Glycidyl Azide Polymer and its Derivatives-Versatile Binders for Explosives and Pyrotechnics: Tutorial Review of Recent Progress
Abstract
1. Introduction
2. Synthesis, Modification and Properties of GAP
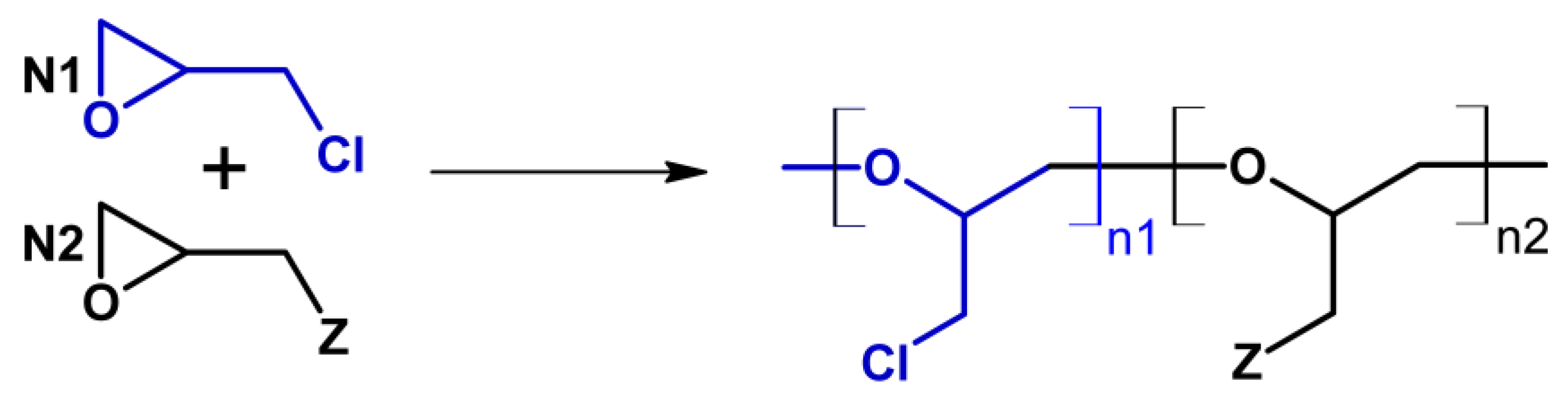
2.1. Recent Trends in the Synthesis of Glycidyl Azide Polymers
2.2. Modification of GAP through Curing
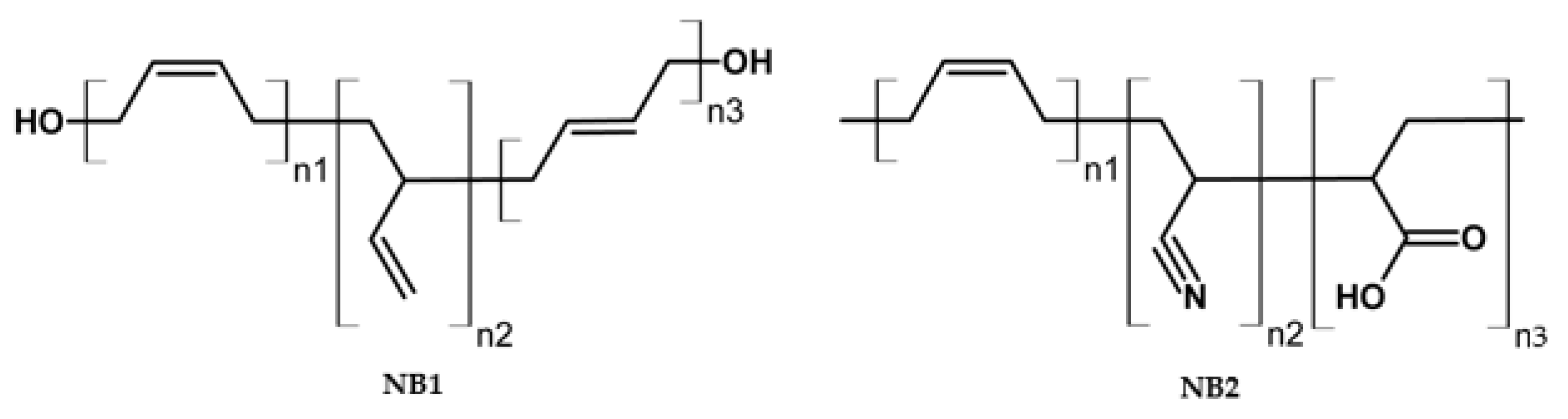
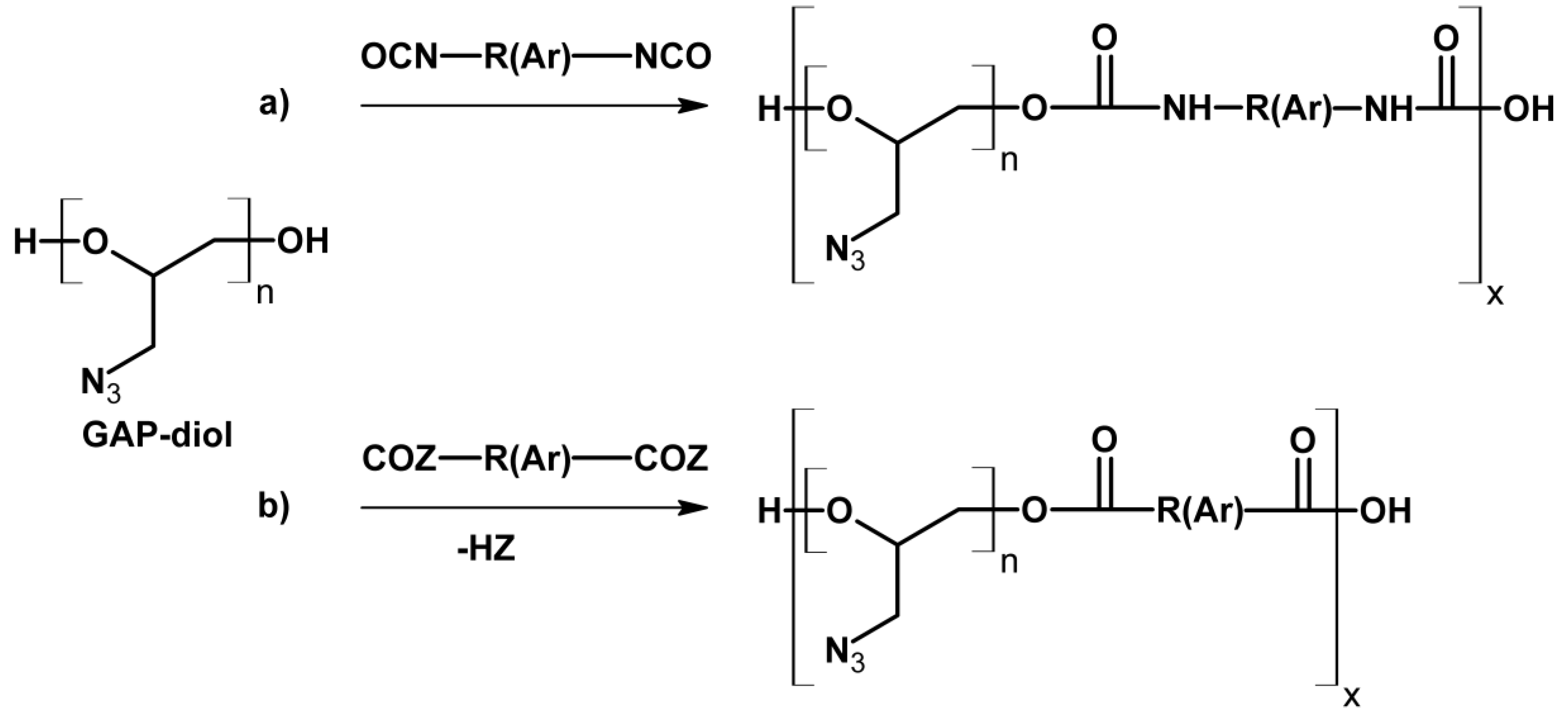
2.3. Development of GAP Derivatives and Plasticizers
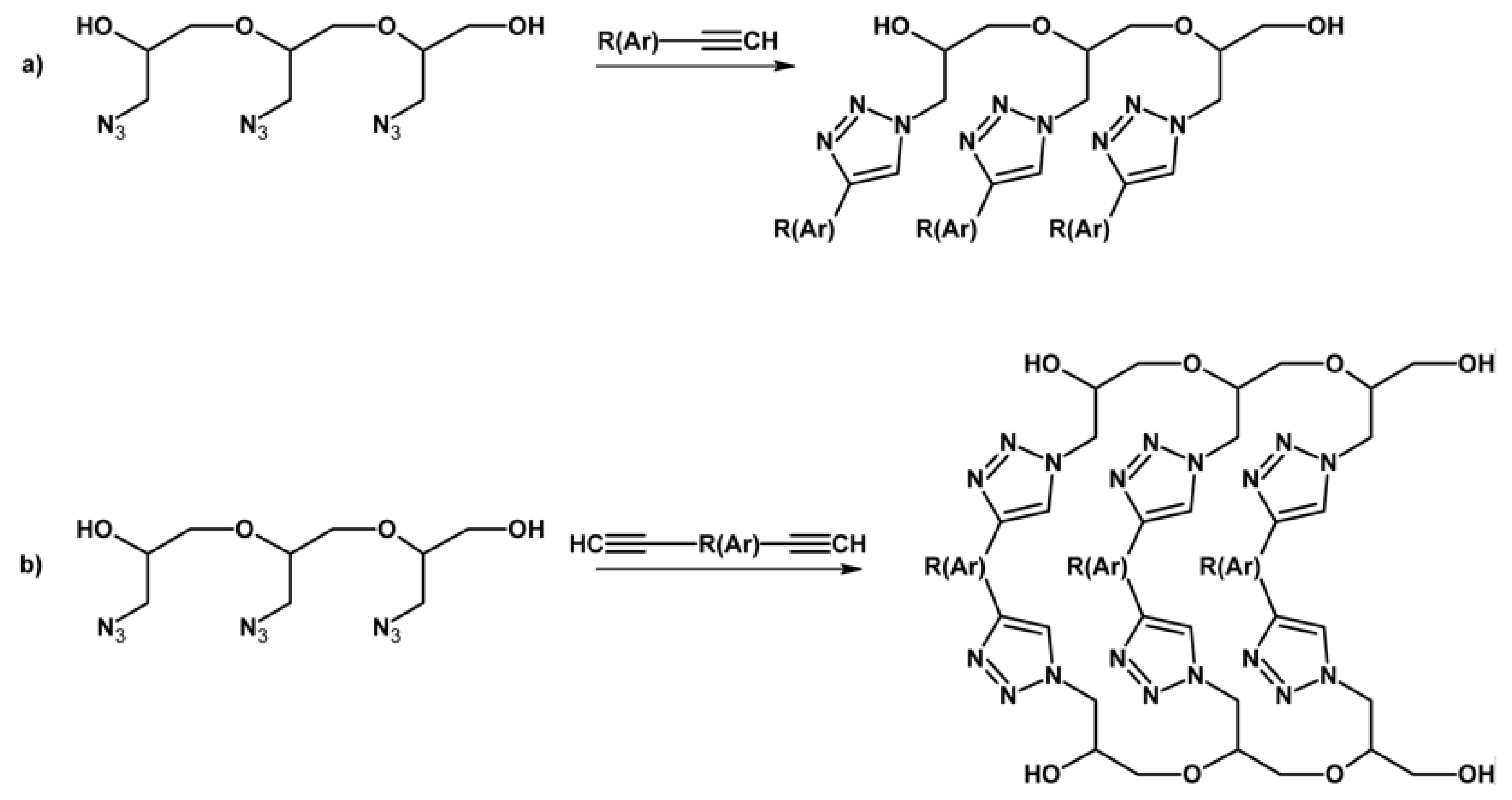
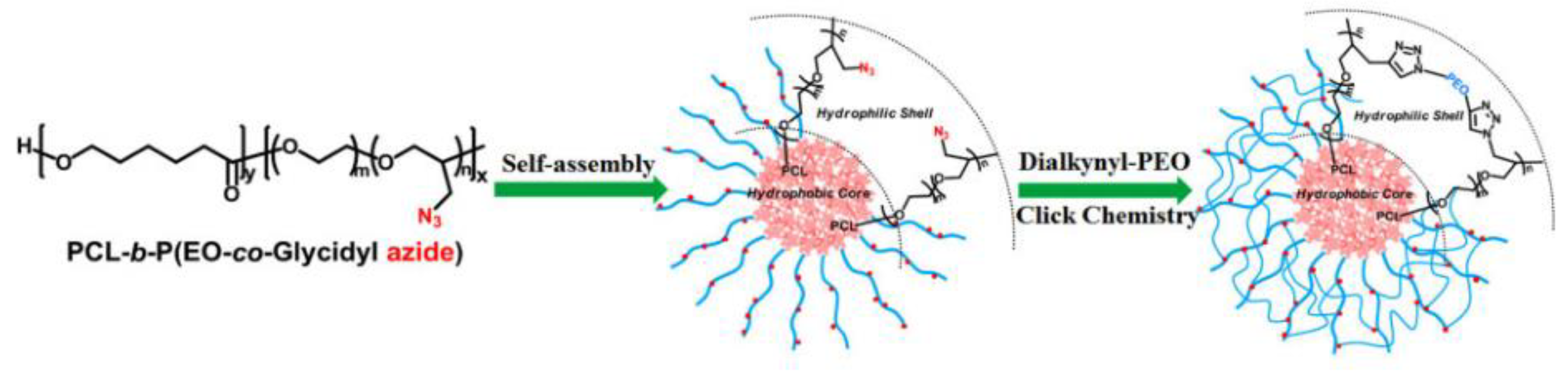
