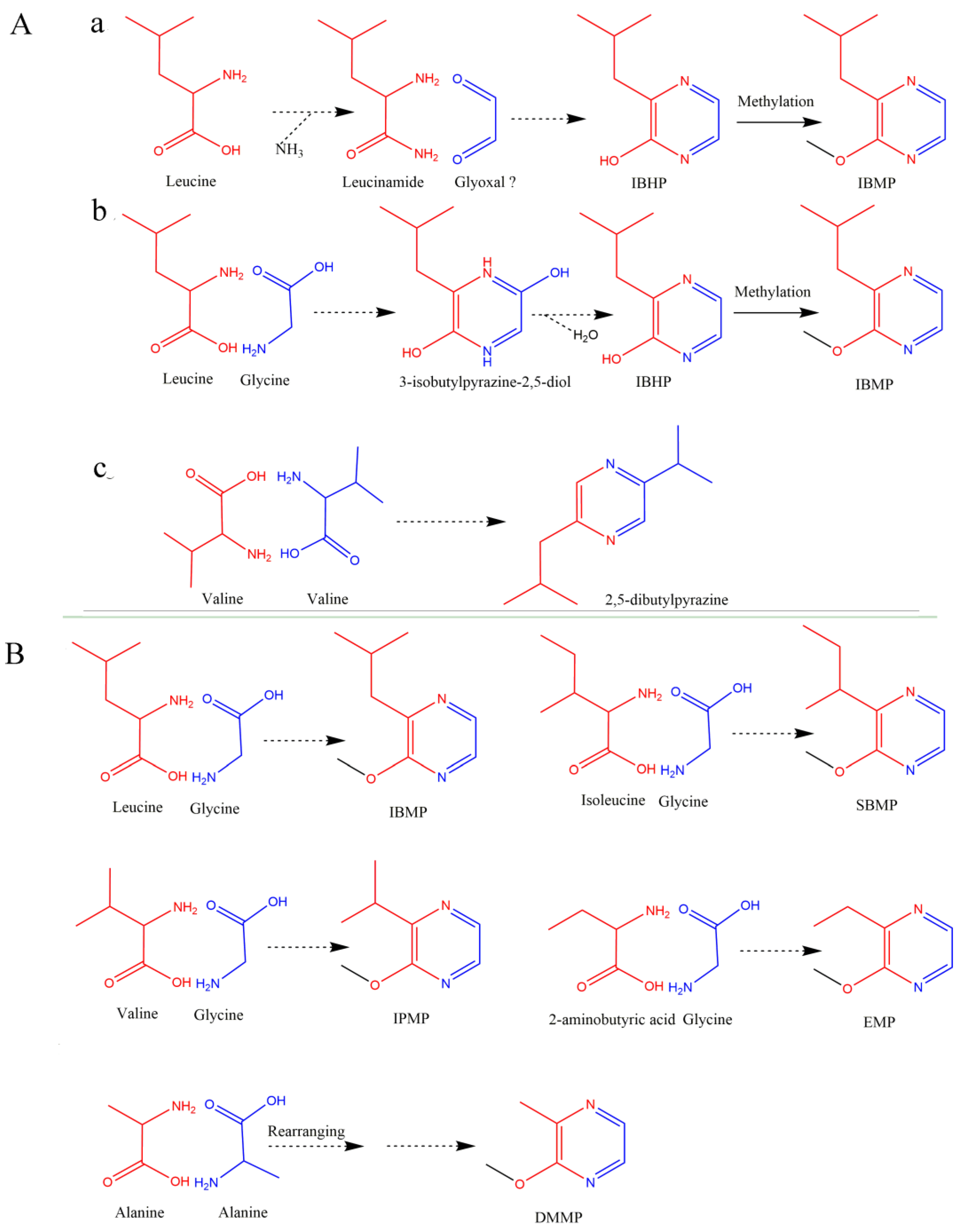
3.1.1. Precursors to and Formation of the Pyrazine Ring
3.1.2. States of HPs in Grapes
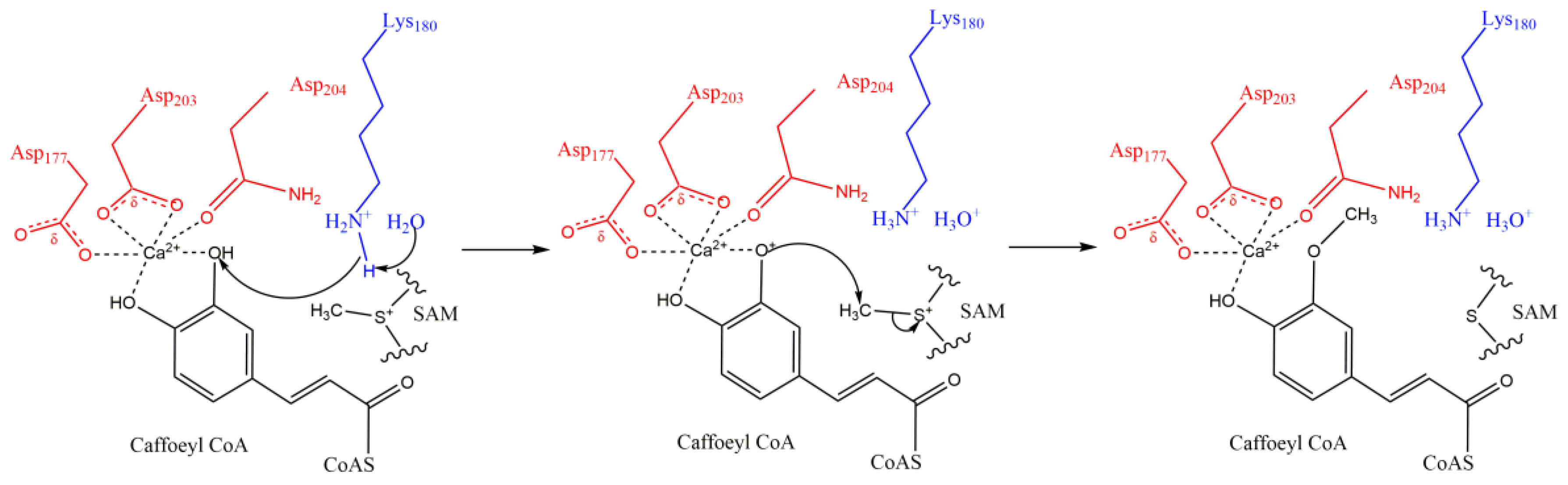
3.1.3. O-Methylation
- (i)
- (ii)
- (iii)
- The substrate molecule has one or more cyclic group (benzene or pyrazine ring, Figure 1A). On the ring, there are often at least two branched pendant groups: a hydroxyl group, which serves as the methyl receptor; and an assistant group that binds to Mg2+ or Ca2+, the catalytically active center, by ionic bonding [74].
