NOx-, IL-1β-, TNF-α-, and IL-6-Inhibiting Effects and Trypanocidal Activity of Banana (Musa acuminata) Bracts and Flowers: UPLC-HRESI-MS Detection of Phenylpropanoid Sucrose Esters
Abstract
:1. Introduction
2. Results
2.1. Biological Activities
2.1.1. Anti-Inflammatory Activity
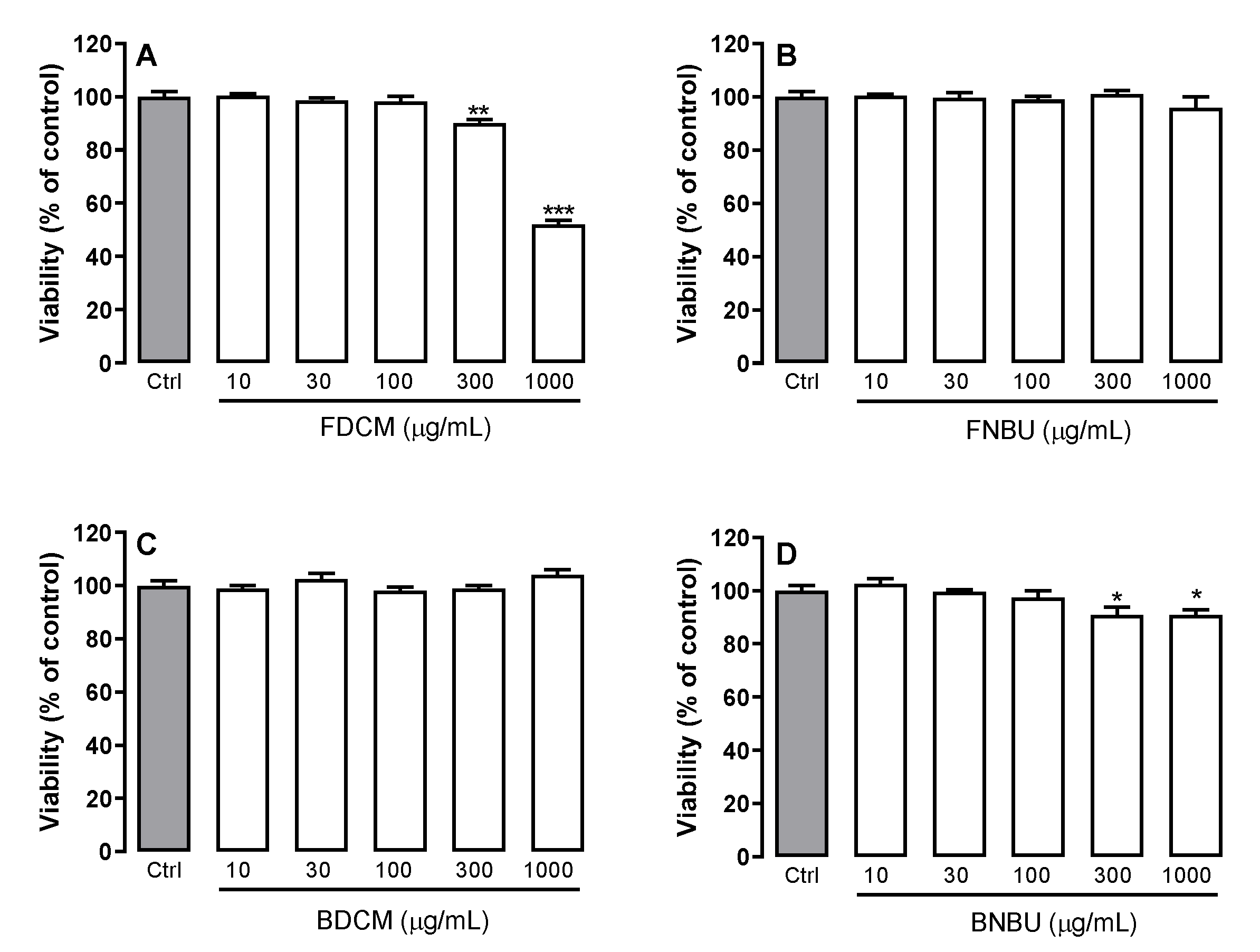
Cell Viability
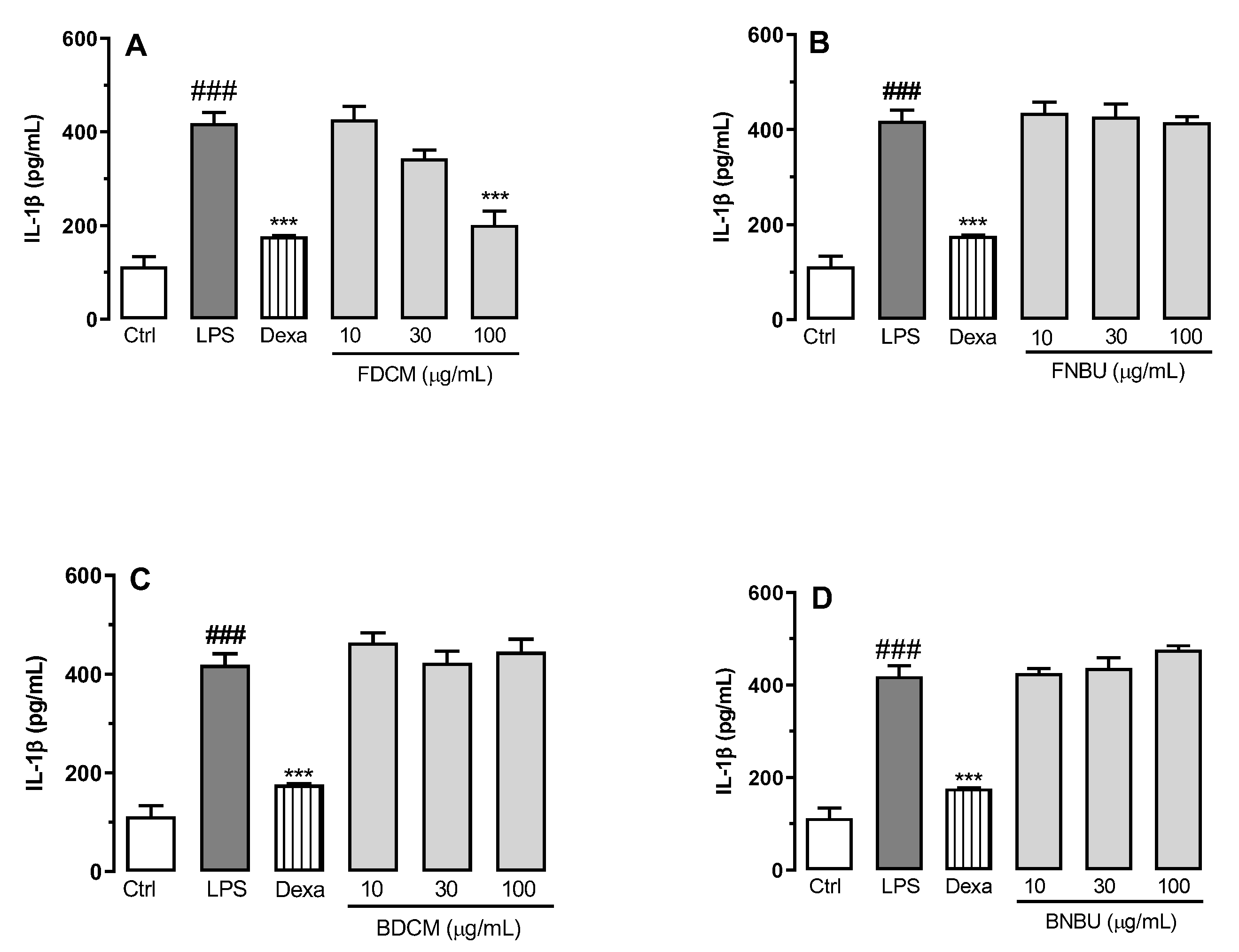
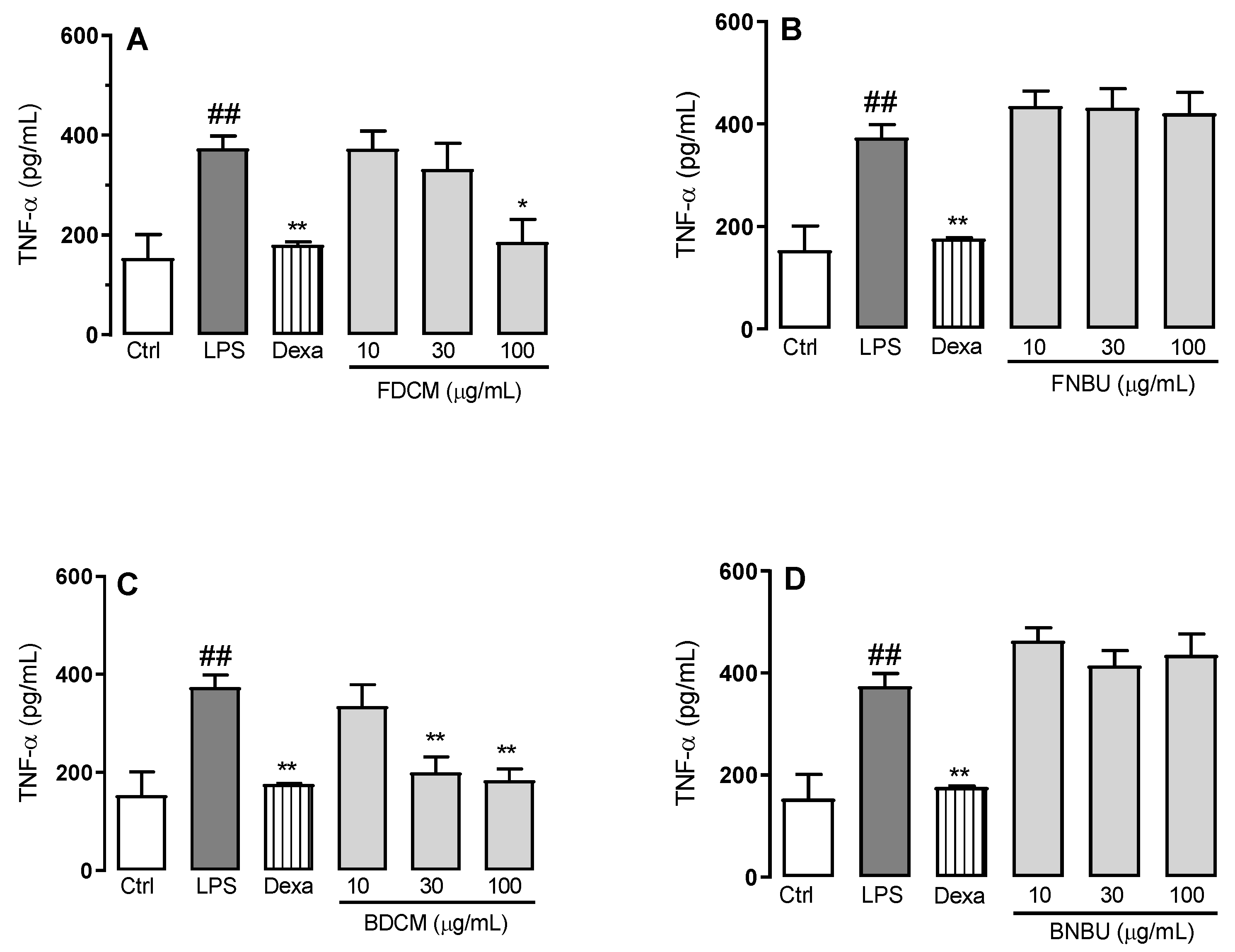
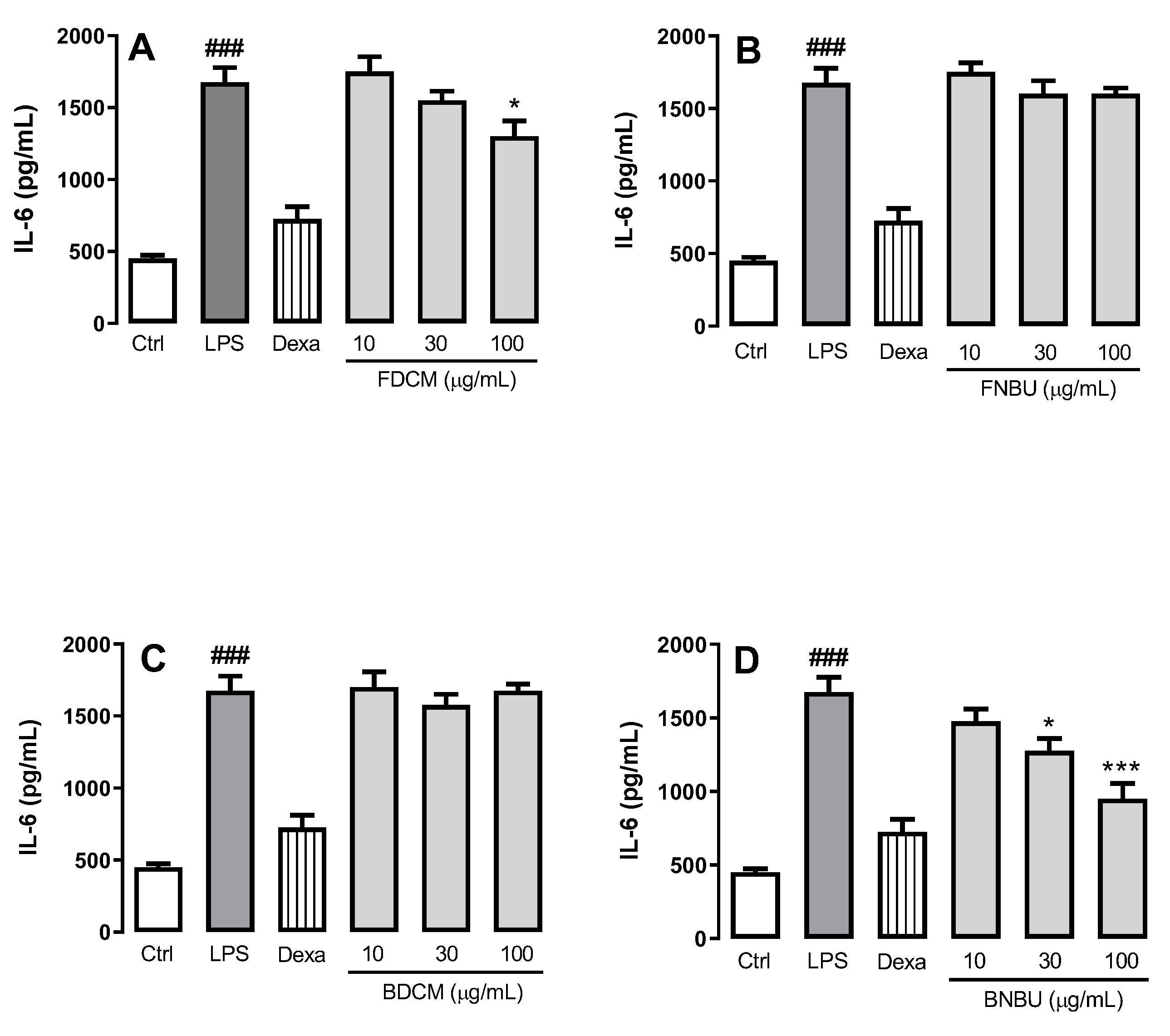
Inhibition Study on Cytokine Secretion (IL-1β, TNF-α, and IL-6)
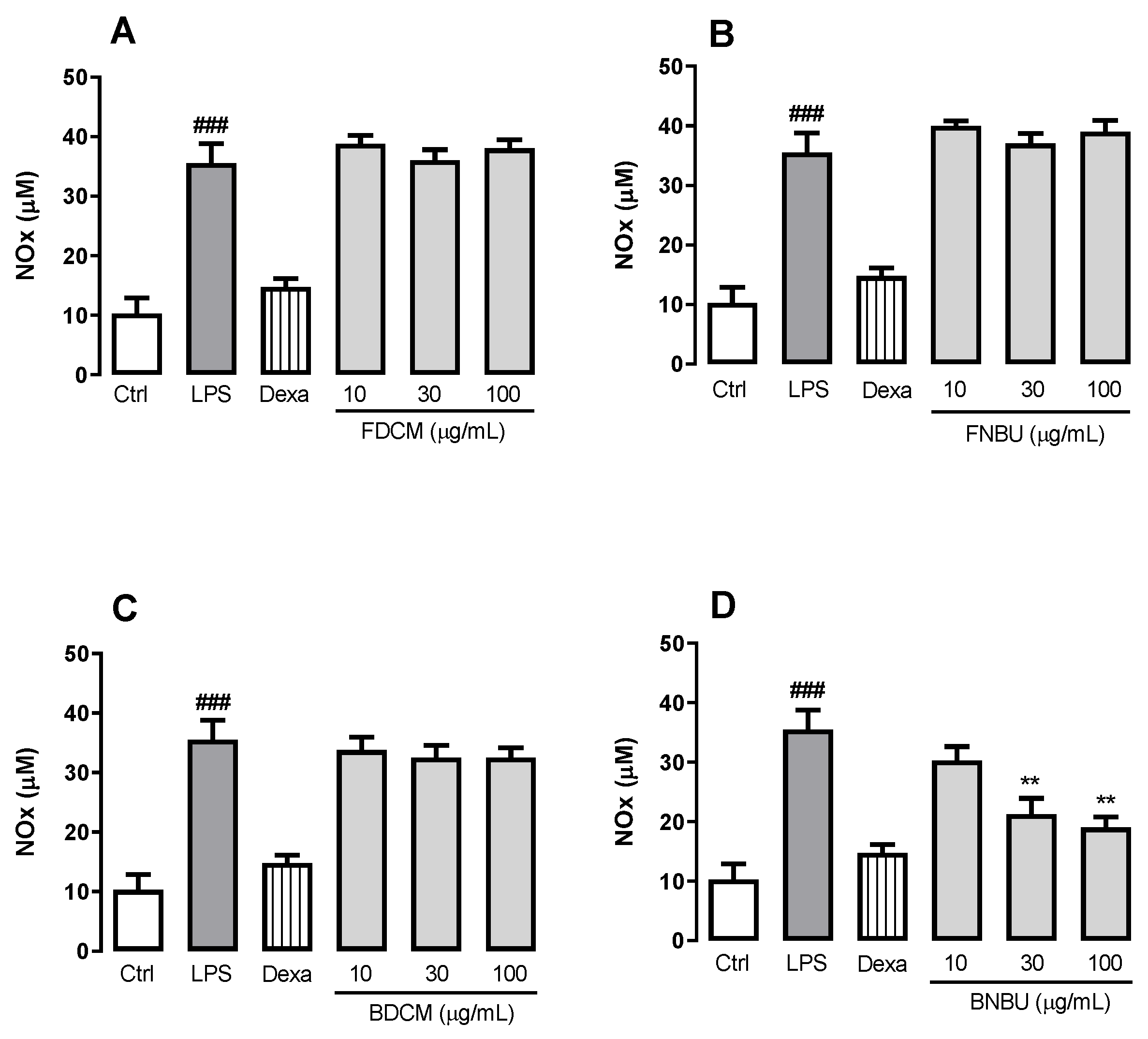
Measurement of NOx Production by Mouse Neutrophils
2.1.2. Antitrypanosomal and Antileishmanial Activities
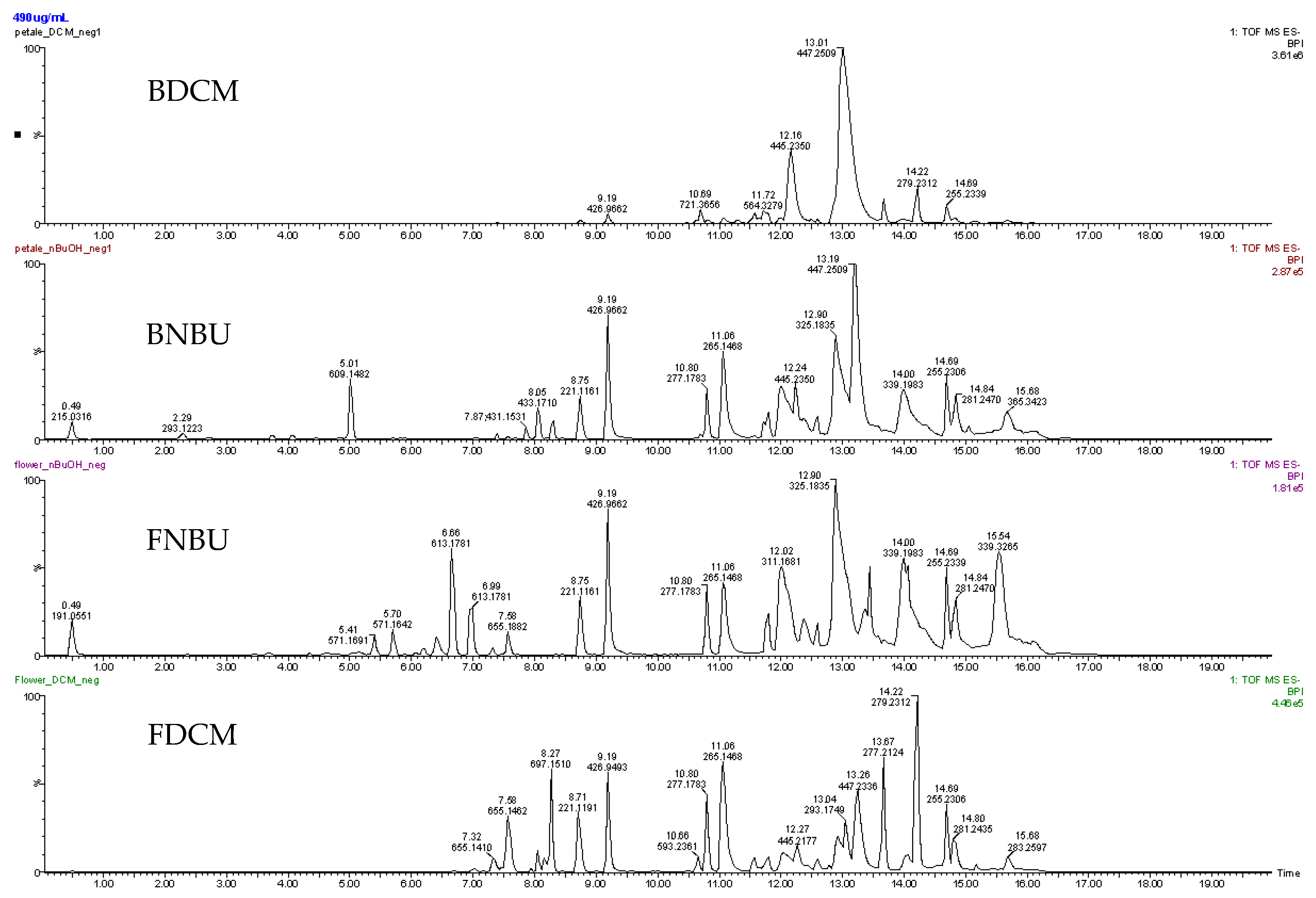
2.2. Chemistry
2.3. Discussion
3. Materials and Methods
3.1. Plant Identification
3.2. Anti-Inflammatory Assays
3.2.1. Mouse Neutrophil Isolation and Primary Culture
3.2.2. Lipopolysaccharide Stimulation of Isolated Neutrophils
3.2.3. Cell Viability Assay Using the Isolated Neutrophilis
3.2.4. Cell Inflammation Assay on Isolated Neutropils
3.2.5. Measurement of NOx Production in Neutrophils
3.2.6. Quantification of Pro-Inflammatory Cytokines Levels (IL-1β, TNF-α, and IL-6) in Neutrophils
3.3. Antiparasitic Assays
3.3.1. In Vitro Antitrypanosomal and Antileishmanial Assays
3.3.2. Cell Viability Assay (MTT)
3.4. LCMS analysis
3.4.1. Chemicals
3.4.2. Extraction, Fractionation, and Sample Preparation
3.4.3. LC-MS Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nunes, H. PANC gourmet: Ensaios culinários; Instituto Plantarum: São Paulo, Brazil, 2017; pp. 88–89. [Google Scholar]
