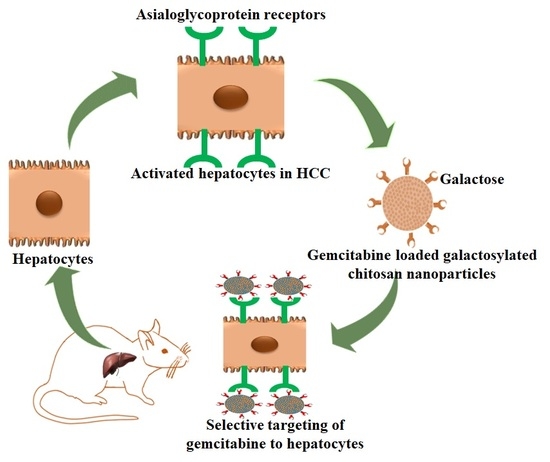
Development of Asialoglycoprotein Receptor-Targeted Nanoparticles for Selective Delivery of Gemcitabine to Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Result and Discussion
2.1. Synthesis of Galactosylated Chitosan
2.2. Preparation of Gemcitabine Nanoparticles
2.3. Characterization of Gemcitabine Nanoparticles
2.4. FTIR
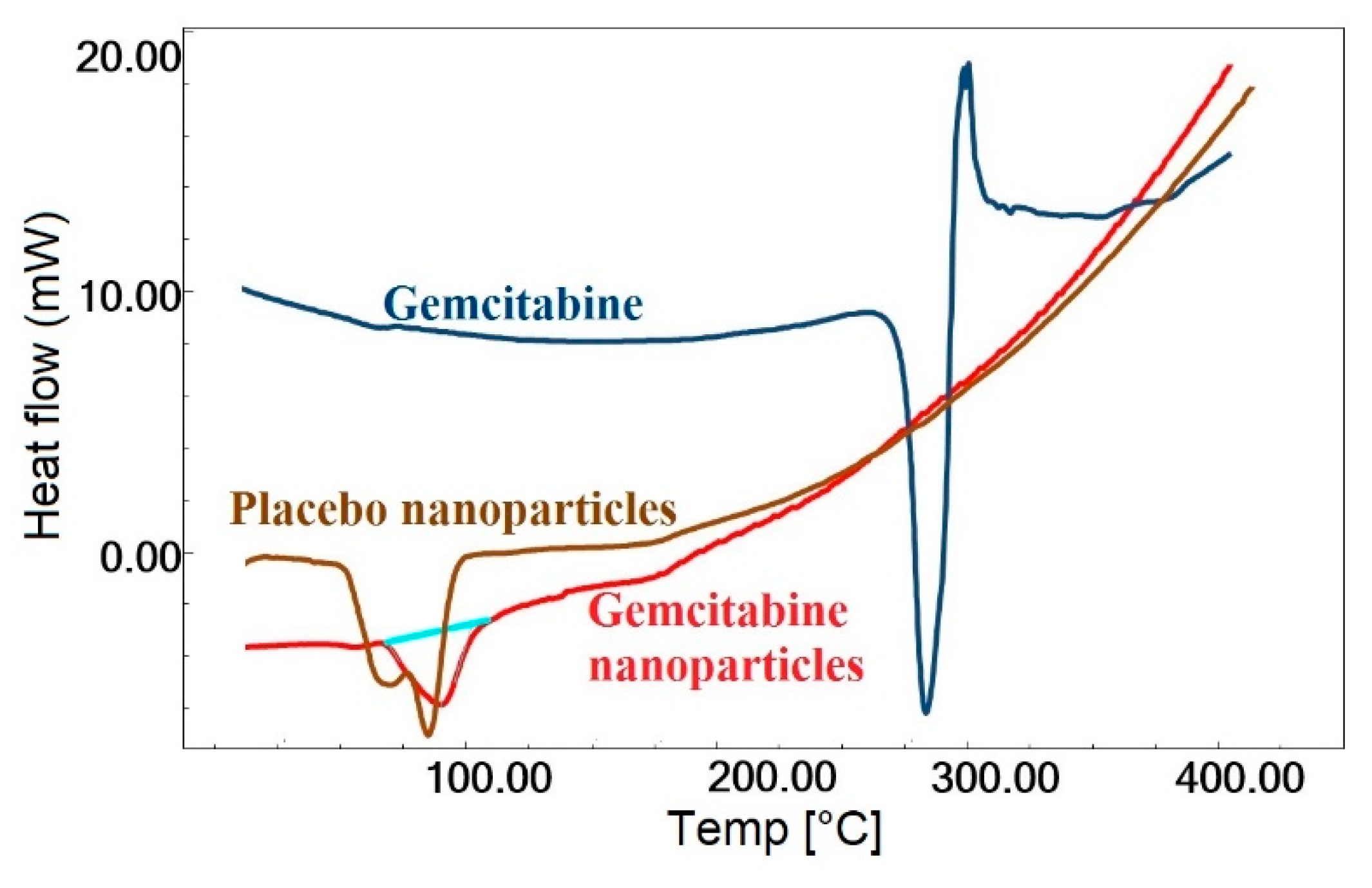
2.5. DSC
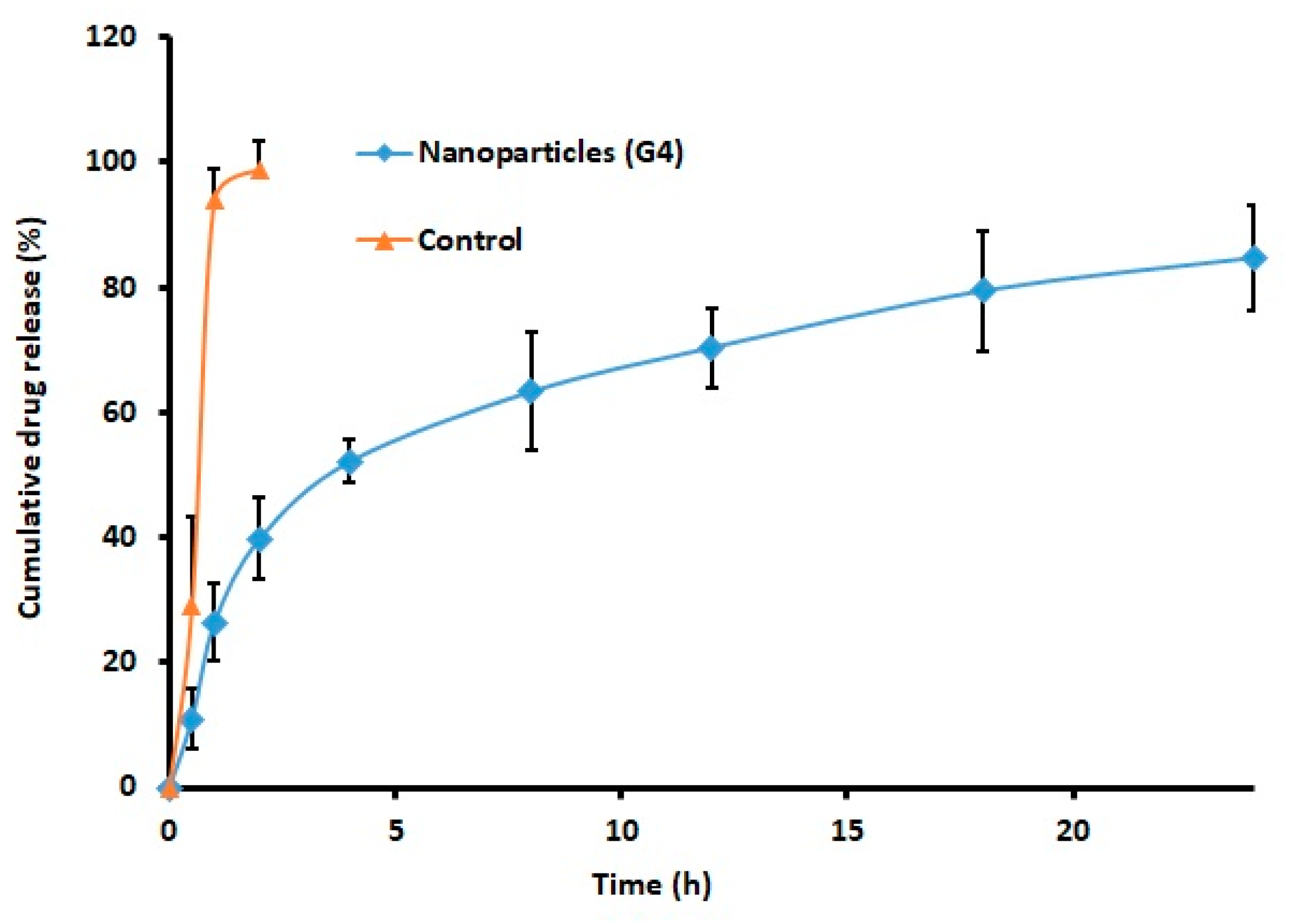
2.6. Drug Release
2.7. Blood Disappearance and Organ Distribution
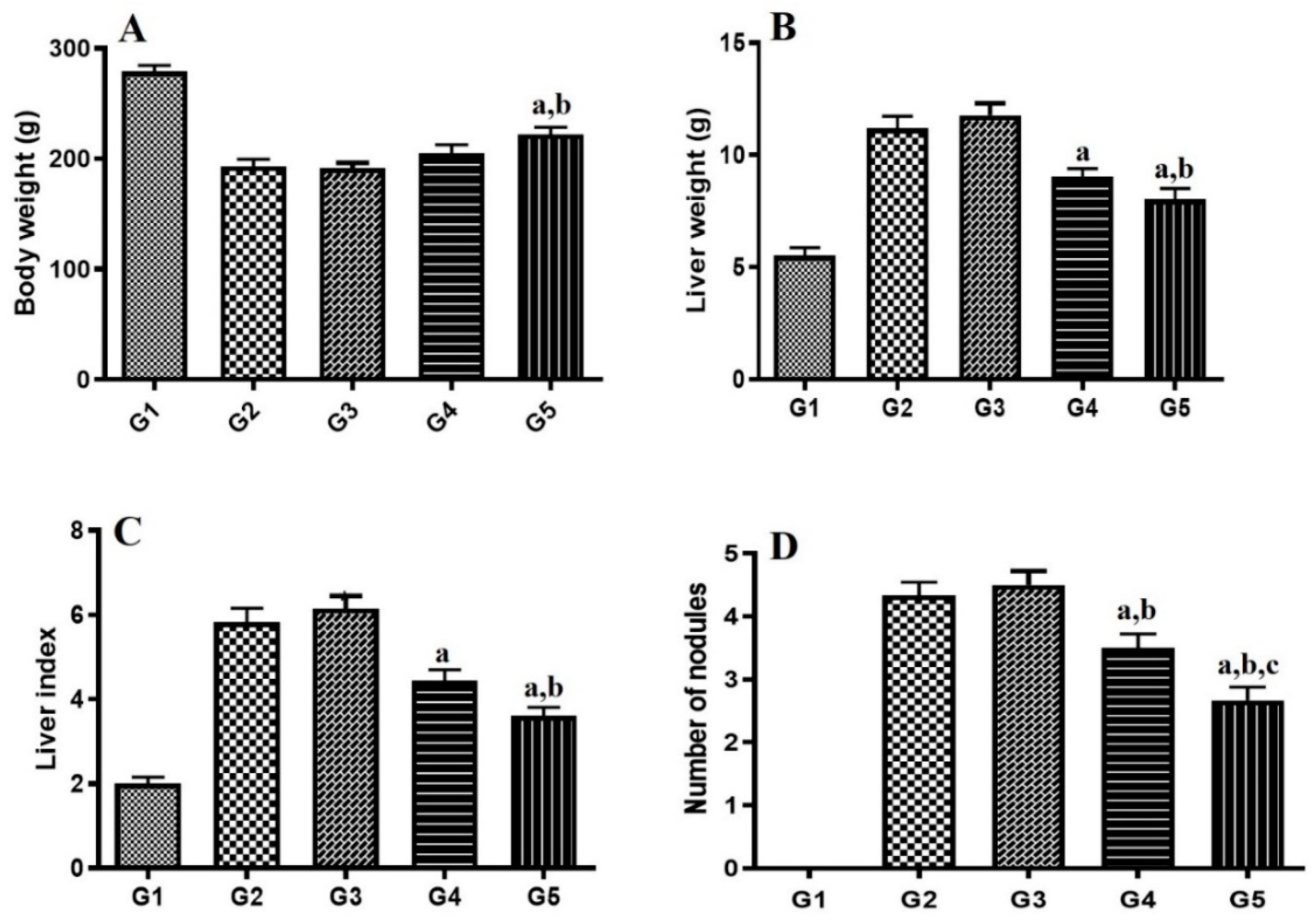
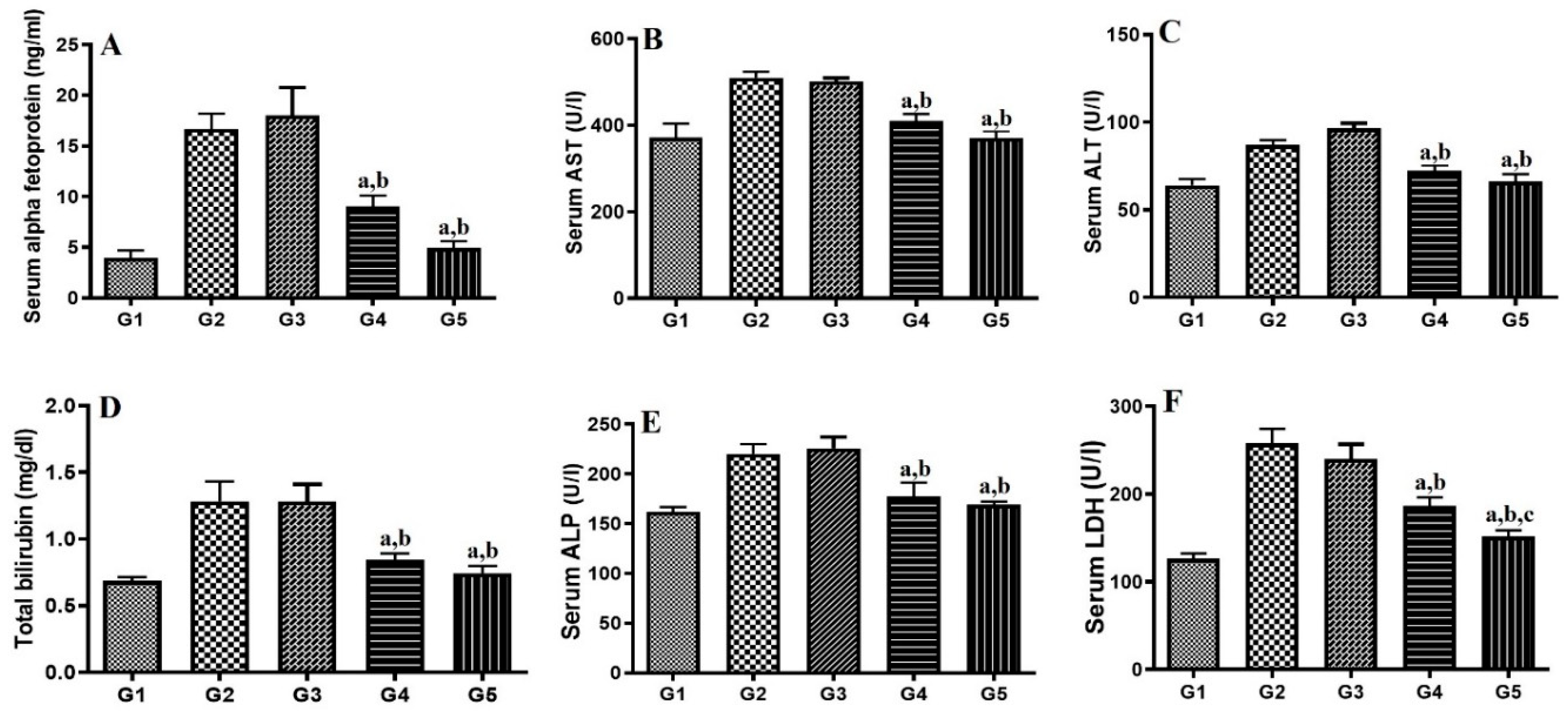
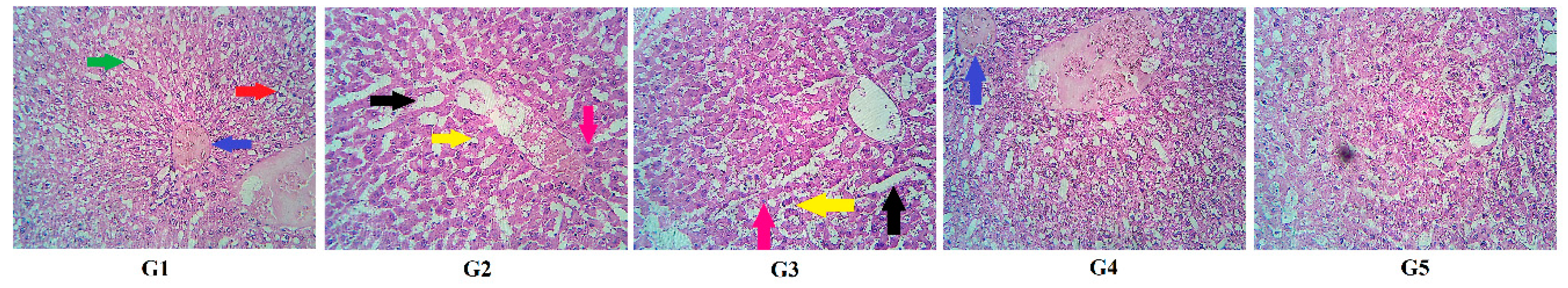
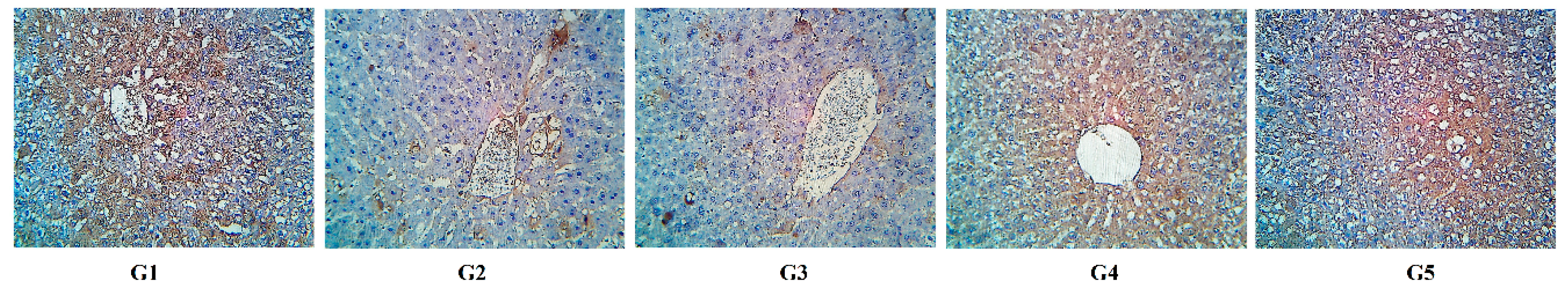
2.8. HCC Measurements.
3. Materials and Methods.
3.1. Materials
3.2. Analysis of Gemcitabine
3.3. Synthesis of Galactosylated Chitosan
3.4. Preparation of Gemcitabine Nanoparticles
3.5. Characterization of Gemcitabine Nanoparticles
3.5.1. Percentage Yield
3.5.2. Drug Entrapment Efficiency (EE) and Drug Loading (DL)
3.5.3. Drug Content
3.5.4. Transmission Electron Microscopy (TEM)
3.5.5. Particle Size Characterization and Zeta Potential
3.5.6. FTIR Spectroscopy
3.5.7. Differential Scanning Calorimetry (DSC)
3.6. In Vitro Drug Release and Kinetics
- Zero order model Q = Q0 + kt
- First order model Q = Q0 × ekt
- Higuchi model Q = k × t0.5
- Korsmeyer–Peppas model Q = k × tn
- Weibull model Q = 1 − exp[−(t)b/a]
3.7. Stability
3.8. Blood Disappearance and Organ Distribution
3.9. HCC Experimental Protocol
