The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties
Abstract
:1. Introduction
2. Results
2.1. EOs Composition
2.2. Antimicrobial Susceptibility Testing to Antibiotics and Antimycotics
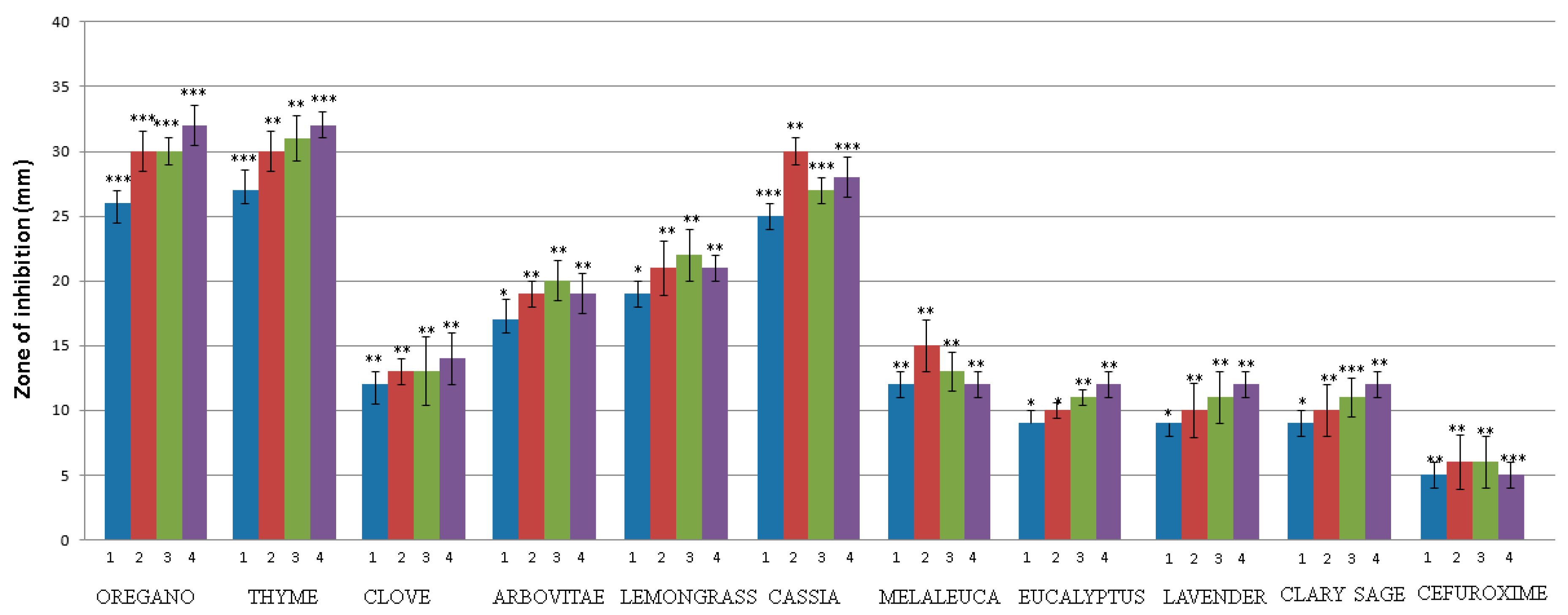
2.3. Screening of EOs Antimicrobial Ability
2.4. MIC, MBC and MFC Values Determination
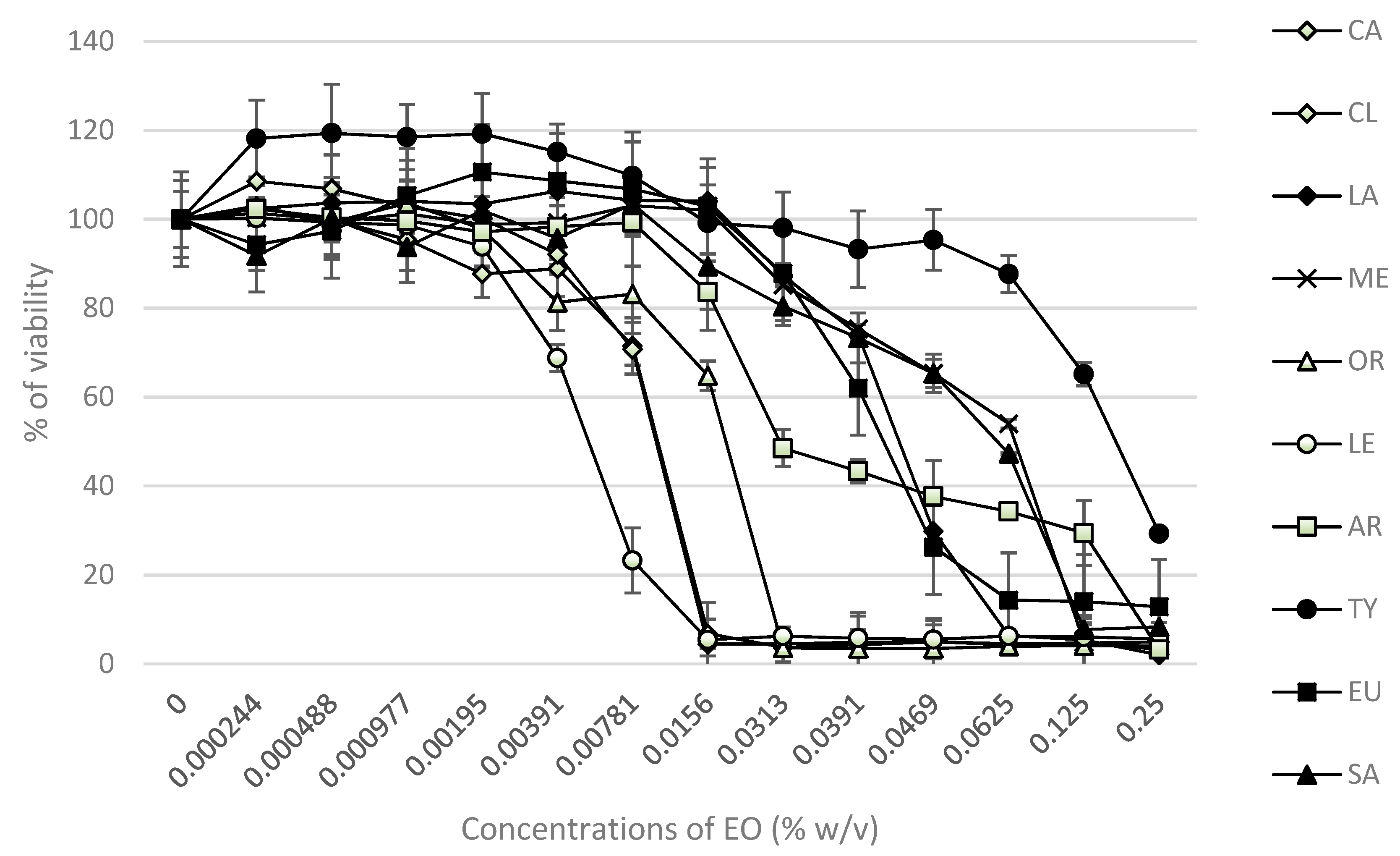
2.5. Cytotoxic and DNA-Damaging Effects of EOs
2.6. Total Antioxidant Status Level of Essential Oils
3. Discussion
4. Materials and Methods
4.1. Essential Oils
4.2. Gas Chromatography–Mass Spectrometry Analysis–Chemical composition
4.3. Antimicrobial Activity
4.4. EOs Agar Disc Diffusion Assay
4.5. Determining Minimal Inhibitory Concentrations (MIC) of EOs
4.6. Cell Culture
4.7. Determination of Cytotoxicity
4.8. Determination of Genotoxicity
4.9. Determination of Total Antioxidant Status (TAS)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Millikan, L.E. Complementary medicine in dermatology. Clin. Dermatol. 2002, 20, 602–605. [Google Scholar] [CrossRef]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polly, S.X.Y.; Ping, H.C.; Hua, S.; Lim, E. Essential oils, A new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar]
- Halcon, L.; Milkus, K. Staphylococcus aureus and wounds: A review of tea tree oil as a promising antimicrobial. Am. J. Infect. Control. 2004, 32, 402–408. [Google Scholar] [CrossRef]
- Kirby, J.P.; Mazuski, J.E. Prevention of surgical site infection. Surg. Clin. N. Am. 2009, 89, 365–389. [Google Scholar] [CrossRef]
- Fuzi, M. Editorial: The global challenge posed by the multiresistant international clones of bacterial pathogens. Front. Microbiol. 2017, 8, 817. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.C.; Perez, R. Cosmeceuticals and natural products: Wound healing. Clin. Dermatol. 2009, 27, 502–506. [Google Scholar] [CrossRef]
- Mayaud, L.; Carricajo, A.; Zhiri, A.; Aubert, G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett. Appl. Microbiol. 2008, 47, 167–173. [Google Scholar] [CrossRef]
- Bučková, M.; Puškárová, A.; Kalászová, V.; Kisová, Z.; Pangallo, D. Essential oils against multidrug resistant gram-negative bacteria. Biologia 2018, 73, 803–808. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Loran, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [Green Version]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Takahashi, T.; Kokubo, R.; Sakaino, M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004, 39, 60–64. [Google Scholar] [CrossRef]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, I.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289–23295. [Google Scholar] [CrossRef]
