Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin
Abstract
:1. Introduction
2. Results and Discussion
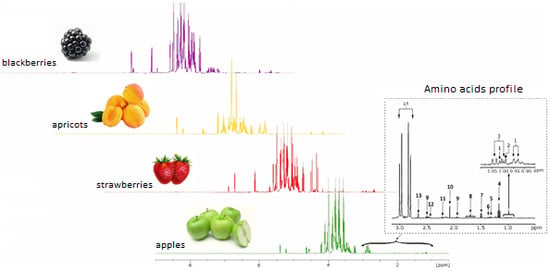
2.1. General Presentation of the 1H-NMR Spectra of Fruit Juices
2.2. Amino Acid Profile: Assignment of the Interest Peaks and Statistical Elaboration
2.3. Botanical Origin Discrimination
3. Materials and Methods
3.1. Fruit Juice Samples
3.2. Sample Preparation for NMR Analysis
3.3. NMR Analysis
3.4. Software and Data Pre-Processing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, F.; Vasas, M.; Hatzakis, E.; Spyros, A. Magnetic resonance applications in food analysis. Annu. Rep. NMR Spectrosc. 2019, 98, 239–306. [Google Scholar]
- Girelli, C.R.; Accogli, R.; Coco, L.D.; Angile, F.; Bellis, L.D.; Fanizzi, F.P. 1H-NMR based metabolomic profiles of different sweet melon (Cucumis melo L.) Salento varieties: Analysis and comparison. Food Res. Int. 2018, 114, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Mannina, L.; Proietti, N.; Carradori, S.; Daglia, M.; Giusti, A.M.; Antiochia, R.; Capitani, D. Untargeted NMR-based methodology in the study of fruit metabolites. Molecules 2015, 20, 4088–4108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khakimov, B.; Mongi, R.J.; Sørensen, K.M.; Ndabikunze, B.K.; Chove, B.E.; Engelsen, S.B. A comprehensive and comparative GC–MS metabolomics study of non-volatiles in Tanzanian grown mango, pineapple, jackfruit, baobab and tamarind fruits. Food Chem. 2016, 213, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Lieb, V.M.; Esquivel, P.; Castillo, E.C.; Carle, R.; Steingass, C.B. GC–MS profiling, descriptive sensory analysis, and consumer acceptance of Costa Rican papaya (Carica papaya L.) fruit purees. Food Chem. 2018, 248, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Fernández, E.; González-Fernández, J.J.; Hormaza, J.I.; Bajoub, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Targeted LC-MS approach to study the evolution over the harvesting season of six important metabolites in fruits from different avocado cultivars. Food Anal. Methods 2016, 9, 3479–3491. [Google Scholar] [CrossRef]
