The Biochemical and Functional Characterization of M28 Aminopeptidase Protein Secreted by Acanthamoeba spp. on Host Cell Interaction
Abstract
:1. Introduction
2. Results
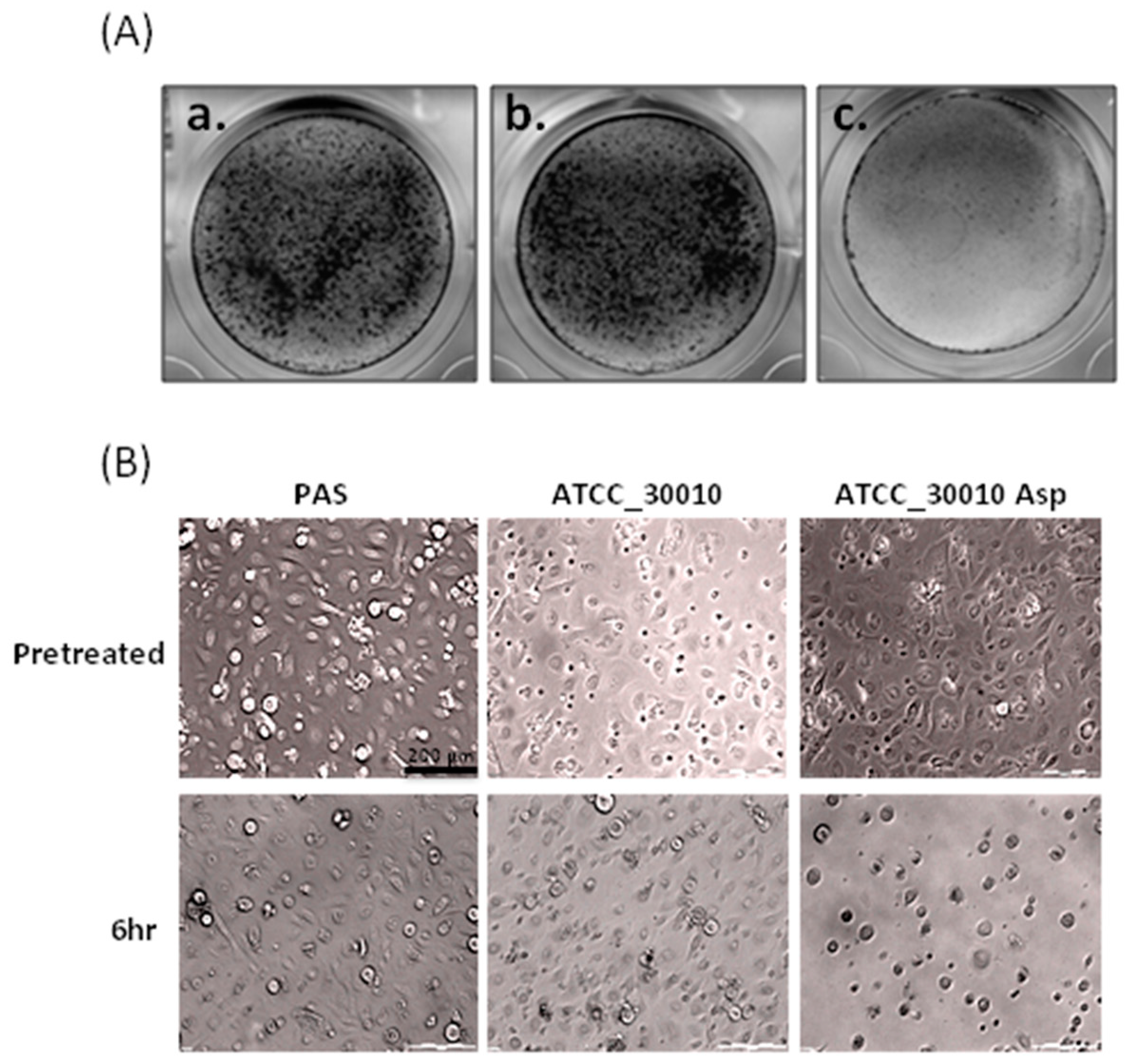
2.1. The Virulence of Asp Induces Cell Damage in Human Primary Corneal Epithelial Cells
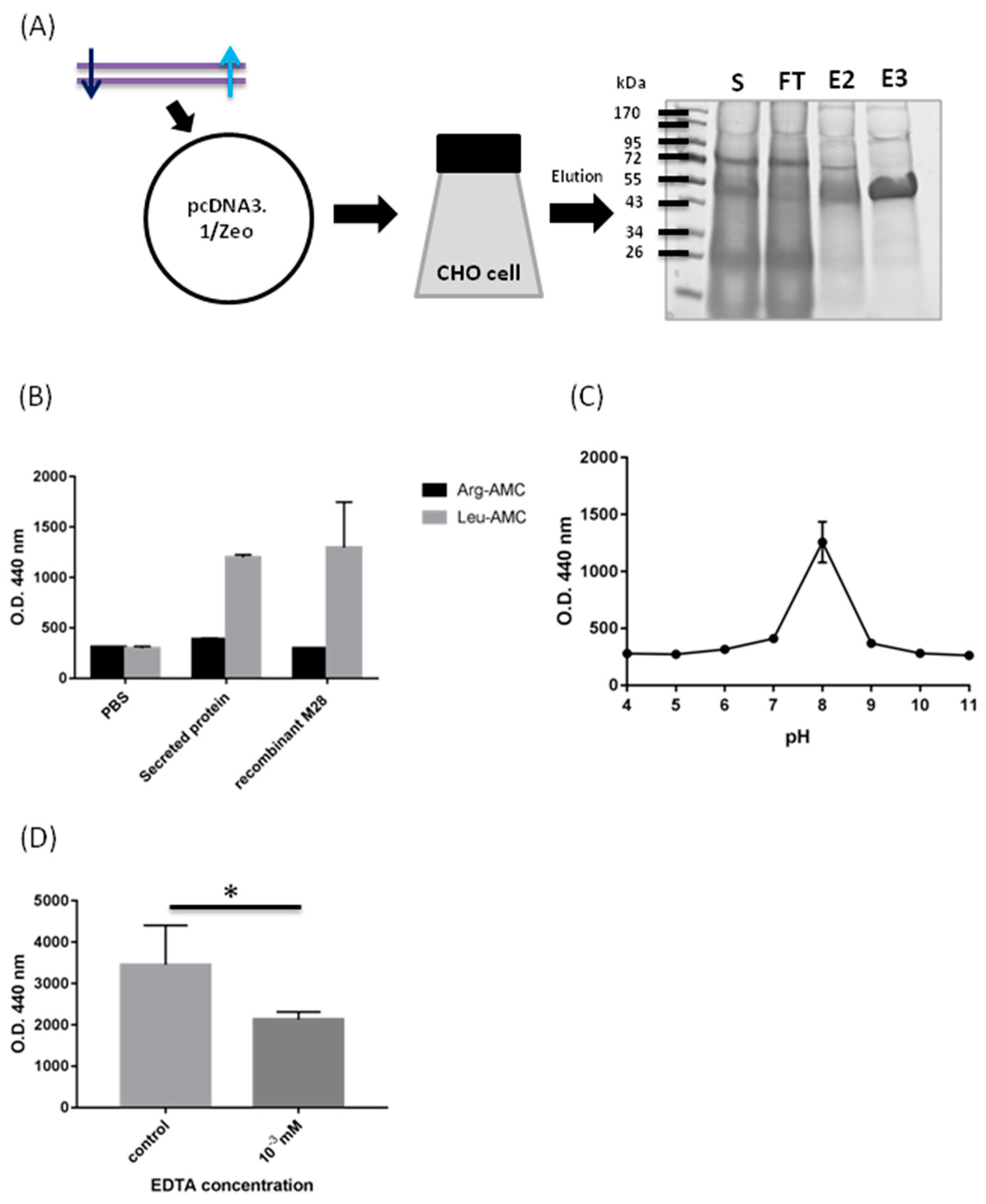
2.2. Biochemical Characterization of M28AP
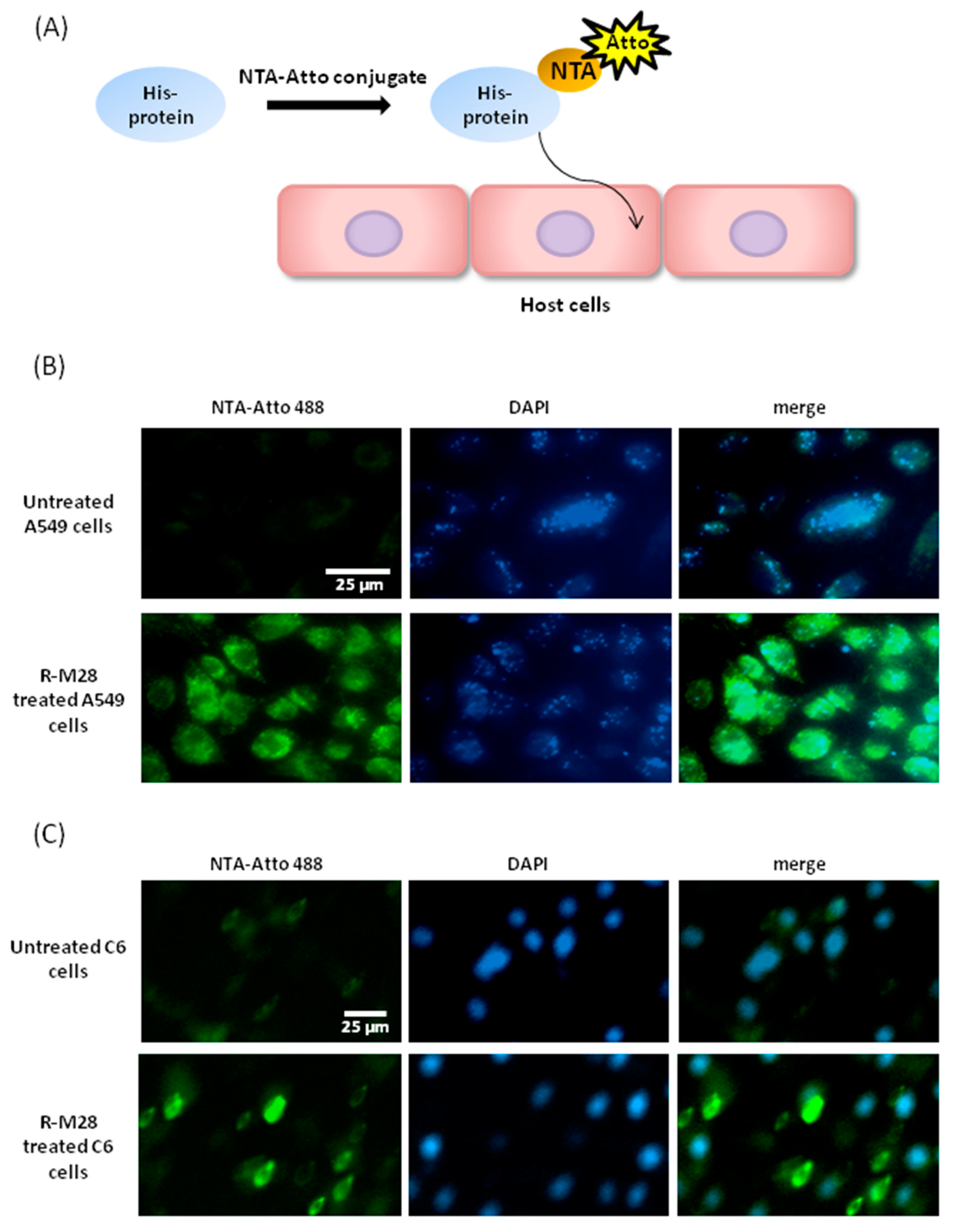
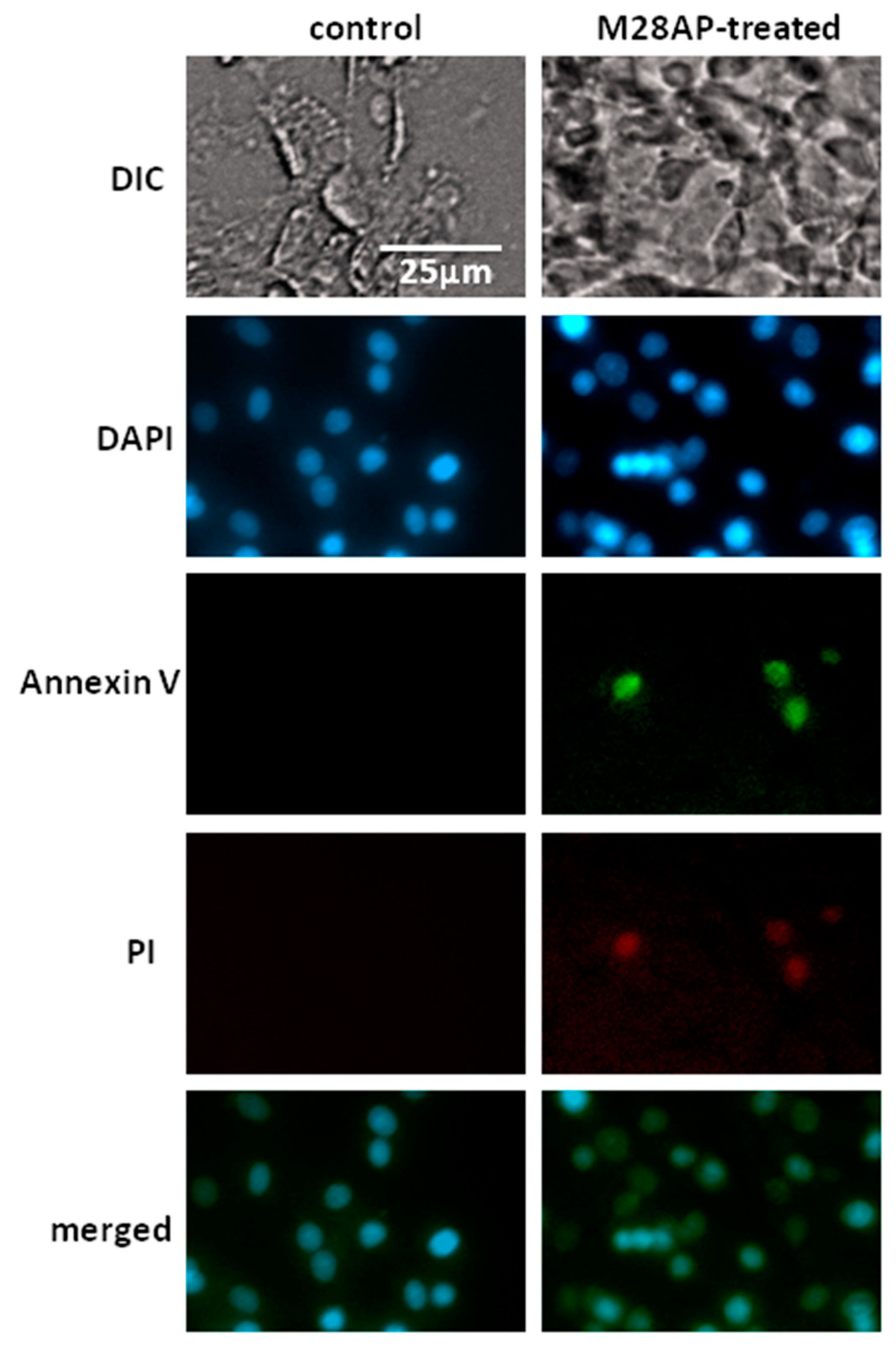
2.3. The Role of M28Ap as Virulence Factor and Host Cell Damage
3. Discussion
3.1. Biochemical Characterization of M28AP
3.2. Cell Line Apoptosis by M28AP
4. Materials and Methods
4.1. Culture of Acanthamoeba Strains
4.2. Culture of C6 Glioma Rat Cell Line, A549 Human Cell Line and Human Primary Corneal Epithelial Cells
4.3. Isolation of Secreted Proteins
4.4. Cytopathic Effect Assay (CPE) and Giemsa Stain
4.5. Total RNA Isolation and cDNA Synthesis
4.6. DNA Construction, Cell Culture and Transient Transfection
4.7. SDS-PAGE and Coomassie Blue Staining
4.8. Biochemical Properties of Recombinant Protein M28AP
4.9. Ni-NTA-Atto Conjugates for Recombinant Protein M28AP- Cell Line Interaction
4.10. Annexin V Apoptosis Assay
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCulley, J.P.; Alizadeh, H.; Niederkorn, J.Y. Acanthamoeba Keratitis. Eye. Contact. Lens. 1995, 21, 73–76. [Google Scholar]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and Opportunistic Free-Living Amoebae: Acanthamoeba spp., Balamuthia Mandrillaris, Naegleria Fowleri, and Sappinia Diploidea. FEMS Immunol. Med. Mic. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.; Cursons, R.; Keys, E. Amoebae from Antarctic Soil and Water. Appl. Environ. Microbiol. 1982, 44, 491–493. [Google Scholar]
- PAGE, F.C. Re-Definition of the Genus Acanthamoeba with Descriptions of Three Species. J. Protozool. 1967, 14, 709–724. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as Agents of Disease in Humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.J.; Visvesvara, G.S. Free-living, Amphizoic and Opportunistic Amebas. Brain. Pathol. 1997, 7, 583–598. [Google Scholar] [CrossRef]
- Salameh, A.; Bello, N.; Becker, J.; Zangeneh, T. Fatal granulomatous amoebic encephalitis caused by Acanthamoeba in a patient with kidney transplant: A case report. Open Forum Infect. Dis. 2015, 2, ofv104. [Google Scholar] [CrossRef]
- Broxton, P.; Woodcock, P.; Gilbert, P. A study of the Antibacterial Activity of Some Polyhexamethylene Biguanides towards Escherichia Coli ATCC 8739. J. Appl. Bacteriol. 1983, 54, 345–353. [Google Scholar] [CrossRef]
- Auran, J.D.; Starr, M.B.; Jakobiec, F.A. Acanthamoeba Keratitis. Cornea 1987, 6, 2–26. [Google Scholar] [CrossRef]
- Allen, M.J.; Morby, A.P.; White, G.F. Cooperativity in the Binding of the Cationic Biocide Polyhexamethylene Biguanide to Nucleic Acids. Biochem. Biophys. Res. Commun. 2004, 318, 397–404. [Google Scholar] [CrossRef]