- Tsiri, D.; Graikou, K.; Pobłocka-Olech, L.; Krauze-Baranowska, M.; Spyropoulos, K.; Chinou, I. Chemosystematic value of essential oil compostion of Thuja species cultivated in Poland-antimicrobial activity. Molecules 2009, 14, 4707–4715. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia 2005, 76, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Siqueira Barreto, R.S.; Guimarães, A.G. Effects of carvacrol, thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Pharmacol. 2019, 71, 141–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattnaik, S.; Subramanyam, V.R.; Bapaji, M.; Kole, C.R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 1997, 89, 39–46. [Google Scholar] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J. Antimicrob. Chemother. 1998, 42, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zu, Y.G.; Chen, L.Y.; Shi, X.G.; Wang, Z. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007, 21, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Edris, E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, S.; Vivot, W.; Szusz, W.; Albo, G. Antifungal activity of essential oils against Candida species isolated from clinical Samples. Mycopathologia 2019, 184, 615–625. [Google Scholar] [CrossRef]
- Aelenei, P.; Miron, A.; Trifan, A.; Bujor, A.; Gille, E.; Aprotosoaie, A. Essential oils and their components as modulators of antibiotic activity against Gram-negative bacteria. Medicines 2016, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Poaty, B.; Lahlah, J.; Porqueres, F.; Bouafif, H. Composition, antimicrobial and antioxidant activities of seven essential oils from the North American boreal forest. World J. Microbiol. Biotechnol. 2015, 31, 907–919. [Google Scholar] [CrossRef]
- Sharifzadeh, A.; Hojjatollah, S. Antifungal activity of essential oils from Iranian plants against fluconazole-resistant and fluconazolesusceptible Candida albicans. J. Phytomed. 2016, 6, 215–222. [Google Scholar]
- Maida, I.; Nostro, A.L.; Pesavento, G. Exploring the anti-Burkholderia cepacia complex activity of essential oils: A preliminary analysis. Evid. Based Complement. Altern. Med. 2014, 2014, 573518. [Google Scholar] [CrossRef]
