Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco
Abstract
1. Introduction
2. Results
2.1. Essential Oil Yields and Composition
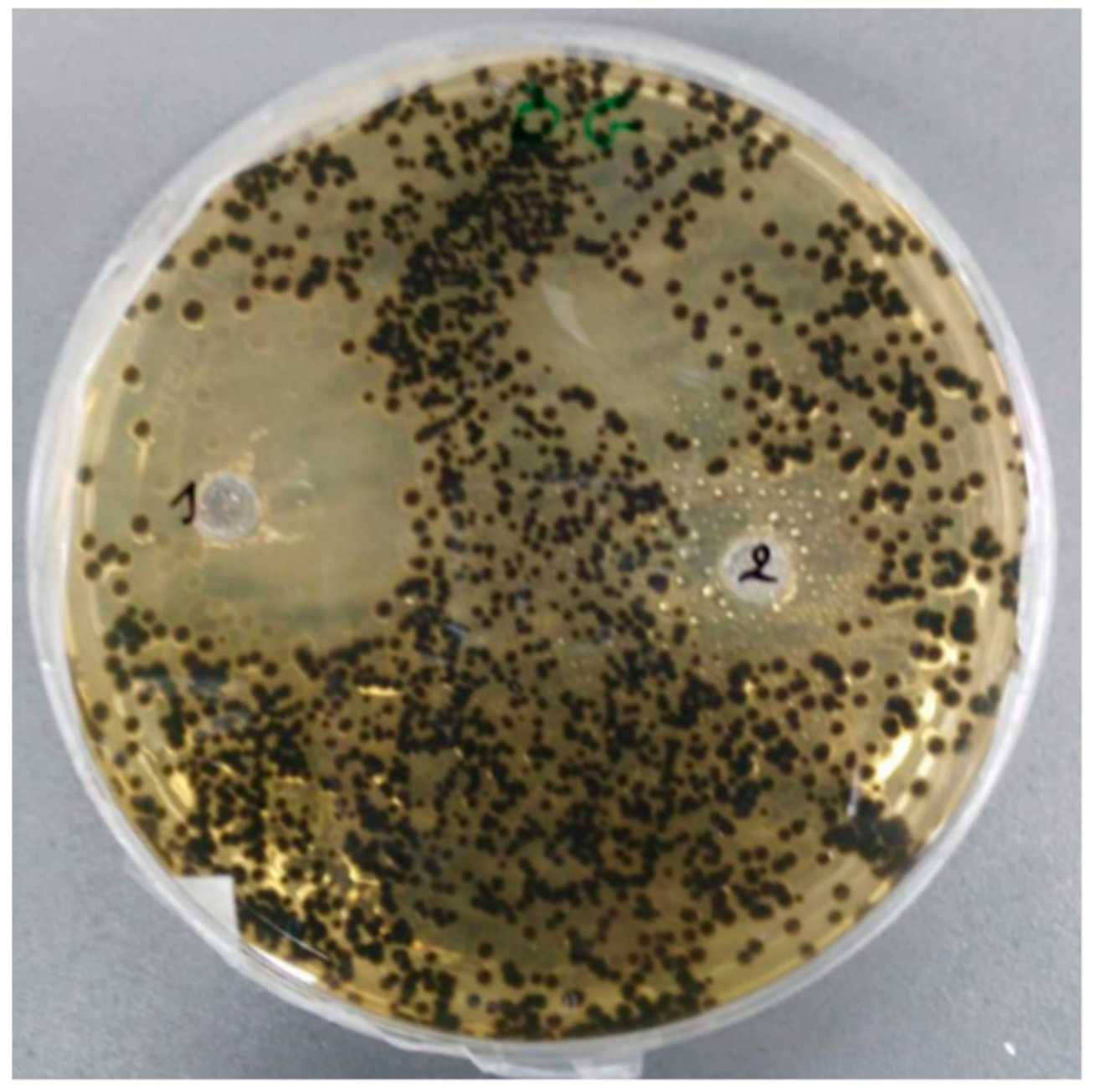
2.2. Antimicrobial Activity
2.3. Antifungal Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Extraction
4.3. GC-FID Analysis
4.4. GC/MS Analysis
4.5. Identification of the Essential Oil Components
4.6. Antibacterial Assay
4.7. Antifungal Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rassem, H.; Nour, A. Techniques for Extraction of Essential Oils From Plants. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Gonzalez-Coloma, A.; Reina, M. Natural Product-Based Biopesticides for Insect Control. Modul. Chem. Mol. Sci. Chem. Eng. 2013, 3, 237–268. [Google Scholar]
- Kaloustian, J.; Chevalier, J. Étude de Six Huiles Essentielles: Composition Chimique et Activité Antibactérienne. Phytothérapie 2008, 6, 160–164. [Google Scholar] [CrossRef]
- Santana, O.; Andrés, M.F.; Sanz, J.; Errahmani, N.; Abdeslam, L.; González-Coloma, A. Valorization of essential oils from Moroccan aromatic plants. Nat. Prod. Commun. 2014, 9, 1109–1114. [Google Scholar] [CrossRef]
- Mohamed, A.E.H.H.; El-Sayed, M.; Hegazy, M.E.; Helaly, S.E.; Esmail, A.M.; Mohamed, N.S. Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod. 2010, 4, 1–25. [Google Scholar]
- Yazdanparast, R.; Shahriyary, L. Comparative effects of Artemisia dracunculus, Satureja hortensis and Origanum majorana on inhibition of blood platelet adhesion, aggregation and secretion. Vasc. Pharmacol. 2008, 48, 32–37. [Google Scholar] [CrossRef]
- Abdollahi Fard, M.; Shojaii, A. Efficacy of Iranian traditional medicine in the treatment of epilepsy. BioMed Res. Int. 2013, 692751, 1–8. [Google Scholar] [CrossRef][Green Version]
- Sbayou, H.; Ababou, B. Chemical Composition and Antibacterial Activity of Artemisia Herba-Albaand Mentha pulegium Essential oils. J. Life Sci. 2014, 8, 35–41. [Google Scholar]
- Aghaie, M.; Alizadeh, M. Chemical composition of essential oil of Artemisia Herba-Alba from west Azerbaijan, Iran. J. Environ. Agric. Food Chem. 2011, 10, 2413–2416. [Google Scholar]
- Majdouli, K.; Elhazzouzi, H. Chemical composition and antibacterial activity of Artemisia Herba Alba Hugeii essential oil from south of Morocco. Indon. J. Chem. Res. 2015, 7, 14397–14404. [Google Scholar]