- Azam, F.M.S.; Biswas, A.; Mannan, A.; Afsana, N.A.; Jahan, R.; Rahmatullah, M. Are Famine Food Plants Also Ethnomedicinal Plants? An Ethnomedicinal Appraisal of Famine Food Plants of Two Districts of Bangladesh. Evid Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kinupp, V.F.; Lorenzi, H. Plantas Alimentícias Não Convencionais (PANC) no Brasil: Guia de identificação, aspectos nutricionais e receitas ilustradas; Instituto de Plantarum de Estudos de Flora: São Paulo, Brasil, 2014; pp. 538–542. [Google Scholar]
- Mathew, N.S.; Negi, P.S. Traditional uses, phytochemistry and pharmacology of wild banana (Musaacuminata Colla). A Review. J. Ethnopharmacol. 2017, 196, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Chintamunnee, V.; Mahomoodally, M.F. Herbal medicine commonly used against non-communicable diseases in the tropical island of Mauritius. J. Herb Med. 2012, 2, 113–125. [Google Scholar] [CrossRef]
- China, R.; Dutta, S.; Sen, S.; Chakrabarti, R.; Bhowmik, D.; Ghosh, S.; Dhar, P. In vitro Antioxidant Activity of Different Cultivars of Banana Flower (Musa paradicicus L.) Extracts Available in India. J Food Sci. 2011, 76, C1292–C1299. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.S.; Dhanabal, S.P.; Zameer, F.; Dhananjaya, B.L.; Prasad, M.N.N. Investigation of Antihyperglycaemic Activity of Banana (Musa sp. Var. Nanjangud rasa bale) Flower in Normal and Diabetic Rats. Pharm. Mag. 2017, 13, S417–S423. [Google Scholar] [CrossRef]
- Liyanage, R.; Gunasegaram, S.; Visvanathan, R.; Jayathilake, C.; Weththasinghe, P.; Jayawardana, B.C.; Vidanarachchi, J.K. Banana Blossom (Musa acuminate Colla) Incorporated Experimental Diets Modulate Serum Cholesterol and Serum Glucose Level in Wistar Rats Fed with Cholesterol. Cholesterol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Otálvaro, F.; Nanclares, J.; Vásquez, L.E.; Quiñones, W.; Echeverri, F.; Arango, R.; Schneider, B. Phenalenone-type compounds from Musa acuminata var. “Yangambi km 5” (AAA) and their activity against Mycosphaerella fijiensis. J. Nat. Prod. 2007, 70, 887–890. [Google Scholar]