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and future treatment of hepatocellular carcinoma: An updated comprehensive review. J. Clin. Transl. Hepatol. 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ng, H.L.H.; Lu, A.; Lin, C.; Zhou, L.; Lin, G.; Zhang, Y.; Yang, Z.; Zhang, H. Drug delivery system targeting advanced hepatocellular carcinoma: Current and future. Nanomedicine 2016, 12, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, W.; Wang, B.; Gao, Y.; Song, Z.; Zheng, Q.C. Ligand-based targeted therapy: A novel strategy for hepatocellular carcinoma. Int. J. Nanomed. 2016, 11, 5645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef]
- Wysocki, P.J. Targeted therapy of hepatocellular cancer. Expert Opin. Investig. Drugs 2010, 19, 265–274. [Google Scholar] [CrossRef]
- Dutta, R.; Mahato, R.I. Recent advances in hepatocellular carcinoma therapy. Pharmacol. Ther. 2017, 173, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Prabhu, R.H.; Patravale, V.B.; Joshi, M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001. [Google Scholar]
- Cervello, M.; McCubrey, J.A.; Cusimano, A.; Lampiasi, N.; Azzolina, A.; Montalto, G. Targeted therapy for hepatocellular carcinoma: Novel agents on the horizon. Oncotarget 2012, 3, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toschi, L.; Finocchiaro, G.; Bartolini, S.; Gioia, V.; Cappuzzo, F. Role of gemcitabine in cancer therapy. Future Oncol. 