2.4. Chemical Transformation of the Azide Groups of GAP
2.5. Properties of GAP, Cured GAP and its Derivatives
3. Applications as a Component of Solid Propellants
3.1. Double-Base Propellants
3.2. Composite Propellants
3.3. Special Propulsion Applications
4. Applications as an Energetic Binder for Polymer-Bonded Explosives
5. Other Applications and Modifications of GAP
6. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, N.S.; Fleming, R.W.; Derr, R.L. Role of binders in solid propellant combustion. AIAA J. 1974, 12, 212–218. [Google Scholar] [CrossRef]
- Mark Hoffman, D.; Caley, L.E. Polymer blends as high explosive binders. Polym. Eng. Sci. 1986, 26, 1489–1499. [Google Scholar] [CrossRef]
- Shankar, R.M.; Roy, T.K.; Jana, T. Terminal functionalized hydroxyl-terminated polybutadiene: An energetic binder for propellant. J. Appl. Polym. Sci. 2009, 114, 732–741. [Google Scholar] [CrossRef]
- Sell, T.; Vyazovkin, S.; Wight, C.A.; Abbreviations, L.O.F. Thermal decomposition kinetics of PBAN-binder and composite solid rocket propellants. Combust. Flame 1999, 181, 174–181. [Google Scholar] [CrossRef]
- Colclough, M.E.; Desai, H.; Millar, R.W.; Paul, N.C.; Stewart, M.J.; Golding, P. Energetic polymers as binders in composite propellants and explosives. Polym. Adv. Technol. 1994, 5, 554–560. [Google Scholar] [CrossRef]
- Stacer, R.G.; Husband, D.M. Molecular structure of the ideal solid propellant binder. Propellants Explos. Pyrotech. 1991, 16, 167–176. [Google Scholar] [CrossRef]
- Mach I Product Bulletin for “GAP-5527 Energetic Binder”. Available online: http://www.machichemicals.com/pdf/3M_GAP-5527.pdf (accessed on 28 October 2019).