- Hölscher, D.; Buerkert, A.; Schneider, B. Phenylphenalenones Accumulate in Plant Tissues of Two Banana Cultivars in Response to Herbivory by the Banana Weevil and Banana Stem Weevil. Plants 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Kamo, T.; Kato, N.; Hirai, N.; Tsuda, M.; Fujioka, D.; Ohigashi, H. Phenylphenalenone-type Phytoalexins from Unripe Buñgulan Banana Fruit. Biosci Biotechnol Biochem. 1998, 62, 95–101. [Google Scholar] [CrossRef]
- Oliveira, W.N.; Ribeiro, L.E.; Schrieffer, A.; Machado, P.; Carvalho, E.M.; Bacellar, O. The role of inflammatory and anti-inflammatory cytokines inthe pathogenesis of human tegumentary leishmaniasis. Cytokine 2014, 66, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Morgado, F.N.; de Carvalho, L.M.V.; Leite-Silva, J.; Seba, A.J.; Pimentel, M.I.F.; Fagundes, A.; Madeira, M.F.; Lyra, M.R.; Oliveira, M.M.; Schubach, A.O.; et al. Unbalanced inflammatory reaction could increase tissue destruction and worsen skin infectious diseases—a comparative study of leishmaniasis and sporotrichosis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, B.P.; Vazquez, T.P.; Miguel, C.B.; Rodrigues, W.F.; Mendes, M.T.; de Oliveira, C.J.F.; Javier Chica, E.L. Inflammatory responses and intestinal injury development during acute Trypanosoma cruzi infection are associated with the parasite load. Parasites Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Pinho, R.T.; da Silva, W.S.; de Castro Cortes, L.M.; da Silva Vasconcelos Sousa, P.; de Araujo Soares, R.O.; Alve, C.R. Production of MMP-9 and inflammatory cytokines by Trypanosoma cruzi-infected macrophages. Exp. Parasitol. 2014, 147, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Abrankó, L.; García-Reyes, J.F.; Molina-Díaz, A. In-source fragmentation and accurate mass analysis of multiclass flavonoid conjugates by electrospray ionization time-of-flight mass spectrometry. J. Mass Spectrom. 2011, 46, 478–488. [Google Scholar] [CrossRef]