3.2. Release and Biodegradation
3.3. HP–MP Cycle
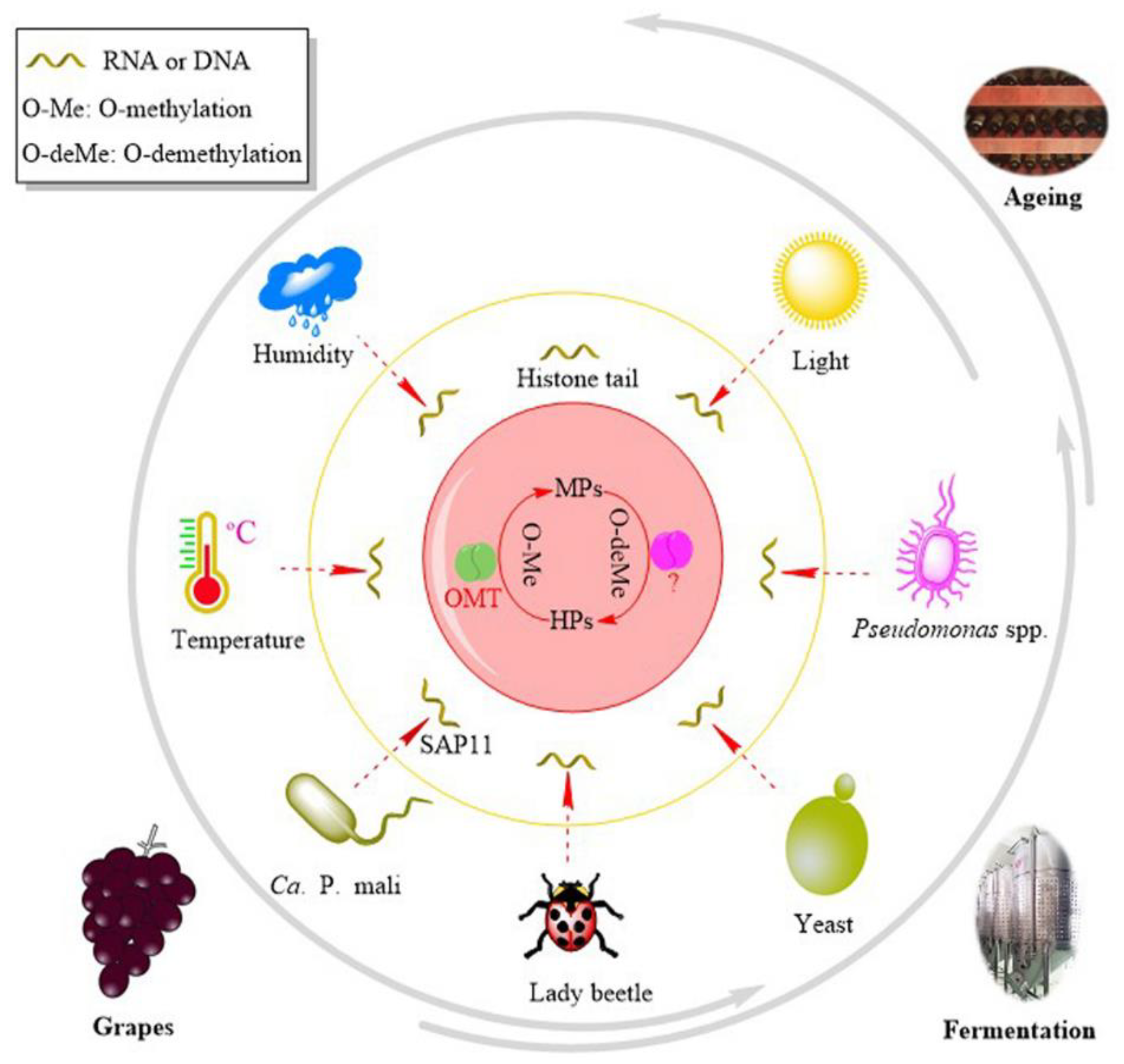
4. Regulation
4.1. Gene Regulation
4.2. Environmental Stimuli
4.2.1. Light
4.2.2. Temperature
4.2.3. Rainfall, Humidity, and Irrigation
4.2.4. Nitrogen Fertilizers
4.3. Biotic Factors
4.3.1. Lady Beetles
4.3.2. Yeasts and Microbes
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DMMP | 3,5-dimethyl-2-methoxypyrazine |
| EMP | 3-ethyl-2-methoxypyrazine |
| HPs | 3-alkyl-2-hydroxypyrazines |
| IBHP | 3-isobutyl-2-hydroxypyrazine |
| IBMP | 3-isobutyl-2-methoxypyrazine |
| IPHP | 3-isopropyl-2-hydroxypyrazine |
| IPMP | 3-isopropyl-2-methoxypyrazine |
| LBT | the ladybird taint |
| MPs | 3-alkyl-2-methoxypyrazines |
| OBP | odorant binding proteins |
| OMT | O-methyltransferase |
| SAM | S-adenosyl-l-methionine |
| SBMP | 3-secbutyl-2-methoxypyrazine |
| UDP | Uridine Diphosphate |
References
- Allen, M.S.; Lacey, M.J.; Harris, R.L.N.; Brown, W. Contribution of methoxypyrazines to Sauvignon blanc wine aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar]
- Maga, J.A. Sensory and stability properties of added methoxypyrazines to model and authentic wines. Dev. Food Sci. 1990, 61–70. [Google Scholar]
- Villière, A.; Le Roy, S.; Fillonneau, C.; Prost, C. Innoscent system: Advancing flavor analysis using an original gas chromatographic analytical device. J. Chromatogr. A 2018, 153, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Roujou de Boubée, D.; Leeuwen, C.V.; Dubourdieu, D. Organoleptic impact of 2-methoxy-3-isobutylpyrazine on red bordeaux and loire wines. Effect of environmental conditions on concentrations in grapes during ripening. J. Agric. Food Chem. 2000, 48, 4830–4834. [Google Scholar] [CrossRef] [PubMed]
- Lund, C.M.; Thompson, M.K.; Benkwitz, F.; Wohler, M.W.; Triggs, C.M.; Gardner, R.; Heymann, H.; Nicolau, L. New Zealand Sauvignon blanc distinct flavor characteristics: Sensory, chemical, and consumer aspects. Am. J. Enol. Vitic. 2009, 60, 1–12. [Google Scholar]
- King, E.S.; Osidacz, P.; Curtin, C.; Bastian, S.E.P.; Francis, I.L. Assessing desirable levels of sensory properties in Sauvignon Blanc wines—Consumer preferences and contribution of key aroma compounds. Aust. J. Grape Wine Res. 2011, 17, 169–180. [Google Scholar] [CrossRef]
- Simpson, R.F.; Capone, D.L.; Sefton, M.A. Isolation and identification of 2-methoxy-3,5-dimethylpyrazine, a potent musty compound from wine corks. J. Agric. Food Chem. 2004, 52, 5425–5430. [Google Scholar] [CrossRef]
- Botezatu, A.; Pickering, G.J. Determination of ortho- and retronasal detection thresholds and odor impact of 2,5-dimethyl-3-methoxypyrazine in wine. J. Food Sci. 2012, 77, S394–S398. [Google Scholar] [CrossRef]
- Wagner, R.; Czerny, M.; Bielohradsky, J.; Grosch, W. Structure odour-activity relationships of alkylpyrazines. Z. Lebensm. Lebensm.-Unters. Und-Forsch. A Food Res. Technol. 1999, 208, 308–316. [Google Scholar] [CrossRef]
- Wailzer, B.; Klocker, J.; Buchbauer, G.; Ecker, G.; Wolschann, P. Prediction of the aroma quality and the threshold values of some pyrazines using artificial neural networks. J. Med. Chem. 2001, 44, 2805–2813. [Google Scholar] [CrossRef]