- Li, M.-L.; Shih, M.-H.; Huang, F.-C.; Tseng, S.-H.; Chen, C.-C. Treatment of early Acanthamoeba Keratitis with Alcohol-Assisted Epithelial Debridement. Cornea 2012, 31, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An Update on Acanthamoeba Keratitis: Diagnosis, Pathogenesis and Treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, D.W.; Niederkorn, J.Y. The Pathophysiology of Acanthamoeba Keratitis. Trends. Parasitol. 2006, 22, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Biology and Pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A. Acanthamoeba: Biology and Increasing Importance in Human Health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef] [Green Version]
- Wynter-Allison, Z.; Lorenzo Morales, J.; Calder, D.; Radlein, K.; Ortega-Rivas, A.; Lindo, J.F. Acanthamoeba Infection as a Cause of Severe Keratitis in a Soft Contact Lens Wearer in Jamaica. Am. J. Trop. Med. Hyg. 2005, 73, 92–94. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and Pathophysiology of Matrix Metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [Green Version]
- Sbardella, D.; Fasciglione, G.F.; Gioia, M.; Ciaccio, C.; Tundo, G.R.; Marini, S.; Coletta, M. Human Matrix Metalloproteinases: An Ubiquitarian Class of Enzymes involved in Several Pathological Processes. Mol. Aspects. Med. 2012, 33, 119–208. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Cheng, Z.; Jia, H.; Zheng, Y. Characterization of Aspartyl Aminopeptidase from Toxoplasma Gondii. Sci. Rep. 2016, 6, 34448. [Google Scholar] [CrossRef]