- Lin, L.; Yang, W.; Wei, X.; Wang, Y.; Zhang, L.; Zhang, Y.; Zhao, M. Enhancement of Solasodine Extracted from Fruits of Solanum nigrum L. by Microwave-Assisted Aqueous Two-Phase Extraction and Analysis by High-Performance Liquid Chromatography. Molecules 2019, 24, 2294. [Google Scholar] [CrossRef] [Green Version]
- Botoran, O.R.; Popescu, R.; Costinel, D.; Miricioiu, M.; Ionete, R.E. Assessing the origin of vegetables based on ¹H/13C-NMR profiling and chemometrics: Preliminary results. Prog. Cryog. Isot. Sep. 2019, 22, 63–76. [Google Scholar]
- Popescu, R.; Ionete, R.E.; Botoran, O.R.; Costinel, D.; Bucura, F.; Geana, E.I.; Botu, M. ¹H-NMR Profiling and Carbon Isotope Discrimination as Tools for the Comparative Assessment of Walnut (Juglans regia L.) Cultivars with Various Geographical and Genetic Origins—A Preliminary Study. Molecules 2019, 24, 1378. [Google Scholar] [CrossRef] [Green Version]
- Botoran, O.R.; Popescu, R.; Costinel, D.; Ionete, R.E. O-H-C stable isotopes as discriminant markers for fruits botanical origin. J. Biotechnol. 2017, 256, S104. [Google Scholar] [CrossRef]
- Bo, Y.; Feng, J.; Xu, J.; Huang, Y.; Cai, H.; Cui, X.; Chen, Z. High-resolution pure shift NMR spectroscopy offers better metabolite discrimination in food quality analysis. Food Res. Int. 2019, 125, 108574. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Pellati, F.; Benvenuti, S.; Bertelli, D. Use of 1H NMR to Detect the Percentage of Pure Fruit Juices in Blends. Molecules 2019, 24, 2592. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.E.; Gaudillere, J.-P.; Van Leeuwen, C.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H NMR metabolite fingerprints of grape berry: Comparison of vintage and soil effects in Bordeaux grapevine growing areas. Anal. Chim. Acta 2006, 563, 346–352. [Google Scholar] [CrossRef]
- Popescu, R.; Dinca, O.R.; Costinel, D.; Ionete, R.E. Spirits classification using 1H NMR and chemometrics. Prog. Cryog. Isot. Sep. 2014, 17, 35–41. [Google Scholar]