- Yoshii, F.; Hirono, S. Construction of a quantitative three-dimensional model for odor quality using comparative molecular field analysis (CoMFA). Chem. Senses 1996, 21, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Goleblowski, J.; Antonczak, S.; Cabrol-Bass, D. Molecular dynamics studies of odorant binding protein free of ligand and complexed to pyrazine and octenol. J. Mol. Struct. Theochem 2006, 763, 165–174. [Google Scholar] [CrossRef]
- Paolini, S.; Tanfani, F.; Fini, C.; Bertoli, E.; Pelosi, P. Porcine odorant-binding protein: Structural stability and ligand affinities measured by Fourier-transform infrared spectroscopy and fluorescence spectroscopy. Biochim. Et Biophys. ACTA-Protein Struct. Mol. Enzymol. 1999, 1431, 179–188. [Google Scholar] [CrossRef]
- Pelosi, P.; Baldaccini, N.E.; Pisanelli, A.M. Identification of a specific olfactory receptor for 2-isobutyl-3-methoxypyrazine. Biochem. J. 1982, 201, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Iannario, M.; Manisera, M.; Piccolo, D.; Zuccolotto, P. Sensory analysis in the food industry as a tool for marketing decisions. Adv. Data Anal. Classif. 2012, 6, 303–321. [Google Scholar] [CrossRef]
- Zakarya, D.; Farhaoui, L.; Hamidi, M.; Bouachrine, M. Structure-olfactive threshold relationships for pyrazine derivatives. J. Mol. Model. 2006, 12, 985. [Google Scholar] [CrossRef]
- Alberts, P.; Stander, M.A.; Paul, S.O.; De Villiers, A. Survey of 3-alkyl-2-methoxypyrazine content of south african sauvignon blanc wines using a novel LC-APCI-MS/MS method. J. Agric. Food Chem. 2009, 57, 9347–9355. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Baumes, R.L.; Bertrand ASkouroumounis, G.K. Quantitative determination of 2-methoxy-3-isobutylpyrazine in red wines and grapes of Bordeaux using a stable isotope dilution assay. J. Chromatogr. A 1999, 841, 229–237. [Google Scholar] [CrossRef]
- Belancic, A.; Agosin, E. Methoxypyrazines in grapes and wines of vitis vinifera cv. carmenere. Am. J. Enol. Vitic. 2007, 58, 462–469. [Google Scholar]
- Roujou de Boubée, D.; Cumsille, A.M.; Pons, M.; Dubourdieu, D. Location of 2-methoxy-3-isobutylpyrazine in Cabernet Sauvignon grape bunches and its extractability during vinification. Am. J. Enol. Vitic. 2002, 53, 1–5. [Google Scholar]
- Koundouras, S. Environmental and Viticultural Effects on Grape Composition and Wine Sensory Properties. Elements 2018, 14, 173–178. [Google Scholar] [CrossRef]
- Hashizume, K.; Samuta, T. Grape maturity and light exposure affect berry methoxypyrazine concentration. Am. J. Enol. Vitic. 1999, 50, 194–198. [Google Scholar]
- Ryona, I.; Pan, B.S.; Intrigliolo, D.S.; Lakso, A.N.; Sacks, G.L. Effects of cluster light exposure on 3-isobutyl-2-methoxypyrazine accumulation and degradation patterns in red wine grapes (Vitis vinifera L. Cv. Cabernet Franc). J. Agric. Food Chem. 2008, 56, 10838–10846. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Busto, O.; Guasch, J.; Zamora, F. Contents of 3-alkyl-2-methoxypyrazines in musts and wines from Vitis vinifera variety Cabernet Sauvignon: Influence of irrigation and plantation density. J. Sci. Food Agric. 2005, 85, 1131–1136. [Google Scholar] [CrossRef]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes, musts, and wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Šuklje, K.; Antalick, G.; Coetzee, Z.; Schmidtke, L.M.; Cesnik, H.B.; Brandt, J.; Du Toit, W.J.; Lisjak, K.; Deloire, A. Effect of leaf removal and ultraviolet radiation on the composition and sensory perception of Vitis vinifera L. cv. Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2014, 20, 223–233. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, P.; Liu, C.; Wu, B.; Li, S.; Liang, Z. Sunlight exclusion from Muscat grape alters volatile profiles during berry development. Food Chem. 2014, 164, 242–250. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines biosynthesis and metabolism in grape: A review. Food Chem. 2018, 245, 1141–1147. [Google Scholar] [CrossRef]