- Fujimoto, K.; Nakamura, S.; Matsumoto, T.; Ohta, T.; Yoshikawa, M.; Ogawa, K.; Kashiwazaki, E.; Matsuda, H. Structures of acylated sucroses from the flower buds of Prunus Mume. J. Nat. Med. 2014, 68, 481–487. [Google Scholar] [CrossRef]
- Nakamura, S.; Fujimoto, K.; Matsumoto, T.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Structures of acylated sucroses and an acylated flavonol glycoside and inhibitory effects of constituents on aldose reductase from the flower buds of Prunus mume. J. Nat. Med. 2013, 67, 799–806. [Google Scholar] [CrossRef]
- Shimazaki, N.; Mimaki, Y.; Sashida, Y. Prunasin and acetylated phenylpropanoic acid sucrose esters, bitter principles from the fruits of Prunus jamasakura and P. Maximowiczii. Phytochemistry 1991, 30, 1475–1480. [Google Scholar] [CrossRef]
- Bonta, R.K. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef]
- Kayano, S.; Kikuzaki, H.; Hashimoto, S.; Kasamatsu, K.; Ikami, T.; Nakatani, N. Glucosyl terpenates from the dried fruits of Prunus domestica L. Phytochem. Lett. 2014, 8, 132–136. [Google Scholar] [CrossRef]
- Sanz, M.; de Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, T.; Estrella, I.; Pinto, E. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. J. Mass Spectrom. 2012, 47, 905–918. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.-X.; Yu, L.-H.; Liu, Q.-B.; Li, L.-Z.; Sun, Q.; Song, S.-J. Tomensides A–D, new antiproliferative phenylpropanoid sucrose esters from Prunus tomentosa leaves. Bioorg. Med. Chem. Lett. 2014, 24, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, D.; Schneider, B. A resveratrol dimer from Anigozanthos preissii and Musa Cavendish. Phytochemistry 1996, 43, 471–473. [Google Scholar] [CrossRef]