2005, 1, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Allouache, D.; Gawande, S.R.; Tubiana-Hulin, M.; Tubiana-Mathieu, N.; Piperno-Neumann, S.; Mefti, F.; Bozec, L.; Genot, J.Y. First-line therapy with gemcitabine and paclitaxel in locally, recurrent or metastatic breast cancer: A phase II study. BMC Cancer 2005, 5, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toschi, L.; Cappuzzo, F. Gemcitabine for the treatment of advanced nonsmall cell lung cancer. OncoTargets Ther. 2009, 2, 209. [Google Scholar]
- Thota, R.; Pauff, J.M.; Berlin, J.D. Treatment of metastatic pancreatic adenocarcinoma: A review. Oncology 2014, 28, 70–74. [Google Scholar]
- Guan, Z.; Wang, Y.; Maoleekoonpairoj, S.; Chen, Z.; Kim, W.S.; Ratanatharathorn, V.; Reece, W.H.H.; Kim, T.W.; Lehnert, M. Prospective randomised phase II study of gemcitabine at standard or fixed dose rate schedule in unresectable hepatocellular carcinoma. Br. J. Cancer 2003, 89, 1865. [Google Scholar] [CrossRef] [Green Version]
- Ulrich-Pur, H.; Kornek, G.V.; Fiebiger, W.; Schüll, B.; Raderer, M.; Scheithauer, W. Treatment of advanced hepatocellular carcinoma with biweekly high-dose gemcitabine. Oncology 2001, 60, 313–315. [Google Scholar] [CrossRef]
- Chia, W.K.; Ong, S.; Toh, H.C.; Hee, S.W.; Choo, S.P.; Poon, D.Y.; Tay, M.H.; Tan, C.K.; Koo, W.H.; Foo, K.F. Phase II trial of gemcitabine in combination with cisplatin in inoperable or advanced hepatocellular carcinoma. Ann. Acad. Med. Singap. 2008, 37, 554. [Google Scholar]
- Zaanan, A.; Williet, N.; Hebbar, M.; Dabakuyo, T.S.; Fartoux, L.; Mansourbakht, T.; Dubreuil, O.; Rosmorduc, O.; Cattan, S.; Bonnetain, F.; et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: A large multicenter AGEO study. J. Hepatol. 2013, 58, 81–88. [Google Scholar] [CrossRef]