- Murase, L.S.; de Souza, J.V.P.; de Lima Neto, Q.A.; de Mello, T.F.P.; Cardoso, B.M.; Lera-Nonose, D.S.S.L.; Teixeira, J.J.V.; Lonardoni, M.V.C.; Demarchi, I.G. The Role of Metalloproteases in Leishmania Species Infection in the New World: A Systematic Review. Parasitology 2018, 145, 1499–1509. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Na, B.-K.; Moon, E.-K.; Song, S.-M.; Joo, S.-Y.; Kong, H.-H.; Goo, Y.-K.; Chung, D.-I.; Hong, Y. Essential Role for an M17 Leucine Aminopeptidase in Encystation of Acanthamoeba Castellanii. PLoS ONE 2015, 10, e0129884. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Ortega-Rivas, A.; Foronda, P.; Abreu-Acosta, N.; Ballart, D.; Martinez, E.; Valladares, B. RNA Interference (RNAi) for the Silencing of Extracellular Serine Proteases Genes in Acanthamoeba: Molecular Analysis and Effect on Pathogenecity. Mol. Biochem. Parasitol. 2005, 144, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Mattana, A.; Cappai, V.; Alberti, L.; Serra, C.; Fiori, P.L.; Cappuccinelli, P. ADP and Other Metabolites Released from Acanthamoeba Castellanii Lead to Human Monocytic Cell Death through Apoptosis and Stimulate the Secretion of Proinflammatory Cytokines. Infect. Immun. 2002, 70, 4424–4432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-M.; Liao, C.-C.; Kuo, C.-C.; Chen, L.-R.; Huang, L.; Shin, J.-W.; Lin, W.