- Ono, M.; Uenosono, Y.; Umaoka, H.; Shiono, Y.; Ikeda, T.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; Nohara, T. Five New Steroidal Glycosides from the Stems of Solanum sodomaeum. Chem Pharm Bull. 2009, 57, 759–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guccione, C.; Bergonzi, M.C.; Piazzini, V.; Bilia, A.R. A Simple and Rapid HPLC-PDA MS Method for the Profiling of Citrus Peels and Traditional Italian Liquors*. Planta Med. 2016, 82, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, Y.; Fan, C.; Ye, W.; Luo, J. Two new iridoid glucosides from Hedyotis diffusa. Fitoterapia 2010, 81, 515–517. [Google Scholar] [CrossRef]
- Hussein, S.R.; Latif, R.R.A.; Marzouk, M.M.; Elkhateeb, A.; Mohammed, R.S.; Soliman, A.A.F.; Abdel-Hameed, E.-S.S. Spectrometric analysis, phenolics isolation and cytotoxic activity of Stipagrostis plumosa (Family Poaceae). Chem Pap. 2018, 72, 29–37. [Google Scholar] [CrossRef]
- Shan, M.; Yu, S.; Yan, H.; Guo, S.; Xiao, W.; Wang, Z.; Zhang, L.; Ding, A.; Wu, Q.; Li, S.F.Y. A Review on the Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology of Geniposide, a Natural Product. Molecules 2017, 22. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.L.; Zhang, L.J.; Chen, R.Y.; Kuo, L.M.; Huang, J.P.; Huang, H.C.; Lee, K.H.; Wu, Y.C.; Kuo, Y.H. Antioxidant and anti-inflammatory phenylpropanoid derivatives from Calamus quiquesetinervius. J. Nat. Prod. 2010, 73, 1482–1488. [Google Scholar] [CrossRef]