- Chi, D.C.; Brogan, F.; Turenne, I.; Zelonis, S.; Schwartz, L.; Saif, M.W. Gemcitabine-induced pulmonary toxicity. Anticancer Res. 2012, 32, 4147–4149. [Google Scholar]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naskar, S.; Sharma, S.; Koutsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Cheng, M.; He, B.; Wan, T.; Zhu, W.; Han, J.; Zha, B.; Chen, H.; Yang, F.; Li, Q.; Wang, W.; et al. 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS ONE 2012, 7, e47115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, M.; Zheng, X.; Tu, L.; Ma, Z.; Wang, Z.; Yan, D.; Shen, Z. The liver-targeting study of the N-galactosylated chitosan in vivo and in vitro. Artif. Cells Nanomed. Biotechnol. 2014, 42, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Shen, Y.; Liao, M.M.; Mao, X.; Mi, G.J.; You, C.; Guo, Q.Y.; Li, W.J.; Wang, X.Y.; Lin, N.; et al. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: Enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine 2019, 15, 86–97. [Google Scholar] [CrossRef]
- Pranatharthiharan, S.; Patel, M.D.; Malshe, V.C.; Pujari, V.; Gorakshakar, A.; Madkaikar, M.; Ghosh, K.; Devarajan, P.V. Asialoglycoprotein receptor targeted delivery of doxorubicin nanoparticles for hepatocellular carcinoma. Drug Deliv. 2017, 24, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Nantz, M.H.; Zern, M.A. Targeting hepatocytes for drug and gene delivery: Emerging novel approaches and applications. Front. Biosci. 2002, 7, 717–725. [Google Scholar]