- Aldosary, N.H.; Omar, D.; Awang, R.M.; Adam, N.A. Chemical Profiling and Insecticidal Activity of Artemisia Herba-Alba Essential Oil Against Papaya Mealybug Paracoccus marginatus (Hemiptera: Pseudococcidae). Res. J. Appl. Sci., Engin. Technol. 2018, 15, 261–269. [Google Scholar] [CrossRef]
- Qnais, E.Y.; Alatshan, A.Z.; Bseiso, Y.G. Chemical composition, antinociceptive and anti-inflammatory effects of Artemisia herba-alba essential oil. J. Food Agric. Environ. 2016, 14, 20–27. [Google Scholar]
- Bertella, A.; Benlahcen, K. Artemisia herba-alba Asso: Essential oil antibacterial activity and acute toxicity. Ind. Crops Prod. 2018, 116, 137–143. [Google Scholar] [CrossRef]
- Younsi, F.; Mehdi, S.; Aissi, O.; Rahali, N.; Jaouadi, R.; Boussaid, M.; Messaoud, C. Essential Oil Variability in Natural Populations of Artemisia campestris (L.) and Artemisia herba-alba (Asso) and Incidence on Antiacetylcholinesterase and Antioxidant Activities. Chem. Biodivers. 2017, 14, e1700017. [Google Scholar] [CrossRef] [PubMed]
- Dahmani-Hamzaoui, N.; Baaliouamer, A. Chemical Composition of Algerian Artemisia herba-alba Essential Oils Isolated by Microwave and Hydrodistillation. J. Essent. Oil Res. 2010, 22, 514–517. [Google Scholar] [CrossRef]
- Mohsen, H.; Ali, G. Essential Oil Composition of Artemisia herba-alba from Southern Tunisia. Molecules 2009, 14, 1585–1594. [Google Scholar]
- Amri, I.; De Martino, L.; Marandino, A.; Lamia, H.; Mohsen, H.; Scandolera, E.; De Feo, V.; Mancini, E. Chemical composition and biological activities of the essential oil from Artemisia herba-alba growing wild in Tunisia. Nat. Prod. Commun. 2013, 8, 407–410. [Google Scholar] [CrossRef]
- Boutkhil, S.; El Idrissi, M. Chemical composition of the essential oil of Artemisia herba alba (Asso) populations. J. Phys. Chem. C. 2009, 47, 133–137. [Google Scholar]
- Aljaiyash, A.; Kasrati, A. Effect of cultivation on chemical composition and bioactivities of essential oils from Artemisia herba-alba Asso grown in Morocco. Biochem. Syst. Ecol. 2018, 81, 74–79. [Google Scholar] [CrossRef]
- Rekkab, S.; Abaza, I.S. Chemical composition of the essential oil of aerial parts of Artemisia herba-alba Asso from Oum El-Bouaghi (Algeria) and chemotaxonomic survey. J. Mater. Environ. Sci. 2016, 7, 4383–4390. [Google Scholar]
- Janackovic, P.; Novacovic, J. Composition and antimicrobial activity of essential oils of Artemisia judaica, A. herba-alba and A. arborescens from Libya. Arch. Biol. Sci. 2015, 67, 455–466. [Google Scholar] [CrossRef]