- Grullon, J.; Mack, J.; Rojtman, A. Using essential oils to combat the threat of multi-drug resistant bacteria, Pseudomonas aeruginosa. Inter. J. Pharm. Pharm. Sci. 2016, 8, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Soares, H.; Loreto, É.S.; Rossato, L.; Mario, D.N.; Venturini, T.P.; Baldissera, F.; Santurio, J.M.; Alves, S.H. In vitro activity of essential oils extracted from condiments against fluconazole-resistant and -sensitive Candida glabrata. J. Mycol. Med. 2015, 25, 213–217. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Morocon plants and the mechanism of action of secondary metabolites from plant. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of lavender essential oils by using gas chromatography—Mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2002, 970, 225–234. [Google Scholar] [CrossRef]
- Cai, J.; Lin, P.; Zhu, X.; Su, Q. Comparative analysis of clary sage (S. sclarea L.) oil volatiles by GC–FTIR and GC–MS. Food Chem. 2006, 99, 401–407. [Google Scholar] [CrossRef]
- Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knežević-Vukčević, J.; Stanković, S.; Simić, D. Comparative study on the antibacterial activity of volatiles from sage (Salvia officinalis L.). Arch. Biol. Sci. 2005, 57, 173–178. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.A. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Biomed. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential Oil of Artemisia annua L. An Extraordinary Component with Numerous Antimicrobial Properties. Evid. Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, J.; de Bont, J.A.M.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar]
- Pauli, A. Antimicrobial properties of essential oil constituents. Int. J. Aromather. 2001, 11, 126–133. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Enig, M.; Brook, I.; Elliott, T.B. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol. Cell Biochem. 2005, 272, 29–34. [Google Scholar] [CrossRef]
- Al-Mariri, A.; Safi, M. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran. J. Med. Sci. 2014, 39, 36–43. [Google Scholar]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [Green Version]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotech. 2016, 100, 9619–9627. [Google Scholar] [CrossRef] [Green Version]
- Schnitzler, P.; Koch, C.; Reichling, J. Susceptibility of drugresistant clinical HSV-1 strains to essential oils of Ginger, Thyme, Hyssop and Sandalwood. Antimicrob. Agents Chemother. 2007, 51, 1859–1862. [Google Scholar] [CrossRef] [Green Version]
- Allahghadri, T.; Rasooli, I.; Owlia, P.; Jalali Nadooshan, M.; Ghazanfari, T.; Taghizadeh, M.; Alipoor Astaneh, S.D. Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J. Food Sci. 2010, 75, 54–61. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef] [Green Version]