- Wang, Q.; Zhang, L.; Hu, W.; Hu, Z.H.; Bei, Y.Y.; Xu, J.Y.; Wang, W.J.; Zhang, X.N.; Zhang, Q. Norcantharidin-associated galactosylated chitosan nanoparticles for hepatocyte-targeted delivery. Nanomedicine 2010, 6, 371–381. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Attimarad, M.; Harsha, S. Poly (lactic acid-co-glycolic acid) Nanospheres improved the oral delivery of candesartan cilexetil. Indian J. Pharm. Educ. Res. 2017, 51, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zan, X.; Wang, Z.; Wu, H.; Yin, D.; Liao, C.; Wan, Y. Galactosylated chitosan–polycaprolactone nanoparticles for hepatocyte-targeted delivery of curcumin. Carbohydr. Polym. 2013, 94, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Derakhshandeh, K.; Fathi, S. Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int. J. Pharm. 2012, 437, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ping, Q.; Ding, Y.; Cheng, Y.; Shen, J. Synthesis, characterization, and microsphere formation of galactosylated chitosan. J. Appl. Polym. Sci. 2004, 91, 659–665. [Google Scholar] [CrossRef]
- Sawada, S.; Okano, J.I.; Imamoto, R.; Yasunaka, Y.; Abe, R.; Koda, M.; Murawaki, Y.; Isomoto, H. Preventive effect of geraniol on diethylnitrosamine-induced hepatocarcinogenesis in rats. Yonago Acta Med. 2016, 59, 37. [Google Scholar]