- Dunlevy, J.D.; Soole, K.L.; Perkins, M.V.; Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Boss, P.K. Two O-methyltransferases involved in the biosynthesis of methoxypyrazines: Grape-derived aroma compounds important to wine flavour. Plant Mol. Biol. 2010, 74, 77–89. [Google Scholar] [CrossRef]
- Dunlevy, J.D.; Soole, K.L.; Perkins, M.V.; Nicholson, E.L.; Maffei, S.; Boss, P.K. Determining the methoxypyrazine biosynthesis variables affected by light exposure and crop level in Cabernet Sauvignon. Am. J. Enol. Vitic. 2013, 64, 450–458. [Google Scholar] [CrossRef]
- Guillaumie, S.; Ilg, A.; Réty, S.; Brette, M.; Trossat-Magnin, C.; Decroocq, S.; Léon, C.; Keime, C.; Ye, T.; Baltenweck-Guyot, R.; et al. Genetic analysis of the biosynthesis of 2-methoxy-3-isobutylpyrazine, a major grape-derived aroma compound impacting wine quality. Plant Physiol. 2013, 162, 604–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.M.; Li, C.H.; Tsao, N.W.; Su, L.W.; Lu, Y.T.; Chang, S.H.; Lin, Y.Y.; Liou, J.C.; Hsieh, L.C.; Yu, J.Z.; et al. Phytoplasma SAP11 alters 3-isobutyl-2-methoxypyrazine biosynthesis in Nicotiana benthamiana by suppressing NbOMT1. J. Exp. Bot. 2016, 67, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Gregan, S.M.; Jordan, B. Methoxypyrazine accumulation and O-methyltransferase gene expression in Sauvignon blanc grapes: The role of leaf removal, light exposure, and berry development. J. Agric. Food Chem. 2016, 64, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Campo, E.; Farina, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.; Sen, A.; Grosch, W. Potent odorants of the roasted powder and brew of Arabica coffee. Z. Lebensm-Unters. Und-Forsch. 1992, 195, 239–245. [Google Scholar] [CrossRef]
- Slabizki, P.; Legrum, C.; Wegmannherr, P.; Fischer, C.; Schmarr, H.G. Quantification of cork off-flavor compounds in natural cork stoppers and wine by multidimensional gas chromatography mass spectrometry. Eur. Food Res. Technol. 2015, 242, 1–10. [Google Scholar] [CrossRef]
- Koch, A.L. Quantification of the Effects of Genotype and the Environment on 2-Methoxy-3-Isobutylpyrazine in the Fruit of Vitis vinifera L. cv. Cabernet Sauvignon. Ph.D. Thesis, University of California, Oakland, CA, USA, 2009. [Google Scholar]
- Pevsner, J.; Trifiletti, R.R.; Strittmatter, S.M.; Snyder, S.H. Isolation and characterization of an olfactory receptor protein for odorant pyrazines. Proc. Natl. Acad. Sci. USA 1985, 82, 3050–3054. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, C. Oxidation Treatments Affecting Sauvignon Blanc Wine Sensory and Chemical Composition. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Lawless, H. Sensory Evaluation of Food: Principles and Practices; Heymann, H., Ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- ISO. 11136. Sensory analysis:Methodology. In General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. 2014. Available online: https://www.iso.org (accessed on 3 May 2018).