- Spagnoletti, A.; Guerrinia, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical Composition and Bio-efficacy of Essential Oils from Italian Aromatic Plants: Mentha suaveolens, Coridothymus capitatus, Origanum hirtum and Rosmarinus officinalis. Nat. Prod. Commun. 2016, 11, 1517–1520. [Google Scholar] [CrossRef] [Green Version]
- LLana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Jos, Á.; Cameán, A.M. Evaluation of the mutagenicity and genotoxic potential of carvacrol and thymol using the Ames Salmonella test and alkaline, Endo III- and FPG-modified comet assays with the human cell line Caco-2. Food Chem. Toxicol. 2014, 72, 122–128. [Google Scholar] [CrossRef]
- Slamenová, D.; Horváthová, E.; Sramková, M.; Marsálková, L. DNA-protective effects of two components of essential plant oils carvacrol and thymol on mammalian cells cultured in vitro. Neoplasma 2007, 54, 108–112. [Google Scholar]
- Kozics, K.; Klusová, V.; Srančíková, A.; Mučaji, P.; Slameňová, D.; Hunáková, L.; Kusznierewicz, B.; Horváthova, E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef]
- Horvathova, E.; Srančíková, A.; Regendová-Sedláčková, E.; Melušová, M.; Meluš, V.; Netriová, J.; Krajčovičová, Z.; Slameňová, D.; Pastorek, M.; Kozics, K. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis 2016, 31, 51–59. [Google Scholar]
- Gutteridge, J.M.; Halliwell, B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann. N. Y. Acad. Sci. 2000, 899, 136–147. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants—Determination of radical—Scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Nagata, H.; Takekoshi, S.; Takagi, T.; Honma, T.; Watanabe, K. Antioxidative action of flavonoids, quercetin and catechin, mediated by the activation of glutathione peroxidase. Tokai J. Exp. Clin. Med. 1999, 24, 1–11. [Google Scholar]
- Kozics, K.; Srancikova, A.; Sedlackova, E.; Horvathova, E.; Melusova, M.; Melus, V.; Krajcovicova, Z.; Sramkova, M. Antioxidant potential of essential oil from Lavandula angustifolia in in vitro and ex vivo cultured liver cells. Neoplasma 2017, 64, 485–493. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, X.; Zhang, K.; Sekaran, G.; Cao, B.; Zhao, Q.; Zhang, S.; Kirby, G.M.; Zhang, X. The Essential Oils and Eucalyptol from Artemisia vulgaris L. Prevent Acetaminophen-Induced Liver Injury by Activating Nrf2-Keap1 and Enhancing APAP Clearance Through Non-Toxic Metabolic Pathway. Front. Pharmacol. 