- Ben Salha, G.; Herrera Díaz, R. Deterpenation of Origanum majorana L. essential oil by reduced pressure steam distillation. Ind. Crops Prod. 2017, 109, 116–122. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and Phytotoxic Activity of Origanum heracleoticum and O. majorana Essential Oils Growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Mighri, H. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog. 2016, 95, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Y. Volatile Oil Composition of Natural Spice, Origanum majorana L., Grown in China. J. Essent. Oil Bear. Plants 2011, 14, 458–462. [Google Scholar] [CrossRef]
- El-Akhal, F.; Lalami, A.E.O.; Zoubi, Y.E.; Greche, H.; Guemmouh, R. Chemical composition and larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4, 746–750. [Google Scholar] [CrossRef]
- Nurzyñska-Wierdak, R.; Zawislak, G. The Content and Composition of Essential Oil of Origanum majorana L. Grown in Poland Depending on Harvest Tme and Method of Raw Material Preparation. J. Essent. Oil Bear. Plants 2015, 18, 1482–1489. [Google Scholar] [CrossRef]
- Alizadeh, A.; Khosh-Khui, M. Chemical composition of the essential oil, total phenolic content and antioxidant activity in Origanum majorana L. (Lamiaceae) cultivated in Iran. Adv. Environ. Biol. 2011, 5, 2326–2331. [Google Scholar]
- Lakehal, S.; Meliani, A. Essential Oil Composition and Antimicrobial Activity of Artemisia herba-alba Asso Grown in Algeria. Med. Chem. 2016, 6, 435–439. [Google Scholar] [CrossRef]
- Karabörklü, S.; Ayvaz, A.; Yilmaz, S.; Akbulut, M. Chemical composition and fumigant toxicity of some essential oils against Ephestia kuehniella. J. Econom. Entomol. 2011, 104, 1212–1219. [Google Scholar] [CrossRef]
- Lin, P.C.; Lee, J.J.; Chang, I.J. Essential oils from Taiwan: Chemical composition and antibacterial activity against Escherichia coli. J. Food Drug Anal. 2016, 24, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Rojas, L. Chemical Composition and Antibacterial Activity of Origanum majorana L. Essential Oil from the Venezuelan Andes. J. Essent. Oil Res. 2011, 23, 45–49. [Google Scholar] [CrossRef]