- Dawé, A.; Mbiantcha, M.; Yakai, F.; Jabeen, A.; Ali, M.S.; Lateef, M.; Ngadjui, B.T. Flavonoids and triterpenes from Combretum fragrans with anti-inflammatory, antioxidant and antidiabetic potential. Z Nat. C. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Anti-inflammatory activities of flavonoids and a triterpene caffeate isolated from Bauhinia variegata. Phytother Res. 2008, 22, 957–962. [Google Scholar] [CrossRef]
- Deng, Y.H.; Alex, D.; Huang, H.Q.; Wang, N.; Yu, N.; Wang, Y.T.; Leung, G.P.; Lee, S.M. Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4’-trimethoxystilbene. Phytother Res. 2011, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Pae, H.O.; Oh, G.S.; Choi, B.M.; Jeong, S.; Jang, S.I.; Oh, H.; Kwon, T.O.; Song, C.E.; Chung, H.T. Inhibition of TNF-alpha, IL-1beta, and IL-6 productions and NF-kappa B activation in lipopolysaccharide-activated RAW 264.7 macrophages by catalposide, an iridoid glycoside isolated from Catalpa ovata G. Don (Bignoniaceae). Int. Immunopharmacol. 2002, 2, 1137–1181. [Google Scholar] [CrossRef]
- Kim, M.K.; Yun, K.J.; Lim, D.H.; Kim, J.; Jang, Y.P. Anti-Inflammatory Properties of Flavone di-C-Glycosides as Active Principles of Camellia Mistletoe, Korthalsella japonica. Biomol. 2016, 24, 630–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, F.R.S.; Mineo, T.W.P.; Pavanelli, W.R.; Guedes, P.M.M.; Silva, J.S. The effects of nitric oxide on the immune system during Trypanosoma cruzi infection. Mem. Inst. Oswaldo Cruzrio De Jan. 2009, 104, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Machado, I.D.; Santin, J.R.; de Melo, I.L.; Pedrosa, G.V.; Genovese, M.I.; Farsky, S.H.; Mancini-Filho, J. Aqueous extract of Rosmarinus officinalis L. inhibits neutrophil influx and cytokine secretion. Phytother Res. 2015, 29, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P. Replacement, reduction and refinement. ALTEX 2002, 19, 73–78. [Google Scholar] [CrossRef]