- Frankel, M.B.; Grant, L.R.; Flanagan, J.E. Historical development of glycidyl azide polymer. J. Propuls. Power 1992, 8, 560–563. [Google Scholar] [CrossRef]
- Eroglu, M.S.; Bostan, M.S. GAP pre-polymer, as an energetic binder and high performance additive for propellants and explosives: A review. Org. Commun. 2017, 10, 135–143. [Google Scholar] [CrossRef]
- Ikeda, T. Glycidyl Triazolyl Polymers: Poly (ethylene glycol) Derivatives Functionalized by Azide–Alkyne Cycloaddition Reaction. Macromol. Rapid Commun. 2018, 39, 1700825. [Google Scholar] [CrossRef]
- Mura, C.; Fruci, S.; Lamia, P.; Cappello, M.; Filippi, S.; Polacco, G. Synthesis of GAP and PAMMO Homopolymers from Mesylate Polymeric Precursors. J. Energy Mater. 2016, 34, 216–233. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Masuhara, E.; Iwakura, Y. Some Reactions of the Glycidyl Esters of Sulfonic Acids. Bull. Chem. Soc. Jpn. 1966, 39, 413–417. [Google Scholar] [CrossRef]
- Kazemi, F.; Massah, A.R.; Javaherian, M. Chemoselective and scalable preparation of alkyl tosylates under solvent-free conditions. Tetrahedron 2007, 63, 5083–5087. [Google Scholar] [CrossRef]
- Xu, X.; Liu, M.; Yin, Y.; Zheng, C.; Deng, P.; Xue, D. Synthesis of glycidyl azide polymers (GAPs) via binary ionic liquid-water mixtures without catalysts. Green Chem. 2016, 18, 1364–1367. [Google Scholar] [CrossRef]
- Grinevich, T.V.; Solov’Yanov, A.A.; Vinogradov, D.B.; Bulatov, P.V.; Kuznetsov, G.P.; Assovskii, I.G.; Berlin, A.A.; Tartakovskii, V.A. Oligo (glycidyl azides): New approaches to synthesis and properties. Dokl. Chem. 2014, 454, 39–41. [Google Scholar] [CrossRef]
- Soman, R.R.; Athar, J.; Agawane, N.T.; Shee, S.; Gore, G.M.; Sikder, A.K. Synthesis, characterization and rheology of tetrafunctional glycidyl azide polymer vis-à-vis difunctional GAP. Polym. Bull. 2016, 73, 449–461. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, C.; Guo, X.; Che, Y.; Huang, J. Structure and mechanical properties of novel composites based on glycidyl azide polymer and propargyl-terminated polybutadiene as potential binder of solid propellant. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Hu, C.; Guo, X.; Jing, Y.; Chen, J.; Zhang, C.; Huang, J. Structure and mechanical properties of crosslinked glycidyl azide polymers via click chemistry as potential binder of solid propellant. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Sonawane, S.; Anniyappan, M.; Athar, J.; Singh, A.; Talawar, M.B.; Sinha, R.K.; Banerjee, S.; Sikder, A.K. Isocyanate-Free Curing of Glycidyl Azide Polymer with Bis–propargylhydroquinone. Propellants Explos. Pyrotech. 2017, 42, 386–393. [Google Scholar] [CrossRef]
- Sonawane, S.H.; Anniyappan, M.; Athar, J.; Banerjee, S.; Sikder, A.K. Synthesis of bis (propargyl) aromatic esters and ethers: A potential replacement for isocyanate based curators. RSC Adv. 2016, 6, 8495–8502. [Google Scholar] [CrossRef]
- Sonawane, S.H.; Banerjee, S.; Sider, A.K.; Talawar, M.B.; Khan, M.A.S. Understanding the reactivity of bis (propargyl) aromatic esters towards GAP: A theoretical exploration. New J. Chem. 2017, 41, 7886–7892. [Google Scholar] [CrossRef]
- Li, H.; Zhao, F.; Yu, Q.; Wang, B.; Lu, X. A comparison of triazole cross-linked polymers based on poly-AMMO and GAP: Mechanical properties and curing kinetics. J. Appl. Polym. Sci. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Tanver, A.; Huang, M.H.; Luo, Y.; Khalid, S.; Hussain, T. Energetic interpenetrating polymer network based on orthogonal azido-alkyne click and polyurethane for potential solid propellant. RSC Adv. 2015, 5, 64478–64485. [Google Scholar] [CrossRef]
- Tanver, A.; Huang, M.H.; Luo, Y. Energetic interpenetrating polymer network (EIPN): Enhanced thermo-mechanical properties of NCO-fMWCNTs/HTPB PU and alkyne-fMWCNTs/acyl-GAP based nanocomposite and its propellants. RSC Adv. 2016, 6, 49101–49112. [Google Scholar] [CrossRef]