- Culleré, L.; López, R.; Ferreira, V. Red Wine Technology; Morata, A., Ed.; Elsevier Academic Press Inc.: London, UK, 2019. [Google Scholar]
- Goldner, M.C.; Zamora, M.C.; Lira, P.D.L.; Gianninoto, H.; Bandoni, A. Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 2009, 24, 243–257. [Google Scholar] [CrossRef]
- Hartmann, P.J.; Mcnair, H.M.; Zoecklein, B.W. Measurement of 3-alkyl-2-methoxypyrazine by headspace solid-phase microextraction in spiked model wines. Am. J. Enol. Vitic. 2002, 53, 285–288. [Google Scholar]
- Parliment, T.H.; Epstein, M.F. Organoleptic poperties of sme akyl-sbstituted akoxy- and akylthiopyrazines. J. Agric. Food Chem. 1973, 4, 714–716. [Google Scholar] [CrossRef]
- Murray, K.E.; Shipton, J.; Whitfield, F.B. 2-Methoxypyrazines and the flavour of green peas (Pisum sativum). Chem. Ind. 1970, 27, 897–898. [Google Scholar] [PubMed]
- Alberts, P.; Kidd, M.; Stander, M.A.; Nieuwoudt, H.H.; Tredoux, A.G.J.; de Villiers, A. Quantitative survey of 3-alkyl-2-methoxypyrazines and first confirmation of 3-ethyl-2-methoxypyrazine in South African Sauvignon blanc wines. S. Afr. J. Enol. Vitic. 2013, 34, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Seifert, R.M.E.A. Synthesis of some 2-methoxy-3-alkylpyrazines with strong bell pepper-like odors. J. Agric. Food Chem. 1970, 18, 246–249. [Google Scholar] [CrossRef]
- Czerny, M.; Grosch, W. Potent odorants of raw Arabica coffee. Their changes during roasting. J. Agric. Food Chem. 2000, 48, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Slabizki, P.; Legrum, C.; Meusinger, R.; Schmarr, H. Characterization and analysis of structural isomers of dimethyl methoxypyrazines in cork stoppers and ladybugs (Harmonia axyridis and Coccinella septempunctata). Anal. Bioanal. Chem. 2014, 406, 6429–6439. [Google Scholar] [CrossRef] [PubMed]
- Zakarya, D. Use of autocorrelation components and Wiener index in the evaluation of the odor threshold of aliphatic alcohols. New J. Chem. 1992, 16, 1039–1042. [Google Scholar]
- Buchbauer, G.; Klein, C.T.; Wailzer, B.; Wolschann, P. Threshold-based structure-activity relationships of pyrazines with bell-pepper flavor. J. Sci. Food Agric. 2000, 48, 4273–4278. [Google Scholar] [CrossRef]
- Nursten, H.E.; Sheen, M.R. Volatile flavour components of cooked potato. J. Sci. Food Agric. 1974, 25, 643–663. [Google Scholar] [CrossRef]
- Gallois, A.; Kergomard, A.; Adda, J. Study of the biosynthesis of 3-isopropyl-2-methoxypyrazine produced by Pseudomonas taetrolens. Food Chem. 1988, 28, 299–309. [Google Scholar] [CrossRef]
- Cheng, T.; Reineccius, G.; Bjorklund, J.; Leete, E. Biosynthesis of 2-methoxy-3-isopropylpyrazine in Pseudomonas perolens. J. Agric. Food Chem. 1991, 39, 1009–1012. [Google Scholar] [CrossRef]
- Nawrath, T.; Dickschat, J.S.; Kunze, B.; Schulz, S. The biosynthesis of branched dialkylpyrazines in myxobacteria. Chem. Biodivers. 2010, 7, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Roujou de Boubée. Research on 2-Methoxy-3-Isobutylpyrazine in Grapes and Wines; Acadamie Amorim: Bordeaux, Frence, 2003. [Google Scholar]
- Lei, Y.; Xie, S.; Chen, H.; Guan, X.; Zhang, Z. Behavior of 3-isobutyl-2-methoxypyrazine biosynthesis related to proposed precursor and intermediate in wine grape. Food Chem. 2019, 277, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ryona, I.; Leclerc, S.; Sacks, G.L. Correlation of 3-isobutyl-2-methoxypyrazine to 3-isobutyl-2-hydroxypyrazine during maturation of bell pepper (Capsicum annuum) and wine grapes (Vitis vinifera). J. Agric. Food Chem. 2010, 58, 9723–9730. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Ryona, I.; Sacks, G.L. Behavior of 3-isobutyl-2-hydroxypyrazine (IBHP), a key intermediate in 3-isobutyl-2-methoxypyrazine (IBMP) metabolism, in ripening wine grapes. J. Agric. Food Chem. 2012, 60, 11901–11908. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, G.; Scheline, R.R. Metabolism in the rat of some pyrazine derivatives having flavour importance in foods. Xenobiotica 1975, 5, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kennison, K.R.; Gibberd, M.R.; Pollnitz, A.P.; Wilkinson, K.L. Smoke-derived taint in wine: The release of smoke-derived volatile phenols during fermentation of Merlot juice following grapevine exposure to smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef]