- Baydar, H.; Sağdiç, O. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Zouari, S.; Zouari, N. Chemical composition and biological activities of a new essential oil chemotype of Tunisian Artemisia herba alba Asso. J. Med. Plants Res. 2010, 4, 871–880. [Google Scholar]
- Goudjil, M.B.; Ladjel, S.; Bencheikh, S.E.; Zighmi, S.; Hamada, D. Chemical compounds profile, antibacterial and antioxidant activities of the essential oil extracted from the Artemisia herba-alba of Southern Algeria. Int. J. Biol. Chem. 2015, 9, 70–78. [Google Scholar] [CrossRef]
- Bellili, S.; Jazi, S.; Hrira, M.Y.; Lamari, A.; Dhifi, W.; Diouani, M.F.; Mnif, W. Phytochemical identification of volatile fraction, essential oil and screening of antioxidant, antibacterial, allelopathic and insecticidal potential from Artemisia herba-alba leaves. Main Group Chem. 2017, 16, 95–109. [Google Scholar] [CrossRef]
- Ezzeddine, N.B.H.B.; Abdelkefi, M.M.; Aissa, R.B.; Chaabouni, M.M. Antibacterial screening of Origanum majorana L. oil from Tunisia. J. Essent. Oil Res. 2001, 13, 295–297. [Google Scholar] [CrossRef]
- Dorma, H.; Deans, S. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of the essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Natalia, A.; Kim, S. Antioxidant and antibacterial activity of fatty acid vanillyl ester produced by Proteusvulgaris K80 lipase-mediated transesterification. J. Mol. Catal. B Enzym. 2016, 133, 475–481. [Google Scholar] [CrossRef]
- Mockute, D.; Nivinskiene, G. The cis-thujone chemotype of Salvia officinalis L essential oils. Chemija 2003, 14, 216–220. [Google Scholar]
- Cox, S.; Mann, C. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Mehni, M.; Segni, L.; Terzi, V.; Morcia, C.; Ghizzoni, R.; Goudgil, B.; Benchhikh, S. Antifungal Activity of Artemisia herba-alba on Various Fusarium. Phytothérapie 2018, 16, 87–90. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2004; Volume I, p. 217. [Google Scholar]
- Jenning, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: San Francisco, CA, USA, 1980. [Google Scholar]
- Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/mass Spectroscopy; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Johnston, C. The Wiley/NBS Registry of Mass Spectral Data. J. Chem. Educ. 1989, 66, 256. [Google Scholar]
Sample Availability: Samples of A. herba-alba and O. majorana essential oils are available from the authors. |

| Compound | % | Ki a | Ki b | Identification c |
|---|---|---|---|---|
| trans-Arbusculone | 4.5 | 1048 | 1,2 | |