- Schwende, H.; Fitzke, E.; Ambs, P.; Dieter, P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc Biol. 1996, 59, 555–561. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactoside. Antimicrob. Agents Chemother 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Sieuwerts, A.M.; Klijn, J.G.M.; Peters, H.A.; Foekens, J.A. The MTT tetrazolium salt assay scrutinized: How to use this assay reliably to measure metabolic activity of cell cultures in vitro for the assessment of growth characteristics, IC50-values and cell survival. Clin. Chem. Lab. Med. 1995, 33, 813–824. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.; Nennie, E.; Ossenkoppele, G.; Beelen, R.; Langenhuijsen, M. Cell mediated cytotoxicity against U 937 cells by human monocytes and macrophages in a modified colorimetric MTT assay. A Methodol. Study. J. Immunol. Methods 1991, 141, 15–22. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| T. cruzi | L. amazonensis | L. infantum | THP-1 (Human Monocyte Cells) | SI | ||||
|---|---|---|---|---|---|---|---|---|
| Fractions | % inhibition | IC50 (µg/mL) | % inhibition | IC50 (µg/mL) | % inhibition | IC50 (µg/mL) | CC50 (µg/mL) | |
| FDCM | 90.37% ± 1.17% | 37.35 ± 0.97 | 37.13% ± 3.15% | ND | 11.04% ± 1.51% | ND | 341.50 ± 17.20 | 9.14 |
| FNBU | NA | ND | 1.71% ± 0.62% | ND | NA | ND | ND | ND |
| BDCM | NA | ND | NI | ND | NA | ND | ND | ND |
| BNBU | 5.37% ± 0.32% | ND | 5.52% ± 2.30% | ND | NA | ND | ND | ND |
| Benznidazole | 93.48% ± 1.04% | 10.18 ± 0.3 | - | - | - | - | >500 | >49.11 |
| Amphotericin B | - | - | 84.14% ± 1.37% | 0.09 ± 0.02 | 76.42% ± 4.24% | 0.11 ± 0.03 | - | - |
| Group of characterized metabolites | Fractions | |||
|---|---|---|---|---|
| FDCM | FNBU | BDCM | BNBU | |
| Arylpropanoid sucroses | X | X | ||
| Phenolics | X | X | X | |
| Fatty acids | X | X | ||
| Cyclohexanetetrol | X | |||