-C. Pathogenic Acanthamoeba Castellanii Secretes the Extracellular Aminopeptidase M20/M25/M40 Family Protein to Target Cells for Phagocytosis by Disruption. Molecules 2017, 22, 2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-M.; Lin, W.-C.; Li, S.-C.; Shih, M.-H.; Chan, W.-C.; Shin, J.-W.; Huang, F.-C. Comparative Proteomic Analysis of Extracellular Secreted Proteins Expressed by Two Pathogenic Acanthamoeba Castellanii Clinical Isolates and a Non-Pathogenic ATCC Strain. Exp. Parasitol. 2016, 166, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-M.; Chang, Y.-T.; Shih, M.-H.; Lin, W.-C.; Huang, F.-C. Identification and Characterization of a Secreted M28 Aminopeptidase Protein in Acanthamoeba. Parasitol. Res. 2019, 118, 1865–1874. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The Database of Proteolytic Enzymes, their Substrates and Inhibitors. Nucleic Acids Res. 2011, 40, D343–D350. [Google Scholar] [CrossRef] [Green Version]
- Barrett, A.J.; Woessner, J.F.; Rawlings, N.D. Handbook of Proteolytic Enzymes; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Rawlings, N.D.; Barrett, A.J. [13] Evolutionary Families of Metallopeptidases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 248, pp. 183–228. [Google Scholar]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural Properties of Matrix Metalloproteinases. Cell. Mol. Life. Sci. CMLS 1999, 55, 639–652. [Google Scholar] [CrossRef]