| cis-Thujone | 25.5 | 1079 | 1102 | 1,2,3 |
| trans-Thujone | 17.7 | 1111 | 1114 | 1,2,3 |
| Camphor | 4.9 | 1150 | 1146 | 1,2,3 |
| nor-Davanone | 7.8 | 1200 | 1231 | 1,2 |
| cis-Chrysanthenylacetate | 4.7 | 1231 | 1265 | 1,2 |
| Undec-10-en-1-al | 1.3 | 1261 | 1296 | 1,2 |
| Cyclosativene | T | 1342 | 1368 | 1,2 |
| cis, threo-Davanafuran | 5.8 | 1386 | 1415 | 1,2 |
| Vanillyl Alcohol | 11.5 | 1424 | 1447 | 1,2 |
| n-Dodecanol | 3.1 | 1445 | 1470 | 1,2 |
| Isobornyl n-butyrate | 4.9 | 1466 | 1491 | 1,2 |
| <E>-Jasmolactone | 3.4 | 1483 | 1491 | 1,2 |
| Artedouglasia Oxide C | 2.5 | 1496 | 1523 | 1,2 |
| Total | 97.6 | |||
| Oxygenated monoterpene | 56.4 | |||
| Oxygenated sesquiterpenes | 2.5 | |||
| Other compounds | 38.7 |
| Compound | % | KI a | KI b | Identification c |
|---|---|---|---|---|
| α-Pinene | 4.1 | 941 | 932 | 1,2,3 |
| p-Cymene | 2.6 | 950 | 1024 | 1,2,3 |
| iso-Sylvestrene | 0.6 | 952 | 1008 | 1,2,3 |
| β-Pinene | 0.2 | 975 | 974 | 1,2,3 |
| α-Phellandrene | 2.6 | 984 | 1002 | 1,2,3 |
| δ-3-Carene | 1.9 | 1008 | 1011 | 1,2 |
| α-Terpinene | 19.2 | 1021 | 1017 | 1,2,3 |
| Limonene | 0.1 | 1038 | 1029 | 1,2,3 |
| 1,8 Cineole | 3.0 | 1047 | 1031 | 1,2,3 |
| β-Ocimene | 0.1 | 1061 | 1037 | 1,2,3 |
| cis-Sabinene hydrate | 1.3 | 1070 | 1070 | 1,2 |
| Terpinen-4-ol | 34.1 | 1096 | 1149 | 1,2,3 |
| endo-Fenchyl-acetate | 9.8 | 1114 | 1220 | 1,2 |
| Pulegone | 0.7 | 1122 | 1237 | 1,2 |
| trans-Pinocarveol | 0.3 | 1143 | 1139 | 1,2 |
| Terpineol | 8.9 | 1160 | 1133 | 1,2,3 |
| cis-Limonene oxide | T | 1188 | 1136 | 1,2 |
| dihydro-Linalool | 0.1 | 1191 | 1135 | 1,2 |
| cis-Verbenol | T | 1193 | 1141 | 1,2,3 |
| Viridene | 0.1 | 1199 | 1167 | 1,2 |
| (E)-Isocitral | 0.2 | 1205 | 1180 | 1,2 |
| Thymol | 0.2 | 1211 | 1290 | 1,2,3 |
| Carvacrol | 0.3 | 1220 | 1299 | 1,2,3 |
| γ-Elemene | 0.1 | 1233 | 1338 | 1,2,3 |
| α-Terpinyl acetate | 0.8 | 1242 | 1349 | 1,2 |
| Eugenol | T | 1271 | 1359 | 1,2,3 |
| Neryl acetate | 0.2 | 1274 | 1361 | 1,2 |
| α-Copaene | T | 1278 | 1376 | 1,2,3 |
| Geranyl acetate | 0.3 | 1293 | 1381 | 1,2,3 |
| iso-Longifolene | 0.1 | 1303 | 1390 | 1,2 |
| (E)-Caryophillene | 2.1 | 1314 | 1407 | 1,2,3 |
| β-Duprezianene | T | 1321 | 1422 | 1,2 |
| β-Cedrene | T | 1324 | 1420 | 1,2,3 |
| β-Copaene | T | 1327 | 1432 | 1,2,3 |
| α-Guaiene | 0.2 | 1332 | 1439 | 1,2,3 |
| Aromadendrene | 0.3 | 1336 | 1441 | 1,2,3 |