- Carr, B.I.; Guerra, V.; Giannini, E.G.; Farinati, F.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Zoli, M.; Borzio, F.; et al. A liver index and its relationship to indices of HCC aggressiveness. J. Integr. Oncol. 2016, 5, 178–184. [Google Scholar] [CrossRef]
- Zerbini, A.; Pilli, M.; Penna, A.; Pelosi, G.; Schianchi, C.; Molinari, A.; Schivazappa, S.; Zibera, C.; Fagnoni, F.F.; Ferrari, C.; et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006, 66, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Reichl, P.; Mikulits, W. Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma: An update for clinicians. Oncol. Rep. 2016, 36, 613–625. [Google Scholar] [CrossRef]
- Ding, Y.F.; Wu, Z.H.; Wei, Y.J.; Shu, L.; Peng, Y.R. Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular carcinoma induced by diethylnitrosamine. J. Cancer Res. Clin. Oncol. 2017, 143, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, H. Matrine inhibits diethylnitrosamine-induced HCC proliferation in rats through inducing apoptosis via p53, Bax-dependent caspase-3 activation pathway and down-regulating MLCK overexpression. Iran. J. Pharm. Res. 2016, 15, 491. [Google Scholar] [PubMed]
- Wang, L.; Zhang, Y.; Wang, W.; Zhu, Y.; Chen, Y.; Tian, B. Gemcitabine treatment induces endoplasmic reticular (ER) stress and subsequently upregulates urokinase plasminogen activator (uPA) to block mitochondrial-dependent apoptosis in Panc-1 cancer stem-like cells (CSCs). PLoS ONE 2017, 12, e0184110. [Google Scholar] [CrossRef]
- Parshina, N.A.; Pleteneva, T.V.; Baikova, V.N.; Narimanov, M.N.; Tyulyandin, S.A. Quantitative estimation of gemcitabine by HPLC in plasma. Pharm. Chem. J. 2008, 42, 288–290. [Google Scholar] [CrossRef]