- Fricke, B.; Drößler, K.; Willhardt, I.; Schierhorn, A.; Menge, S.; Rücknagel, P. The Cell Envelope-Bound Metalloprotease (camelysin) from Bacillus Cereus is a Possible Pathogenic Factor. BBA-Mol. Basis Dis. 2001, 1537, 132–146. [Google Scholar] [CrossRef] [Green Version]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal human Tear pH by Direct Measurement. Arch. Ophthalmol. 1981, 99, 301. [Google Scholar] [CrossRef]
- Kellum, J.A. Determinants of Blood pH in Health and Disease. Crit. Care 2000, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.A. How Human Pathogenic Fungi Sense and Adapt to pH: The Link to Virulence. Curr. Opin. Microbiol. 2009, 12, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Datta, G.; Kaur, I.; Malhotra, P.; Mohmmed, A. Disruption of Cellular Homeostasis Induces Organelle Stress and Triggers Apoptosis like Cell-Death Pathways in Malaria Parasite. Cell Death Dis. 2015, 6, e1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igney, F.H.; Krammer, P.H. Death and Anti-Death: Tumour Resistance to Apoptosis. Nat. Rev. Cancer. 2002, 2, 277. [Google Scholar] [CrossRef]
- Martinvalet, D.; Zhu, P.; Lieberman, J. Granzyme A induces Caspase-Independent Mitochondrial Damage, a Required First Step for Apoptosis. Immunity 2005, 22, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Bienvenu, A.-L.; Gonzalez-Rey, E.; Picot, S. Apoptosis Induced by Parasitic Diseases. Parasites Vectors 2010, 3, 106. [Google Scholar] [CrossRef] [Green Version]