| allo-Aromadendrene | 1.3 | 1370 | 1460 | 1,2 |
| Valencene | 0.2 | 1401 | 1496 | 1,2,3 |
| Caryophyllene oxide | T | 1436 | 1583 | 1,2,3 |
| Epiglobulol | T | 1445 | 1590 | 1,2 |
| (-)-Spathulenol | T | 1453 | 1578 | 1,2 |
| β-Atlanthol | 1.6 | 1464 | 1608 | 1,2,3 |
| Rosifoliol | 0.1 | 1485 | 1600 | 1,2 |
| Cubenol | T | 1497 | 1646 | 1,2 |
| Total | 97.8 | |||
| Monoterpene hydrocarbons | 33.1 | |||
| Oxygenated monoterpene | 57.9 | |||
| Sesquiterpene hydrocarbons | 5.1 | |||
| Oxygenated sesquiterpenes | 1.7 |
| Strain | Control | Essential Oil | ||||
|---|---|---|---|---|---|---|
| Gentamicin | Tetracyclin | 5 μL | 10 μL | 15 μL | 20 μL | |
| B. clausii 2226 | 11.0 ± 1.0 | 16.3 ± 1.5 | 10.3 ± 0.6 | 14.7 ± 0.6 | 19.7 ± 1.5 a,D | 24.0 ± 1.0 a,A |
| Br. thermosphacta 7R1 | 18.3 ± 1.5 | 19.3 ± 1.2 | na | 6.0 ± 0.0 | 6.0 ± 0.1 | 12.3 ± 0.6 |
| Br. thermosphacta D274 | 6.0 ± 0.0 | 8.7 ± 1.2 | 6.0 ± 0.0 | 11.7 ± 0.6 a,C | 14.7 ± 0.6 a,A | 17.7 ± 0.6 a,A |
| C. maltaromaticum 9P | 6.0 ± 0.0 | 24.3 ± 1.2 | 6.0 ± 0.0 | 9.0 ± 1.0 b | 11.7 ± 0.6 a | 12.3 ± 0.6 a |
| C. maltaromaticum D1203 | 6.0 ± 0.0 | 22.3 ± 0.6 | na | na | na | na |
| C. maltaromaticum F1201 | 6.0 ± 0.0 | 23.3 ± 1.5 | na | na | na | na |
| C. maltaromaticum H1201 | 10.0 ± 0.0 | na | na | na | na | 6.0 ± 0.0 A |
| E. coli 32 | 14.7 ± 0.6 | 18.7 ± 1.2 | na | na | 6.0 ± 0.0 | 6.0 ± 0.0 |
| Ent. faecalis 226 | 6.0 ± 0.0 | 9.0 ± 1.0 | na | na | na | na |
| Ent. faecalis E21 | 6.0 ± 0.0 | 14.7 ± 0.6 | na | na | na | na |
| H. alvei 53M | 11.7 ± 1.5 | 9.7 ± 0.6 | na | na | na | na |
| L. innocua 1770 | 25.3 ± 0.6 | 20.3 ± 1.5 | na | na | na | 6.0 ± 0.0 |
| P. fragi 6P2 | 14.7 ± 0.6 | 17.0 ± 1.0 | na | 6.0 ± 0.0 | 9.3 ± 0.6 | 13.3 ± 2.1 |
| Staph. aureus | 6.0 ± 0.0 | 15.3 ± 0.6 | na | na | na | 6.0 ± 0.0 |
| S. Typhimurium | 9.7 ± 0.6 | 12.7 ± 1.2 | na | 6.0 ± 0.0 | 14.0 ± 1.7 b | 17.7 ± 0.6 a,B |
| Serr. proteamaculans 20P | 12.3 ± 0.6 | 24.3 ± 1.2 | na | 6.0 ± 0.0 | 7.7 ± 0.6 | 10.3 ± 0.6 |
| Str. salivarius | 6.0 ± 0.0 | 18.7 ± 1.2 | na | 6.0 ± 0.0 | 14.0 ± 1.0 a | 18.3 ± 1.5 a |
| Staph. saprophyticus 3S | 24.0 ± 1.0 | 29.0 ± 3.6 | na | na | 6.0 ± 0.0 | 6.0 ± 0.0 |
| Staph. sp.ES1 | 19.3 ± 1.2 | 29.3 ± 1.2 | na | 6.0 ± 0.0 | 8.3 ± 0.6 | 10.3 ± 0.6 |
| Staph.sp.GB1 | 21.3 ± 1.2 | 27.7 ± 2.5 | 6 ± 0.0 | 11.3 ± 1.2 | 14.7 ± 0.6 | 17 ± 1.0 |