- Cheng, M.; Li, Q.; Wan, T.; Hong, X.; Chen, H.; He, B.; Cheng, Z.; Xu, H.; Ye, T.; Zha, B.; et al. Synthesis and efficient hepatocyte targeting of galactosylated chitosan as a gene carrier in vitro and in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 70–80. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Nair, A.B.; Kaushik, A.; Attimarad, M.; Al-Dhubiab, B.E. Enhanced oral bioavailability of calcium using bovine serum albumin microspheres. Drug Deliv. 2012, 19, 277–285. [Google Scholar] [CrossRef]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.; Nair, A.B.; Al-Dhubiab, B.E. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J. Liposome Res. 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Seydi, E.; Rasekh, H.R.; Salimi, A.; Mohsenifar, Z.; Pourahmad, J. Myricetin selectively induces apoptosis on cancerous hepatocytes by directly targeting their mitochondria. Basic Clin. Pharmacol. Toxicol. 2016, 119, 249–258. [Google Scholar] [CrossRef]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| F | Drug: Polymer Ratio | % Yield | % EE | % Drug Loading | % Drug Content | Mean Diameter (nm) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|
| G1 | 1:2 | 68.5 ± 6.1 | 54.6 ± 3.2 | 18.2 ± 2.9 | 93.5 ± 2.5 | 156.8 ± 30.1 | 19.3 ± 2.6 |
| G2 | 1:4 | 72.5 ± 7.8 | 64.2 ± 5.9 | 12.8 ± 1.6 | 94.1 ± 2.1 | 157.2 ± 38.3 | 21.5 ± 3.1 |
| G3 | 1:6 | 73.4 ± 5.9 | 66.4 ± 6.1 | 9.5 ± 2.1 | 94.6 ± 2.8 | 162.0 ± 34.2 | 22.3 ± 2.5 |