- Hashizume, K.; Tozawa, K.; Hiraga, Y.; Aramaki, I. Purification and characterization of a O-methyltransferase capable of methylating 2-hydroxy-3-alkylpyrazine from Vitis vinifera L. (cv. Cabernet Sauvignon). Biosci. Biotechnol. Biochem. 2001, 65, 2213–2219. [Google Scholar] [CrossRef]
- Vallarino, J.G.; Lopez-Cortes, X.A.; Dunlevy, J.D.; Boss, P.K.; Gonzalez-Nilo, F.D.; Moreno, Y.M. Biosynthesis of methoxypyrazines: Elucidating the structural/functional relationship of two Vitis vinifera O-methyltransferases capable of catalyzing the putative final step of the biosynthesis of 3-alkyl-2-methoxypyrazine. J. Agric. Food Chem. 2011, 59, 7310–7316. [Google Scholar] [CrossRef]
- Battilana, J.; Dunlevy, J.D.; Boss, P.K. Histone modifications at the grapevine VvOMT3 locus, which encodes an enzyme responsible for methoxypyrazine production in the berry. Funct. Plant Biol. 2017, 44, 655–664. [Google Scholar] [CrossRef]
- Frato, K.E. Identification of Hydroxypyrazine O-Methyltransferase Genes in Coffea arabica: A Potential Source of Methoxypyrazines That Cause Potato Taste Defect. J. Agric. Food Chem. 2019, 67, 341–351. [Google Scholar] [CrossRef]
- Vallarino, J.G.; Gainza-Cortes, F.; Verdugo-Alegria, C.; Gonzalez, E.; Moreno, Y.M. Abiotic stresses differentially affect the expression of O-methyltransferase genes related to methoxypyrazine biosynthesis in seeded and parthenocarpic fruits of Vitis vinifera (L.). Food Chem. 2014, 154, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Wang, Y.; Zhou, Z.; Bao, S.; Lin, Y.; Gong, W. Crystal structure of SAM-dependent O-methyltransferase from pathogenic bacterium Leptospira interrogans. J. Struct. Biol. 2007, 159, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, M.Z.; Zhu, S.L.; Li, M.; Dong, X.; Luo, X.C.; Kong, Z.; Lu, Y.X.; Wang, S.Y.; Tong, W.Y. Studies on the function and catalytic mechanism of O-methyltransferases SviOMT02, SviOMT03 and SviOMT06 from Streptomyces virginiae IBL14. Enzym. Microb. Technol. 2015, 73, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kopycki, J.G.; Rauh, D.; Chumanevich, A.A.; Neumann, P.; Vogt, T.; Stubbs, M.T. Biochemical and structural analysis of substrate promiscuity in plant Mg2+-dependent O-methyltransferases. J. Mol. Biol. 2008, 378, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.X.; Gao, S.; Zhao, Y.; Lou, H.X.; Cheng, A.X. Functional characterization of a Mg2+-dependent O-methyltransferase with coumarin as preferred substrate from the liverwort Plagiochasma appendiculatum. Plant Physiol. Biochem. 2016, 106, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.L.; Zubieta, C.; Dixon, R.A.; Noel, J.P. Crystal structures of alfalfa caffeoyl coenzyme A 3-O-methyltransferase. Plant Physiol. 2005, 137, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.M.; Sattler, S.A.; Regner, M.; Jones, J.P.; Ralph, J.; Vermerris, W.; Sattler, S.E.; Kang, C. The structure and catalytic mechanism of Sorghum bicolor caffeoyl-CoA O-methyltransferase. Plant Physiol. 2016, 172, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.C.; Ibrahim, R.K.; Behdad, B.; Dayanandan, S. Structure, function, and evolution of plant O-methyltransferases. Gemome 2007, 50, 1001–1013. [Google Scholar]
- Lacey, M.J.; Allen, M.S.; Rln, H.; Brown, W. Methoxypyrazines in Sauvignon blanc grapes and wines. Am. J. Enol. Vitic. 1991, 42, 103–108. [Google Scholar]
- Koch, R.L.; Burkness, E.C.; Burkness, S.J.; Hutchison, W.D. Phytophagous preferences of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) for autumn-ripening fruit. J. Econ. Entomol. 2004, 97, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Cudjoe, E.; Wiederkehr, T.B.; Brindle, I.D. Headspace gas chromatography-mass spectrometry: A fast approach to the identification and determination of 2-alkyl-3-methoxypyrazine pheromones in ladybugs. Analyst 2005, 130, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.P.; Brown, W.V.; Rothschild, M. Methylalkylpyrazines in aposematic insects, their hostplants and mimics. Chemoecology 1990, 1, 43–51. [Google Scholar] [CrossRef]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, L.W.; Lodge, B.A.; By, A.W.; Thomas, B.H. Metabolic disposition of pyrazinamide in the rat: Identification of a novel in vivo metabolite common to both rat and human. Biopharm. Drug Dispos. 