| Strain | Control | Essential Oil | ||||||
|---|---|---|---|---|---|---|---|---|
| Gentamicin | Tetracyclin | 5 µL | 10 µL | 15 µL | 20 µL | 40 µL | 50 µL | |
| B. clausii 2226 | 11.0 ± 1.0 | 16.3 ± 1.5 | na | 6.0 ± 0.0 | 15.3 ± 0.6b | 23.3 ± 1.5 a,B | 24.7 ± 0.6 a,B | 28.3 ± 1.5 a,B |
| Br. thermosphacta D274 | 6.0 ± 0.0 | 8.7 ± 1.2 | 10.6 ± 0.6d | 13.3 ± 1.2 a | 18.3 ± 0.6 a,B | 23.0 ± 1.7 a,B | 24.3 ± 1.2 a,B | 26.3 ± 1.2 a,B |
| Br. thermosphacta 7R1 | 18.3 ± 1.5 | 19.3 ± 1.2 | 11.3 ± 1.2 | 15.3 ± 0.6 | 18.0 ± 0.0 | 20.3 ± 0.6* | 20.0 ± 0.0 | 21.3 ± 0.6c,D |
| C. maltaromaticum 9P | 6.0 ± 0.0 | 24.3 ± 1.2 | na | na | na | 6.0 ± 0.0 | 6.0 ± 0.0 | 9.3 ± 0.6a |
| C.maltaromaticum H1201 | 10.0 ± 0.0 | na | 6.0 ± 0.0A | 6.0 ± 0.0 A | 8.7 ± 1.2 A | 9.7 ± 0.6 A | 9.3 ± 0.6 A | 9.7 ± 0.6 A |
| C. maltaromaticum D1203 | 6.0 ± 0.0 | 22.3 ± 0.6 | na | na | na | 6.0 ± 0.0 | 9.3 ± 0.6 a | 13.3 ± 1.5 a |
| C. maltaromaticum F1201 | 6.0 ± 0.0 | 23.3 ± 1.5 | na | na | na | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 |
| E. coli 32 | 14.7 ± 0.6 | 18.7 ± 1.2 | 9.7 ± 0.6 | 10.7 ± 1.2 | 17.7 ± 0.6 | 20.0 ± 0.0 a | 24.3 ± 1.2 a,B | 26.7 ± 0.6 a,B |
| Ent. faecalis 226 | 6.0 ± 0.0 | 9.0 ± 1.0 | na | na | na | 6.0 ± 0.0 | 6.0 ± 0.1 | 9.7 ± 0.7 |
| Ent. faecalis E21 | 6.0 ± 0.0 | 14.7 ± 0.6 | na | na | na | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 |
| H. alvei 53M | 11.7 ± 1.5 | 9.7 ± 0.6 | 8.3 ± 0.6 | 10.3 ± 0.6 | 11.7 ± 0.6 C | 12.7 ± 0.6 A | 15.0 ± 0.0 b,A | 20.3 ± 0.6 a,B |
| L. innocua 1770 | 25.3 ± 0.6 | 20.3 ± 1.5 | na | 6.0 ± 0.0 | 9.3 ± 0.6 | 11.7 ± 0.6 | 12.3 ± 0.6 | 13.7 ± 1.5 |
| P. fragi 6P2 | 14.7 ± 0.6 | 17.0 ± 1.0 | na | na | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 9.3 ± 0.6 |
| Staph. aureus | 6.0 ± 0.0 | 15.3 ± 0.6 | 10.7 ± 0.6 d | 11.7 ± 1.5 c | 16.3 ± 1.5 a | 24.3 ± 2.1 a,B | 27.7 ± 0.6 a,B | 32.3 ± 2.5 a,B |
| S. Typhimurium | 9.7 ± 0.6 | 12.7 ± 1.2 | 7.7 ± 2.1 | 11.7 ± 0.6 | 14.0 ± 1.7 d | 17.3 ± 1.2 c,D | 23.3 ± 2.9 a,B | 29.7 ± 0.6 a,B |
| Serr.proteamaculans 20P | 12.3 ± 0.6 | 24.3 ± 1.2 | na | na | na | 6.0 ± 0.0 | 16.3 ± 1.5a | 19.3 ± 1.2a |
| Str. salivarius | 6.0 ± 0.0 | 18.7 ± 1.2 | 9.7 ± 0.6 c | 11.3 ± 0.6 a | 13.0 ± 1.0 a | 19.3 ± 1.2 a | 20.7 ± 1.2 a | 24.3 ± 1.2 a,B |
| Staph. saprophyticus 3S | 24.0 ± 1.0 | 29.0 ± 3.6 | 9.9 ± 1.0 | 11.7 ± 0.6 | 19.3 ± 1.2 | 20.3 ± 0.6 | 21.0 ± 1.0 | 24.3 ± 1.2 |