- Tomita, S.; Nemoto, T.; Matsuo, Y.; Shoji, T.; Tanaka, F.; Nakagawa, H. A NMR based, non-targeted multistep metabolic profiling revealed L-rhamnitol as a metabolite that characterised apples from different geographic origins. Food Chem. 2015, 174, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.O.; Pisano, P.L.; Sierra, M.G.; Furlán, R.L. NMR and multivariate data analysis to assess traceability of argentine citrus. Microchem. J. 2018, 141, 264–270. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Quality and Authenticity Control of Fruit Juices—A Review. Molecules 2019, 24, 1014. [Google Scholar] [CrossRef] [Green Version]
- Spraul, M.; Schütz, B.; Rinke, P.; Koswig, S.; Humpfer, E.; Schäfer, H.; Mörtter, M.; Fang, F.; Marx, U.C.; Minoja, A. NMR-Based Multi Parametric Quality Control of Fruit Juices: SGF Profiling. Nutrients 2009, 1, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Villa-Ruano, N.; Pérez-Hernández, N.; Zepeda-Vallejo, L.G.; Quiroz-Acosta, T.; Mendieta-Moctezuma, A.; Montoya-García, C.O.; García-Nava, M.L.; Martínez, E.B. 1H-NMR Based Metabolomics Profiling of Citrus Juices Produced in Veracruz, México. Chem. Biodivers. 2019, 16, e1800479. [Google Scholar] [CrossRef]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C. Phenolic Profile and Bioactive Properties of Carissa macrocarpa (Eckl.) A.D.C.: An In Vitro Comparative Study between Leaves, Stems, and Flowers. Molecules 2019, 24, 1696. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, T.F.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino nitrogen concentration correlates to total yeast assimilable nitrogen concentration in apple juice. Food Sci. Nutr. 2018, 6, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Neilson, A.P.; Lahne, J.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino acid composition of apple juices with potential for cider making as determined by UPLC-PDA. J. Inst. Brew. 2018, 124, 467–476. [Google Scholar] [CrossRef]