1987, 8, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Moriwaki, Y.; Takahashi, S.; Hada, T.; Higashino, K. In vitro conversion of pyrazinamide into 5-hydroxypyrazinamide and that of pyrazinoic acid into 5-hydroxypyrazinoic acid by xanthine oxidase from human liver. Biochem. Pharmacol. 1987, 36, 3317–3318. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Biochemistry and occurrence of o-demethylation in plant metabolism. Front. Physiol. 2010, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Inglis, D.; Brindle, I.D.; Sears, M.; Beh, A.L. Yeast strain affects 3-isopropyl-2-methoxypyrazine concentration and sensory profile in Cabernet Sauvignon wine. Aust. J. Grape Wine Res. 2008, 14, 230–237. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Heymann, H.; Noble, A.C.; Boulton, R.B. Analysis of methoxypyrazines in wines. 1. Development of a quantitative procedure. J. Agric. Food Chem. 1986, 34, 268–271. [Google Scholar] [CrossRef]
- Noble, A.C.; Elliottfisk, D.L.; Allen, M.S. ACS Symposium Series; Rouseff, R.L., Leahy, M.M., Eds.; ACS: Washington, DC, USA, 1995; pp. 226–234. [Google Scholar]
- Allen, M.S.; Lacey, M. Methoxypyrazine grape flavour: Influence of climate, cultivar and viticulture. Die Wein-Wiss. 1993, 48, 211–213. [Google Scholar]
- Szostak, R.; de Souza, E.C.F.; Antunes, S.R.M.; Borges, C.P.F.; de Andrade, A.V.C.; Rodrigues, P.R.P.; Antunes, A.C. Anthocyanin fromvitis labruscagrape used as sensitizer in dssc solar cells. J. Mater. Sci. Mater. Electron. 2015, 26, 2257–2262. [Google Scholar] [CrossRef]
- Morrison, J.C.; Noble, A.C. The effects of leaf and cluster shading on the composition of Cabernet Sauvignon grapes and on fruit and wine sensory properties. Am. J. Enol. Vitic. 1990, 41, 193–200. [Google Scholar]
- Plank, C. Effect of Light Environment on Methoxypyrazine Content of Cabernet Sauvignon. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 2014. [Google Scholar]
- Plank, C.M.; Hellman, E.W.; Montague, T. Light and Temperature Independently Influence Methoxypyrazine Content of Vitis vinifera (cv. Cabernet Sauvignon) Berries. HortScience 2019, 54, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Blake, A.; Kotseridis, Y.; Brindle, I.D.; Inglis, D.; Pickering, G.J. Effect of light and temperature on 3-alkyl-2-methoxypyrazine concentration and other impact odourants of Riesling and Cabernet Franc wine during bottle ageing. Food Chem. 2010, 119, 935–944. [Google Scholar] [CrossRef]
- Pickering, G.; Lin, J.; Reynolds, A.; Soleas, G.; Riesen, R. The evaluation of remedial treatments for wine affected by Harmonia axyridis. Int. J. Food Sci. Technol. 2006, 41, 77–86. [Google Scholar] [CrossRef]
- Hashizume, K.; Umeda, N. Methoxypyrazine content of Japanese red wines. Biosci. Biotechnol. Biochem. 1996, 60, 802–805. [Google Scholar] [CrossRef]
- Hashizume, K.; Kida, S.; Samuta, T. Effect of steam treatment of grape cluster stems on the methoxypyrazine, phenolic, acid, and mineral content of red wines fermented with stems. J. Agric. Food Chem. 1998, 46, 4382–4386. [Google Scholar] [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; Jordans, C.; McCarthy, M.; Ford, C.M.; Dokoozlian, N. Effect of winter rainfall on yield components and fruit green aromas of Vitis vinifera L. cv. Merlot in California. Aust. J. Grape Wine Res. 2014, 20, 100–110. [Google Scholar] [CrossRef]
- Matthews, M.A.; Ishii, R.; Anderson, M.M.; O’Mahony, M. Dependence of wine sensory attributes on vine water status. J. Sci. Food Agric. 1990, 51, 321–335. [Google Scholar] [CrossRef]
- Hakimi Rezaei, J.; Reynolds, A.G. Impact of vine water status on sensory attributes of Cabernet franc wines in the Niagara Peninsula of Ontario. OENO One 2010, 44, 61–75. [Google Scholar] [CrossRef]
- Chapman, D.M.; Roby, G.; Ebeler, S.E.; Guinard, J.X.; Matthews, M.A. Sensory attributes of Cabernet Sauvignon wines made from vines with different water status. Aust. J. Grape Wine Res. 2005, 11, 339–347. [Google Scholar] [CrossRef]
- Helwi, P.; Habran, A.; Guillaumie, S.; Thibon, C.; Hilbert, G.; Gomes, E.; Delrot, S.; Darriet, P.; van Leeuwen, C. Vine nitrogen status does not have a direct impact on 2-methoxy-3-isobutylpyrazine in grape berries and wines. J. Agric. Food Chem. 2015, 63, 9789–9802. [Google Scholar] [CrossRef] [PubMed]