| Staph.sp. ES1 | 19.3 ± 1.2 | 29.3 ± 1.2 | 6.0 ± 0.0 | 6.0 ± 0.0 | 14.7 ± 7.6 | 18.7 ± 1.2 | 20.0 ± 0.0 | 21.0 ± 1.0 |
| Staph.sp.GB1 | 21.3 ± 1.2 | 27.7 ± 2.5 | na | na | 5.7 ± 0.6 | 10 ± 0.0 | 14.7 ± 0.6 | 19.3 ± 1.2 |
| Aspergillus niger | |
|---|---|
| Artemisia herba-alba | 23.6 ± 1.5 |
| Origanum majorana | 14.0 ± 1.0 |
| Gram | Microorganism | Source | Growth Conditions |
|---|---|---|---|
| Positive | B. clausii 2226 | Supplement | TSB 24h at 30 °C |
| Br. thermosphacta 7R1 | Meat | TSB 24h at 20 °C | |
| Br. thermosphacta D274 | Meat | TSB 24h at 20 °C | |
| C. maltaromaticum 9P | Meat | TSB 24h at 20 °C | |
| C. maltaromaticum D1203 | Meat | TSB 24h at 25 °C | |
| C. maltaromaticum F1201 | Meat | TSB 24h at 25 °C | |
| C. maltaromaticum H1201 | Meat | TSB 24h at 25 °C | |
| Ent. faecalis 226 | Milk | TSB 24h at 30 °C | |
| Ent. faecalis E21 | Milk | TSB 24h at 30 °C | |
| Staph. aureus | Meat | TSB 24h at 37 °C | |
| Staph. saprophyticus 3S | Fermented meat | TSB 24h at 37 °C | |
| Staph. sp. ES1 | Fermented meat | TSB 24h at 37 °C | |
| Staph. sp. GB1 | Fermented meat | TSB 24h at 37 °C | |
| L. innocua 1770 | Milk | TSB 24h at 30 °C | |
| E. coli 32 | Meat | TSB 24h at 37 °C | |
| Str. salivarius | Milk | TSB 24h at 30 °C | |
| Negative | H.alvei 53M | Meat | TSB 24h at 30 °C |
| Pseud. fragi 6P2 | Meat | TSB 24h at 20 °C | |
| S. Typhimurium | Chicken meat | TSB 24h at 30 °C | |
| Serr.proteamaculans20P | Meat | TSB 24h at 25 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amor, G.; Caputo, L.; La Storia, A.; De Feo, V.; Mauriello, G.; Fechtali, T. Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules 2019, 24, 4021. https://doi.org/10.3390/molecules24224021
Amor G, Caputo L, La Storia A, De Feo V, Mauriello G, Fechtali T. Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules. 2019; 24(22):4021. https://doi.org/10.3390/molecules24224021
Chicago/Turabian StyleAmor, Ghita, Lucia Caputo, Antonietta La Storia, Vincenzo De Feo, Gianluigi Mauriello, and Taoufiq Fechtali. 2019. "Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco" Molecules 24, no. 22: 4021. https://doi.org/10.3390/molecules24224021
APA StyleAmor, G., Caputo, L., La Storia, A., De Feo, V., Mauriello, G., & Fechtali, T. (2019). Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules, 24(22), 4021. https://doi.org/10.3390/molecules24224021