2019, 25, 782. [Google Scholar] [CrossRef] [Green Version]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Placha, M.; Ryzner, K.; Cobanova, Z.; Faixova, S. Faix Effects of dietary supplementation with sage (Salvia officinalis L.) essential oil on antioxidant status and duodenal wall integrity of laying strain growers. Pol. J. Vet. Sci. 2015, 18, 741–774. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1998, 175, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the essential oils are available from the authors. |

| Oregano | Origanum vulgare | Carvacrol (76.73%), Thymol (11.34%), p-cymene (4.67%) |
| Thyme | Thymus vulgaris | Thymol (53.26%), Carvacrol (6.27%), p-cymene (16.8%), γ-Terpinene (6.48%), Linalool (4.05%), β-Caryophyllene (1.82%) |
| Clove | Eugenia caryophyllata | Eugenol (77.83%), Eugenyl acetate (14.22%), β-Caryophyllene (5.07%) |
| Arborvitae | Thuja plicata | Methyl thujate (55.96%), Methyl myrtenate (6.21%), Terpinen-4-ol (2.97%), α-Terpineol (2.06%) |
| Cassia | Cinnamomum cassia | trans-Cinnamaldehyde (87.85%), o-methoxycinnamaldehyde (5.36%) |
| Lemongrass | Cymbopogon flexuosus | Geranial (45.72%), Neral (34.46%), Geraniol (5.99%), Geranyl acetate (3.83%) |
| Melaleuca | Melaleuca alternifolia | Terpinen-4-ol (44.48%), γ-Terpinene (16.84%), α-Terpinene (6.40%) |
| Eucalyptus | Eucalyptus radiata | Eucalyptol (73.82%), α-Terpineol (9.88%) |
| Clary sage | Salvia sclarea | Linalyl acetate (53.65%), Linalool (22.32%), α-Terpineol (5.93%), Geranyl acetate (4.32%), Neryl acetate (2.37%) |
| Lavender | Lavandula angustifolia | Linalyl acetate (29.15%), Linalool (30.07%), Terpinen-4-ol (4.66%), Lavandulyl acetate (5.56%), β-Caryophyllene (4.16%), cis-β-Ocimene (3.93%) |
| Bacteria | CEF | CTX | CAZ | CPM | SUB | AMP | AMS | TIG | TET | CLM | CIP | COL | GEN | TOB | AMI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. aeruginosa KMB527 | R | R | S | R | R | S | R | S | R | R | R | S | R | R | R |
| P. vulgaris KMB525 | R | R | S | R | S | R | R | R | R | R | R | R | R | R | S |
| K. pneumoniae KMB522 | R | R | R | R | S | R | R | S | S | R | R | S | R | R | S |
| C. koseri KMB526 | R | R | S | R | S | R | R | R | R | R | R | R | R | R | S |
| Candida Strains | FLU | VOR |
|---|---|---|
| C. albicans Nr. 2 | R | S |
| C. parapsilosis Nr. 8 | S | R |
| C. parapsilosis Nr. 52 | R | R |
| Microorganisms | Activity | OR | TY | CL | AR | CA | LE | ME | EU | LA | SA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | P. aeruginosa KMB527 | MIC | 0.125 | 0.125 | 0.5 | 0.25 | 0.125 | 0.25 | 0.5 | 2.5 | - | - |