| Flavonoids | X | X | ||
| Triterpenes | X | |||
| Arylpropanoids | X | |||
| Glycolipids | X | X | ||
| Stilbenes | X | X | ||
| Arylbenzofurans | X | X | ||
| Iridoids | X | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandjo, L.P.; dos Santos Nascimento, M.V.P.; de H. Moraes, M.; Rodrigues, L.M.; Dalmarco, E.M.; Biavatti, M.W.; Steindel, M. NOx-, IL-1β-, TNF-α-, and IL-6-Inhibiting Effects and Trypanocidal Activity of Banana (Musa acuminata) Bracts and Flowers: UPLC-HRESI-MS Detection of Phenylpropanoid Sucrose Esters. Molecules 2019, 24, 4564. https://doi.org/10.3390/molecules24244564
Sandjo LP, dos Santos Nascimento MVP, de H. Moraes M, Rodrigues LM, Dalmarco EM, Biavatti MW, Steindel M. NOx-, IL-1β-, TNF-α-, and IL-6-Inhibiting Effects and Trypanocidal Activity of Banana (Musa acuminata) Bracts and Flowers: UPLC-HRESI-MS Detection of Phenylpropanoid Sucrose Esters. Molecules. 2019; 24(24):4564. https://doi.org/10.3390/molecules24244564
Chicago/Turabian StyleSandjo, Louis P., Marcus V. P. dos Santos Nascimento, Milene de H. Moraes, Luiza Manaut Rodrigues, Eduardo M. Dalmarco, Maique W. Biavatti, and Mario Steindel. 2019. "NOx-, IL-1β-, TNF-α-, and IL-6-Inhibiting Effects and Trypanocidal Activity of Banana (Musa acuminata) Bracts and Flowers: UPLC-HRESI-MS Detection of Phenylpropanoid Sucrose Esters" Molecules 24, no. 24: 4564. https://doi.org/10.3390/molecules24244564
APA StyleSandjo, L. P., dos Santos Nascimento, M. V. P., de H. Moraes, M., Rodrigues, L. M., Dalmarco, E. M., Biavatti, M. W., & Steindel, M. (2019). NOx-, IL-1β-, TNF-α-, and IL-6-Inhibiting Effects and Trypanocidal Activity of Banana (Musa acuminata) Bracts and Flowers: UPLC-HRESI-MS Detection of Phenylpropanoid Sucrose Esters. Molecules, 24(24), 4564. https://doi.org/10.3390/molecules24244564