- Tanver, A.; Rehman, F.; Wazir, A.; Khalid, S.; Ma, S.; Li, X.; Luo, Y.; Huang, M.H. Energetic hybrid polymer network (EHPN) through facile sequential polyurethane curation based on the reactivity differences between glycidyl azide polymer and hydroxyl terminated polybutadiene. RSC Adv. 2016, 6, 11032–11039. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Guo, X.; Tang, G.; Gan, L.; Qi, C.; Huang, J. Effects of crosslinking degree and carbon nanotubes as filler on composites based on glycidyl azide polymer and propargyl-terminated polyether for potential solid propellant application. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Min, B.S.; Kim, C.K.; Ryoo, M.S.; Kim, S.Y. Azide-bearing polymer-based solid composite propellant prepared by a dual curing system consisting of a urethane-forming reaction and a dipolar addition reaction. Fuel 2014, 136, 165–171. [Google Scholar] [CrossRef]
- Hagen, T.H.; Jensen, T.L.; Unneberg, E.; Stenstrøm, Y.H.; Kristensen, T.E. Curing of Glycidyl Azide Polymer (GAP) diol using isocyanate, isocyanate-free, synchronous dual, and sequential dual curing systems. Propellants Explos. Pyrotech. 2015, 40, 275–284. [Google Scholar] [CrossRef]
- Araya-Marchena, M.; St-Charles, J.C.; Dubois, C. Investigations on Non-isocyanate Based Reticulation of Glycidyl Azide Pre-polymers. Propellants Explos. Pyrotech. 2019, 44, 769–775. [Google Scholar] [CrossRef]
- Agawane, N.T.; Soman, R.R.; Wagh, R.M.; Athar, J.; Talawar, M. Optimization of curing agents for linear difunctional Glycidyl Azide Polymer (GAP), with and without isocyanate, for binder applications. Cent. Eur. J. Energy Mater. 2018, 15, 206–222. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Peterson, Z.W.; Eilers, S.D. Analytical and experimental comparisons of HTPB and ABS as hybrid rocket fuels. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 Agust 2011. [Google Scholar] [CrossRef]
- Walters, R.N.; Hackett, S.M.; Lyon, R.E. Heats of combustion of high temperature polymers. Fire Mater. 2000, 24, 245–252. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Ge, Z. Properties and application of a novel type of Glycidyl Azide Polymer (GAP)-modified nitrocellulose powders. Propellants Explos. Pyrotech. 2015, 40, 67–73. [Google Scholar] [CrossRef]
- He, W.; He, L.; Ma, Z.; Guo, Y. Using rheometry to study the curing kinetics of glycidyl azide polymer spherical propellant by non-isothermal method. Rheol. Acta 2016, 55, 365–371. [Google Scholar] [CrossRef]
- He, W.; He, L.; Ma, Z.; Guo, Y. The kinetic and viscosity analysis of glycidyl azide polymer spherical propellant. J. Therm. Anal. Calorim. 2016, 124, 943–950. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Li, G.; Luo, Y. Effect of Bonding Agent on the Mechanical Properties of GAP High-Energy Propellant. Propellants Explos. Pyrotech. 2017, 42, 394–400. [Google Scholar] [CrossRef]
- Ma, S.; Li, Y.; Li, Y.; Li, G.; Luo, Y. Research on the mechanical properties and curing networks of energetic GAP/TDI binders. Cent. Eur. J. Energy Mater. 2017, 14, 708–725. [Google Scholar] [CrossRef]
- Bui, V.T.; Ahad, E.; Rheaume, D.; Raymond, M.P. Energetic polyurethanes from branched glycidyl azide polymer and copolymer. J. Appl. Polym. Sci. 1996, 62, 27–32. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Raju, M.P.; Raju, K.M. Synthesis and Characterization of GAP-PEG Copolymers. Int. J. Polym. Mater. 2005, 54, 651–666. [Google Scholar] [CrossRef]
- Kshirsagar, A.D.; Mahulikar, P.P. Microwave-assisted synthesis of poly (glycidyl azide-co-tetrahydrofuran). Polym. Bull. 2017, 74, 1727–1742. [Google Scholar] [CrossRef]