| MBC | 0.125 | 0.125 | 0.5 | 0.25 | 0.125 | 0.25 | 0.5 | - | - | - | ||
| P. vulgaris KMB525 | MIC | 0.05 | 0.05 | 0.125 | 0.125 | 0.025 | 0.25 | 0.125 | 1.25 | 0.5 | 0.5 | |
| MBC | 0.05 | 0.05 | 0.125 | 0.125 | 0.025 | 0.25 | 0.125 | 1.25 | 0.5 | 0.5 | ||
| K. pneumoniae KMB522 | MIC | 0.05 | 0.05 | 0.125 | 0.125 | 0.125 | 0.25 | 0.125 | 1.25 | 0.5 | 0.5 | |
| MBC | 0.05 | 0.05 | 0.125 | 0.125 | 0.125 | 0.25 | 0.125 | 1.25 | 0.5 | 0.5 | ||
| C. koseri KMB526 | MIC | 0.025 | 0.025 | 0.125 | 0.05 | 0.05 | 0.25 | 0.025 | 1.25 | 0.5 | 0.5 | |
| MBC | 0.025 | 0.025 | 0.125 | 0.05 | 0.05 | 0.25 | 0.025 | 1.25 | 0.5 | 0.5 | ||
| Yeasts | C. albicans Nr. 2 | MIC | 0.05 | 0.05 | 0.5 | 0.05 | 0.125 | 0.125 | 0.125 | 2.5 | - | - |
| MFC | 0.05 | 0.05 | 0.5 | 0.05 | 0.125 | 0.125 | 0.125 | - | - | - | ||
| C. parapsilosis Nr. 8 | MIC | 0.05 | 0.05 | 0.5 | 0.05 | 0.125 | 0.125 | 0.125 | 2.5 | - | - | |
| MFC | 0.05 | 0.05 | 0.5 | 0.05 | 0.125 | 0.125 | 0.125 | - | - | - | ||
| C. parapsilosis Nr. 52 | MIC | 0.05 | 0.05 | 0.5 | 0.05 | 0.125 | 0.125 | 0.125 | 2.5 | - | - | |
| MFC | 0.05 | 0.05 | 0.5 | 0.05 | 0.125 | 0.125 | 0.125 | - | - | - | ||
| EOs | Concentrations of EOs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 6.25 × 10−5 | 1.25 × 10−4 | 2.5 × 10−4 | 5 × 10−4 | 1 × 10−3 | 2 × 10−3 | 4 × 10−3 | 8 × 10−3 | 1.6 × 10−2 | 3.2 × 10−2 | 6.4 × 10−2 | |
| Clary sage | 7.62 ± 0.80 | 8.92 ± 0.10 | 9.21 ± 0.99 | 8.98 ± 0.90 | 8.68 ± 0.11 | 9.59 ± 0.26 | 7.88 ± 0.30 | 6.93 ± 0.90 | 7.71 ± 0.99 | ND | ND | ND |
| Clove | 8.65 ± 0.27 | 9.71 ± 1.09 | 8.91 ± 1.16 | 7.18 ± 0.75 | 8.03 ± 1.42 | 8.50 ± 1.31 | 7.09 ± 1.91 | 8.44 ± 0.63 | 7.61 ± 0.99 | ND | ND | ND |
| Oregano | 7.61 ± 1.15 | 9.29 ± 0.88 | 7.71 ± 1.45 | 8.82 ± 0.86 | 9.73 ± 0.98 | 9.93 ± 1.54 | 7.64 ± 1.47 | 8.27 ± 0.49 | 8.69 ± 0.53 | ND | ND | ND |
| Lemongrass | 9.86 ± 1.22 | 11.21 ± 1.46 | 9.95 ± 1.61 | 11.93 ± 1.16 | 10.28 ± 1.19 | 11.62 ± 1.46 | 10.74 ± 1.73 | 10.92 ± 1.15 | 9.63 ± 1.12 | ND | ND | ND |
| Melaleuca | 8.34 ± 0.58 | 8.29 ± 0.88 | 7.82 ± 0.86 | 7.50 ± 1.31 | 7.55 ± 1.04 | 7.19 ± 0.96 | 8.17 ± 1.22 | 8.18 ± 0.96 | 7.49 ± 0.66 | 7.56 ± 0.37 | 7.20 ± 0.42 | 7.61 ± 0.79 |
| Arborvitae | 8.29 ± 0.67 | 9.31 ± 1.92 | 9.11 ± 2.19 | 10.37 ± 0.89 | 9.07 ± 1.57 | 8.18 ± 1.22 | 10.06 ± 1.60 | 8.89 ± 0.66 | ND | ND | ND | ND |