- Niyyati, M.; Abedkhojasteh, H.; Salehi, M.; Farnia, S.; Rezaeian, M. Axenic Cultivation and Pathogenic Assays of Acanthamoeba Strains using Physical Parameters. Iran. J. Parasitol. 2013, 8, 186. [Google Scholar]
- Thomas, V.; Herrera-Rimann, K.; Blanc, D.S.; Greub, G. Biodiversity of Amoebae and Amoeba-Resisting Bacteria in a Hospital Water Network. Appl. Environ. Microbiol. 2006, 72, 2428–2438. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.K.; Barkowski-Clark, S.; Altman, R.; Johnson, K.; Sun, F.; Zmuda, J.; Liu, C.Y.; Kita, A.; Schulz, R.; Neill, A.; et al. A high Density CHO-S Transient Transfection System: Comparison of ExpiCHO and Expi293. Protein Expr. Purif. 2017, 134, 38–46. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-M.; Chang, Y.-T.; Lin, W.-C. The Biochemical and Functional Characterization of M28 Aminopeptidase Protein Secreted by Acanthamoeba spp. on Host Cell Interaction. Molecules 2019, 24, 4573. https://doi.org/10.3390/molecules24244573
Huang J-M, Chang Y-T, Lin W-C. The Biochemical and Functional Characterization of M28 Aminopeptidase Protein Secreted by Acanthamoeba spp. on Host Cell Interaction. Molecules. 2019; 24(24):4573. https://doi.org/10.3390/molecules24244573
Chicago/Turabian StyleHuang, Jian-Ming, Yao-Tsung Chang, and Wei-Chen Lin. 2019. "The Biochemical and Functional Characterization of M28 Aminopeptidase Protein Secreted by Acanthamoeba spp. on Host Cell Interaction" Molecules 24, no. 24: 4573. https://doi.org/10.3390/molecules24244573