- Peck, G.; McGuire, M.; Boudreau, T.; Stewart, A. Crop load density affects ‘York’ apple juice and hard cider quality. Hortscience 2016, 51, 1098–1102. [Google Scholar] [CrossRef]
- Zielinski, A.F.; Alberti, A.; Nogueira, A. Characterizing fruit juices and fermented fruit beverages using chemometrics tools. In Fruit Juices; Academic Press: Cambridge, MA, USA, 2018; pp. 823–833. [Google Scholar]
- Clausen, M.R.; Pedersen, B.H.; Bertram, H.C.; Kidmose, U. Quality of sour cherry juice of different clones and cultivars (Prunus cerasus L.) determined by a combined sensory and NMR spectroscopic approach. J. Agric. Food Chem. 2011, 59, 12124–12130. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Bianco, A.; Catucci, L.; Cafagna, I.; Gallo, V.; Mastrorilli, P.; Agostiano, A. Non-targeted 1H NMR fingerprinting and multivariate statistical analyses for the characterisation of the geographical origin of Italian sweet cherries. Food Chem. 2013, 141, 3028–3033. [Google Scholar] [CrossRef]
- Grandizoli, C.W.P.d.S.; Campos, F.R.; Simonelli, F.; Barison, A. Grape Juice Quality Control by Means of 1H NMR Spectroscopy and Chemometric Analyses. Quim. Nova 2014, 37, 1227–1232. [Google Scholar]
- Capitani, D.; Sobolev, A.P.; Tomassini, A.; Sciubba, F.; de Salvador, F.R.; Mannina, L.; Delfini, M. Peach fruit: A metabolic comparative analysis of two varieties with different resistance to insect attacks by NMR spectroscopy. J. Agric. Food Chem. 2013, 61, 1718–1726. [Google Scholar] [CrossRef]
- Capitani, D.; Sobolev, A.P.; Delfini, M.; Vista, S.; Antiochia, R.; Proietti, N.; Bubici, S.; Ferrante, G.; Carradori, S.; de Salvador, F.R.; et al. NMR methodologies in the analysis of blueberries. Electrophoresis 2014, 35, 1615–1626. [Google Scholar] [CrossRef]
- McKenzie, J.S.; Donarski, J.A.; Wilson, J.C.; Charlton, A.J. Analysis of complex mixtures using high-resolution nuclear magnetic resonance spectroscopy and chemometrics. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 336–359. [Google Scholar] [CrossRef]