| Cassia | 10.12 ± 0.66 | 9.33 ± 1.06 | 10.04 ± 1.27 | 8.73 ± 0.79 | 7.89 ± 0.78 | 9.86 ± 1.34 | 10.57 ± 0.90 | 8.89 ± 0.52 | 10.58 ± 1.11 | ND | ND | ND |
| Lavender | 9.24 ± 0.60 | 8.29 ± 1.36 | 8.61 ± 0.82 | 9.02 ± 1.32 | 7.69 ± 1.20 | 9.25 ± 1.00 | 8.27 ± 1.30 | 8.76 ± 0.81 | 9.53 ± 1.43 | ND | ND | ND |
| Thyme | 8.27 ± 1.32 | 9.29 ± 0.66 | 9.28 ± 1.04 | 9.02 ± 0.33 | 9.02 ± 1.18 | 9.82 ± 0.29 | 8.60 ± 1.37 | 10.09 ± 0.60 | 9.53 ± 1.43 | ND | ND | ND |
| Eucalyptus | 8.24 ± 0.54 | 7.59 ± 1.20 | 9.32 ± 0.32 | 8.58 ± 1.25 | 8.93 ± 1.58 | 9.69 ± 1.19 | 8.42 ± 1.70 | 8.40 ± 1.04 | 8.04 ± 0.68 | ND | ND | ND |
| Essential Oil | Dose (% w/v) | TAS (mmol/prot) |
|---|---|---|
| Control (−) | - | 0.54 ± 0.04 |
| Control (+) | - | 4.32 ± 0.08 |
| Lemongrass | 8 × 10−3 | 0.56 ± 0.07 |
| 4 × 10−3 | 0.71 ± 0.07 ** | |
| 2 × 10−3 | 0.73 ± 0.03 ** | |
| Clove | 8 × 10−3 | 0.74 ± 0.12** |
| 4 × 10−3 | 1.03 ± 0.08 ** | |
| 2 × 10−3 | 1.45 ± 0.06 ** | |
| Oregano | 8 × 10−3 | 1.53 ± 0.08 ** |
| 4 × 10−3 | 2.07 ± 0.13 *** | |
| 2 × 10−3 | 2.20 ± 0.16 *** | |
| Melaleuca | 6.4 × 10−2 | 0.78 ± 0.12 ** |
| 3.2 × 10−2 | 1.19 ± 0.06 *** | |
| 1.6 × 10−2 | 1.51 ± 0.28 ** | |
| Cassia | 8 × 10−3 | 1.47 ± 0.11 ** |
| 4 × 10−3 | 2.01 ± 0.14 *** | |
| 2 × 10−3 | 2.72 ± 0.09 *** | |
| Arborvitae | 4 × 10−3 | 0.42 ± 0.08 |
| 2 × 10−3 | 0.91 ± 0.13 ** | |
| 1 × 10−3 | 1.01 ± 0.08 ** | |
| Lavender | 8 × 10−3 | 0.61 ± 0.05 * |
| 4 × 10−3 | 0.65 ± 0.09 * | |
| 2 × 10−3 | 0.89 ± 0.07 ** | |
| Thyme | 8 × 10−3 | 0.77 ± 0.11 * |
| 4 × 10−3 | 1.13 ± 0.09 *** | |
| 2 × 10−3 | 1.47 ± 0.12 ** | |
| Clary sage | 8 × 10−3 | 0.75 ± 0.04 ** |
| 4 × 10−3 | 1.31 ± 0.11 ** | |
| 2 × 10−3 | 1.41 ± 0.10 ** | |
| Eucalyptus | 8 × 10−3 | 0.77 ± 0.08 ** |
| 4 × 10−3 | 0.93 ± 0.05 ** | |
| 2 × 10−3 | 1.23 ± 0.05 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. https://doi.org/10.3390/molecules24244570
Kozics K, Bučková M, Puškárová A, Kalászová V, Cabicarová T, Pangallo D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules. 2019; 24(24):4570. https://doi.org/10.3390/molecules24244570
Chicago/Turabian StyleKozics, Katarína, Mária Bučková, Andrea Puškárová, Viktória Kalászová, Terézia Cabicarová, and Domenico Pangallo. 2019. "The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties" Molecules 24, no. 24: 4570. https://doi.org/10.3390/molecules24244570
APA StyleKozics, K., Bučková, M., Puškárová, A., Kalászová, V., Cabicarová, T., & Pangallo, D. (2019). The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules, 24(24), 4570. https://doi.org/10.3390/molecules24244570