- Guti Rrez-Gamboa, G.N.; Garde-Cerd, N.T.; Gonzalo-Diago, A.; Moreno-Simunovic, Y.; Mart Nez-Gil, A.M. Effect of different foliar nitrogen applications on the must amino acids and glutathione composition in Cabernet Sauvignon vineyard. LWT-Food Sci. Technol. 2017, 75, 147–154. [Google Scholar] [CrossRef]
- Galvan, T.L. Feeding Behavior, Phenology, and Management of the Multicolored Asian Lady Beetle, Harmonia axyridis, in Wine Grapes. Ph.D. Thesis, The University of Minnesota, Minneapolis, MN, USA, 2008. [Google Scholar]
- Kögel, S.; Gross, J.; Hoffmann, C.; Ulrich, D. Diversity and frequencies of methoxypyrazines in hemolymph of Harmonia axyridis and Coccinella septempunctata and their influence on the taste of wine. Eur. Food Res. Technol. 2012, 234, 399–404. [Google Scholar] [CrossRef]
- Botezatu, A.I.; Kotseridis, Y.; Inglis, D.; Pickering, G.J. Occurrence and contribution of alkyl methoxypyrazines in wine tainted by Harmonia axyridis and Coccinella septempunctata. J. Sci. Food Agric. 2013, 93, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Koziel, J.A.; O’Neal, M.E. Determination of characteristic odorants from Harmonia axyridis beetles using in vivo solid-phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. J. Chromatogr. A 2007, 1147, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Brindle, I.D.; Sears, M.; Inglis, D. The influence of Harmonia axyridis morbidity on 2-Isopropyl-3-methoxy-pyrazine in Cabernet Sauvignon’ wine. Vitis 2008, 47, 227–230. [Google Scholar]
- Pickering, G.; Lin, J.; Riesen, R.; Reynolds, A.; Brindle, I.; Soleas, G. Influence of Harmonia axyridis on the sensory properties of white and red wine. Am. J. Enol. Vitic. 2004, 55, 153–159. [Google Scholar]
- Galvan, T.L.; Burkness, E.C.; Vickers, Z.; Stenberg, P.; Mansfield, A.K.; Hutchison, W.D. Sensory-based action threshold for multicolored Asian lady beetle-related taint in wine grapes. Am. J. Enol. Vitic. 2007, 58, 518–522. [Google Scholar]
- Kögel, S.; Gross, J.; Hoffmann, C. Sensory detection thresholds of “ladybird taint” in “Riesling” and “Pinot Noir” under different fermentation and processing conditions. Vitis 2012, 51, 27–32. [Google Scholar]
- Pickering, G.J.; Ker, K.; Soleas, J.G. Determination of the critical stages of processing and tolerance limits for Harmonia axyridis for ‘ladybug taint’ in wine. Vitis 2007, 46, 85–90. [Google Scholar]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Acronym | Threshold (ng/L) | Matrix | Reference |
|---|---|---|---|
| IPMP | 2 | White wine | [1] |
| 0.0005–0.001 * | Air | [35] | |
| 1–2 | Water | [18,45,46] | |
| 2 | Red wine | [2] | |
| 2 | Synthetic wine | [2] | |
| IBMP | 0.002–0.004 * | Air | [35] |
| 0.5–16 | Water | [2,18,45] | |
| 1 | White wine | [1] | |
| 10–16 | Red wine | [2,4,18] | |
| 2–6 | Synthetic wine | [2,4] | |
| SBMP | 1–2 | Water | [45] |
| EMP | 425 | Water | [47,48] |
| DMMP | 56 | Air | [49,50] |
| 31 ** | Red wine | [8] | |
| 70 *** | Red wine | [8] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Ju, Y.; Wei, X.; Dong, S.; Sun, X.; Fang, Y. Significance and Transformation of 3-Alkyl-2-Methoxypyrazines Through Grapes to Wine: Olfactory Properties, Metabolism, Biochemical Regulation, and the HP–MP Cycle. Molecules 2019, 24, 4598. https://doi.org/10.3390/molecules24244598
Zhao X, Ju Y, Wei X, Dong S, Sun X, Fang Y. Significance and Transformation of 3-Alkyl-2-Methoxypyrazines Through Grapes to Wine: Olfactory Properties, Metabolism, Biochemical Regulation, and the HP–MP Cycle. Molecules. 2019; 24(24):4598. https://doi.org/10.3390/molecules24244598
Chicago/Turabian StyleZhao, Xianfang, Yanlun Ju, Xiaofeng Wei, Shuo Dong, Xiangyu Sun, and Yulin Fang. 2019. "Significance and Transformation of 3-Alkyl-2-Methoxypyrazines Through Grapes to Wine: Olfactory Properties, Metabolism, Biochemical Regulation, and the HP–MP Cycle" Molecules 24, no. 24: 4598. https://doi.org/10.3390/molecules24244598
APA StyleZhao, X., Ju, Y., Wei, X., Dong, S., Sun, X., & Fang, Y. (2019). Significance and Transformation of 3-Alkyl-2-Methoxypyrazines Through Grapes to Wine: Olfactory Properties, Metabolism, Biochemical Regulation, and the HP–MP Cycle. Molecules, 24(24), 4598. https://doi.org/10.3390/molecules24244598