- Wizgoski, F.J.; Paudel, L.; Rinaldi, P.L.; Reese, R.N.; Ozgen, M.; Tulio, A.Z.; Miller, A.R.; Scheerens, J.C.; Hardy, J.K. Modeling relationships among active components in black raspberry (Rubus occidentalis L.) fruit extracts using high-resolution 1H nuclear magnetic resonance (NMR) spectroscopy and multivariate statistical analysis. J. Agric. Food Chem. 2010, 58, 3407–3414. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, S.J.; Hyun, S.-H.; Yang, S.-O.; Lee, J.; Auh, J.-H.; Kim, J.-H.; Cho, S.-M.; Marriott, P.J.; Choi, H.-K. Biochemical monitoring of black raspberry (Rubus coreanus Miquel) fruits according to maturation stage by 1H NMR using multiple solvent systems. Food Res. Int. 2011, 44, 1977–1987. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Viereck, N.; Bro, R.; Engelsen, S.B. Chemometric Analysis of NMR Spectra. In Modern Magnetic Resonance; Webb, G., Ed.; Springer: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

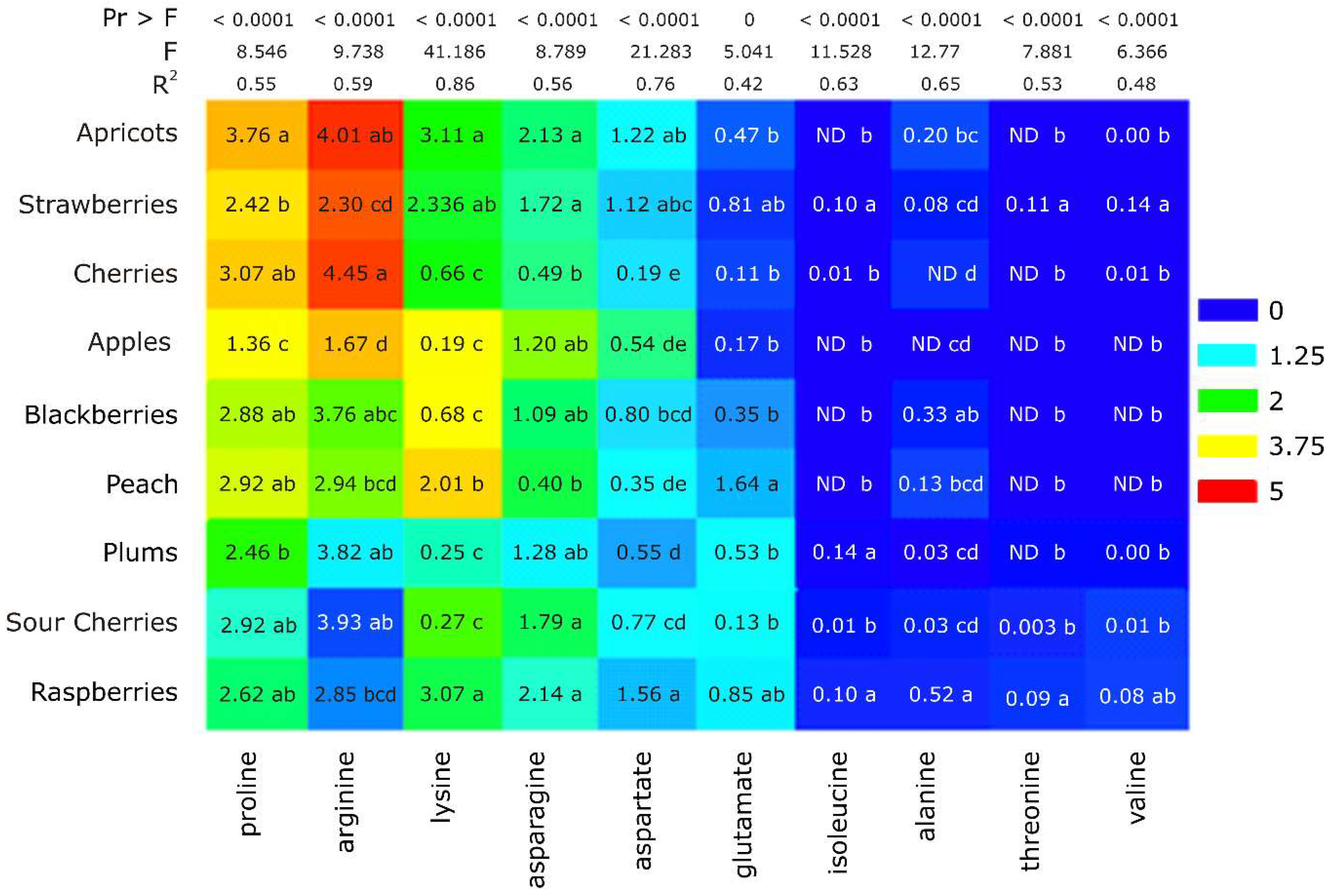
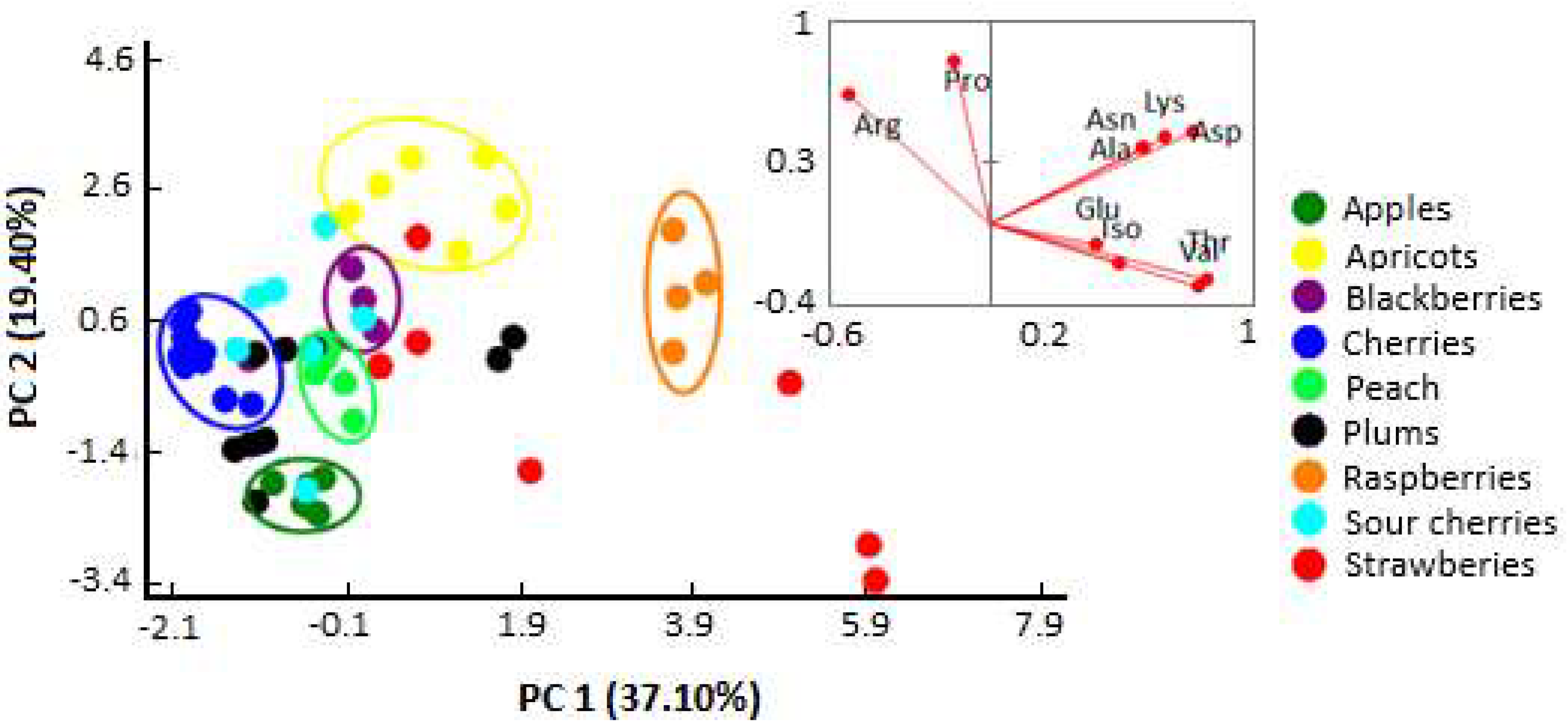
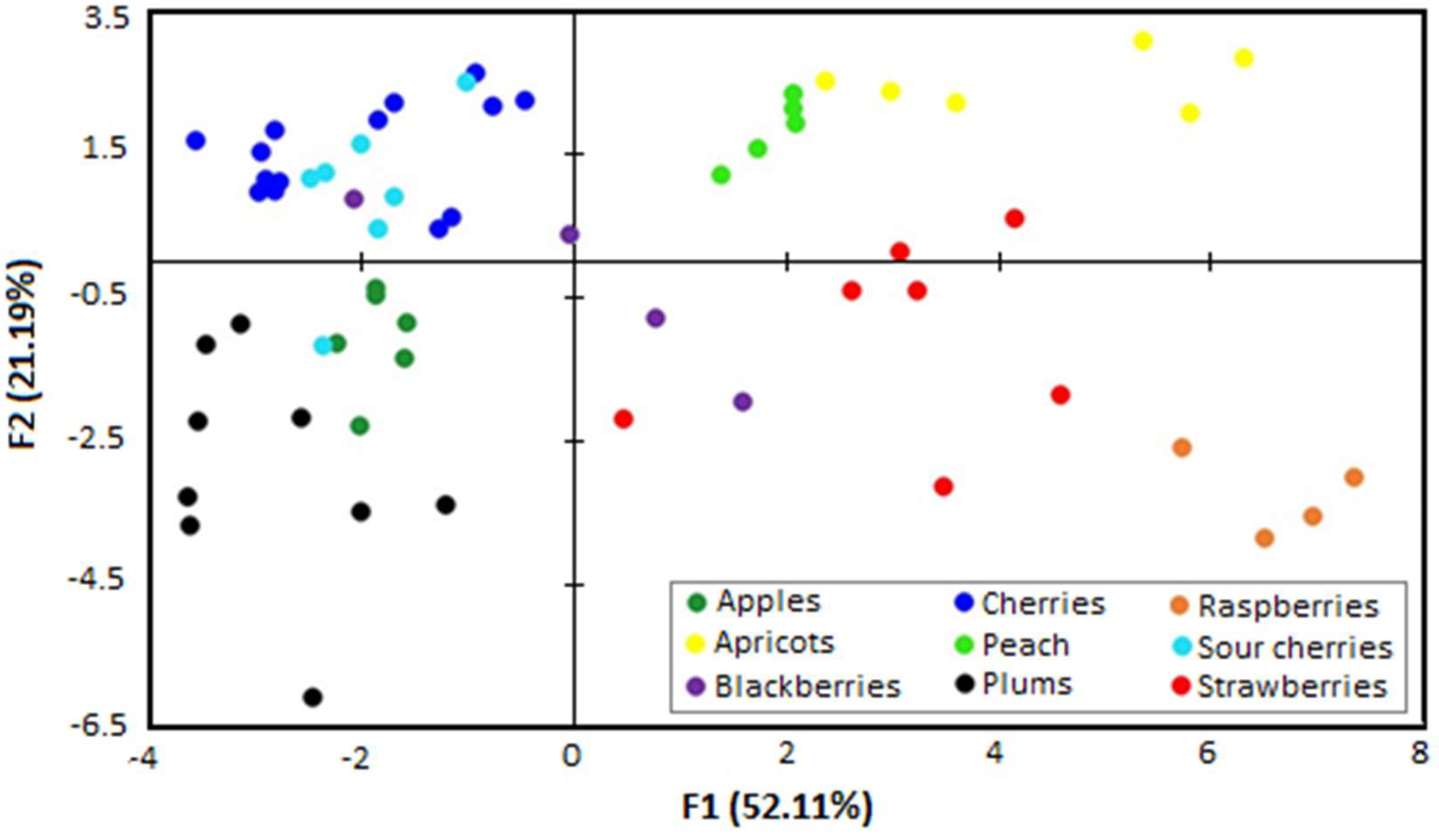
| Variables | Pro | Arg | Lys | Asn | Asp | Glu | Iso | Ala | Thr | Val |
|---|---|---|---|---|---|---|---|---|---|---|
| Genus | ||||||||||
| Prunus | 0.455 | 0.596 | −0.220 | −0.239 | −0.436 | −0.083 | −0.116 | −0.363 | −0.470 | −0.422 |
| Fragaria | −0.147 | −0.360 | 0.347 | 0.199 | 0.324 | 0.181 | 0.275 | −0.034 | 0.616 | 0.631 |
| Malus | −0.576 | −0.503 | −0.272 | −0.019 | −0.099 | −0.147 | −0.177 | −0.180 | −0.109 | −0.110 |
| Rubus | 0.001 | −0.063 | 0.224 | 0.168 | 0.400 | 0.076 | 0.061 | 0.706 | 0.182 | 0.100 |
| From\To | Apple | Apricot | Blackberry | Cherry | Peach | Plum | Raspberry | Sour Cherry | Strawberry | Total | % Correct |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apple | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 100% |
| Apricot | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 100% |
| Blackberry | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 75% |
| Cherry | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 15 | 100% |