| G4 | 1:8 | 74.2 ± 7.4 | 72.1 ± 6.5 | 8.0 ± 2.3 | 95.9 ± 3.2 | 164.1 ± 39.5 | 20.4 ± 3.9 |
| G5 | 1:10 | 73.2 ± 6.5 | 71.9 ± 4.2 | 6.5 ± 2.2 | 94.4 ± 2.8 | 171.3 ± 35.2 | 21.8 ± 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P.; et al. Development of Asialoglycoprotein Receptor-Targeted Nanoparticles for Selective Delivery of Gemcitabine to Hepatocellular Carcinoma. Molecules 2019, 24, 4566. https://doi.org/10.3390/molecules24244566
Nair AB, Shah J, Al-Dhubiab BE, Patel SS, Morsy MA, Patel V, Chavda V, Jacob S, Sreeharsha N, Shinu P, et al. Development of Asialoglycoprotein Receptor-Targeted Nanoparticles for Selective Delivery of Gemcitabine to Hepatocellular Carcinoma. Molecules. 2019; 24(24):4566. https://doi.org/10.3390/molecules24244566
Chicago/Turabian StyleNair, Anroop B., Jigar Shah, Bandar E. Al-Dhubiab, Snehal S. Patel, Mohamed A. Morsy, Vimal Patel, Vishal Chavda, Shery Jacob, Nagaraja Sreeharsha, Pottathil Shinu, and et al. 2019. "Development of Asialoglycoprotein Receptor-Targeted Nanoparticles for Selective Delivery of Gemcitabine to Hepatocellular Carcinoma" Molecules 24, no. 24: 4566. https://doi.org/10.3390/molecules24244566
APA StyleNair, A. B., Shah, J., Al-Dhubiab, B. E., Patel, S. S., Morsy, M. A., Patel, V., Chavda, V., Jacob, S., Sreeharsha, N., Shinu, P., Attimarad, M., & Venugopala, K. N. (2019). Development of Asialoglycoprotein Receptor-Targeted Nanoparticles for Selective Delivery of Gemcitabine to Hepatocellular Carcinoma. Molecules, 24(24), 4566. https://doi.org/10.3390/molecules24244566