| Peach | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 5 | 100% |
| Plum | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 9 | 100% |
| Raspberry | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 100% |
| Sour cherry | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 8 | 87.5% |
| Strawberry | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 5 | 7 | 71.43% |
| Total | 7 | 7 | 3 | 15 | 5 | 10 | 4 | 8 | 5 | 64 | 93.75% |
| Fruit Variety | Fruit Genus | Fruit Species | Harvest Period | Region of Provenance | ||
|---|---|---|---|---|---|---|
| Valcea County | Constanta County | Vrancea County | ||||
| Apricots | Prunus | P. armeniaca | August | 2 | 2 | 2 |
| Cherries | P. avium | June | 5 | 5 | 5 | |
| Peach | P. persica | August | 2 | 2 | 1 | |
| Plums | P. domestica | August | 3 | 3 | 3 | |
| Sour cherries | P. cerasus | June | 3 | 2 | 3 | |
| Raspberries | Rubus | R. idaeus | August | 1 | 2 | 1 |
| Blackberries | R. fruticosus | August | 2 | 1 | 1 | |
| Strawberries | Fragaria | Fragaria sp. | June | 3 | 2 | 2 |
| Apples | Malus | M. domestica | August | 2 | 2 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botoran, O.R.; Ionete, R.E.; Miricioiu, M.G.; Costinel, D.; Radu, G.L.; Popescu, R. Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin. Molecules 2019, 24, 4500. https://doi.org/10.3390/molecules24244500
Botoran OR, Ionete RE, Miricioiu MG, Costinel D, Radu GL, Popescu R. Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin. Molecules. 2019; 24(24):4500. https://doi.org/10.3390/molecules24244500
Chicago/Turabian StyleBotoran, Oana Romina, Roxana Elena Ionete, Marius Gheorghe Miricioiu, Diana Costinel, Gabriel Lucian Radu, and Raluca Popescu. 2019. "Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin" Molecules 24, no. 24: 4500. https://doi.org/10.3390/molecules24244500
APA StyleBotoran, O. R., Ionete, R. E., Miricioiu, M. G., Costinel, D., Radu, G. L., & Popescu, R. (2019). Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin. Molecules, 24(24), 4500. https://doi.org/10.3390/molecules24244500