- Dong, Q.; Li, Y.; Wu, F.; Li, H.; Liu, X.; Huang, C. Synthesis, Characterization and Thermal Properties of Poly (glycidyl azide-r-3-azidotetrahydrofuran) as Azido Binder for Solid Rocket Propellants. Propellants Explos. Pyrotech. 2017, 42, 1143–1148. [Google Scholar] [CrossRef]
- Qi, C.; Tang, G.; Guo, X.; Liu, C.; Pang, A.M.; Gan, L.; Huang, J. Network regulation and properties optimization of glycidyl azide polymer-based materials as a candidate of solid propellant binder via alternating the functionality of propargyl-terminated polyether. J. Appl. Polym. Sci. 2019, 136, 48016. [Google Scholar] [CrossRef]
- Hafner, S.; Keicher, T.; Klapötke, T.M. Copolymers based on GAP and 1, 2-Epoxyhexane as Promising Prepolymers for Energetic Binder Systems. Propellants Explos. Pyrotech. 2018, 43, 126–135. [Google Scholar] [CrossRef]
- Kim, H.; Jang, Y.; Noh, S.; Jeong, J.; Kim, D.; Kang, B.; Kang, T.; Choi, H.; Rhee, H. Ecofriendly synthesis and characterization of carboxylated GAP copolymers. RSC Adv. 2018, 8, 20032–20038. [Google Scholar] [CrossRef]
- Filippi, S.; Mori, L.; Cappello, M.; Polacco, G. Glycidyl Azide-Butadiene Block Copolymers: Synthesis from the Homopolymers and a Chain Extender. Propellants Explos. Pyrotech. 2017, 42, 826–835. [Google Scholar] [CrossRef]
- Cappello, M.; Filippi, S.; Mori, L.; Polacco, G. Glycidyl Azide-butadiene Block Copolymers: 2 Synthesis from a Mesylated Precursor. Propellants Explos. Pyrotech. 2017, 42, 974–981. [Google Scholar] [CrossRef]
- Xu, M.; Ge, Z.; Lu, X.; Mo, H.; Ji, Y.; Hu, H. Structure and mechanical properties of fluorine-containing glycidyl azide polymer-based energetic binders. Polym. Int. 2017, 66, 1318–1323. [Google Scholar] [CrossRef]
- Hu, X.; Fan, J.; Yue, C.Y. Ring-opening polymerization of epichlorohydrin and its copolymerization with other alkylene oxides by quaternary catalyst system. J. Appl. Polym. Sci. 2001, 80, 2446–2454. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Wang, Z.; Zhang, Y.; Ge, Z.; Luo, Y. Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions. Polym. Bull. 2015, 72, 1835–1847. [Google Scholar] [CrossRef]
- Xu, M.; Ge, Z.; Lu, X.; Mo, H.; Ji, Y.; Hu, H. Fluorinated glycidyl azide polymers as potential energetic binders. RSC Adv. 2017, 7, 47271–47278. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhang, Z.; Ge, Z.; Luo, Y. Effect of hard-segment content on rheological properties of glycidyl azide polyol-based energetic thermoplastic polyurethane elastomers. Polym. Bull. 2016, 73, 3095–3104. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhao, B.; Luo, Y. Effect of nitrocellulose (NC) on morphology, rheological and mechanical properties of glycidyl azide polymer based energetic thermoplastic elastomer/NC blends. Polym. Int. 2017, 66, 705–711. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.; Liu, G.; Li, X.; Luo, Y. Mechanical properties and thermal decomposition of PBAMO/GAP random block ETPE. J. Therm. Anal. Calorim. 2016, 126, 717–724. [Google Scholar] [CrossRef]
- Deng, J.; Li, G.; Xia, M.; Lan, Y.; Luo, Y. Improvement of mechanical characteristics of glycidyl azide polymer binder system by addition of flexible polyether. J. Appl. Polym. Sci. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ma, S.; Luo, Y. Compatibility, mechanical and thermal properties of GAP/P(EO-co-THF) blends obtained upon a urethane-curing reaction. Polym. Bull. 2017, 74, 4607–4618. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Deng, J.; Luo, Y. Study on bulk preparation and properties of glycidyl azide polymer with hydroxyl-terminated polyether elastomers obtained through step-wise curing process. Colloid Polym. Sci. 2017, 295, 637–646. [Google Scholar] [CrossRef]
- Pei, J.F.; Zhao, F.Q.; Song, X.D.; Ren, X.N.; Gao, H.X.; An, T.; An, J.; Hu, R.Z. Effects of nano-CuO particles on thermal decomposition behavior and decomposition mechanism of BAMO-GAP copolymer. J. Anal. Appl. Pyrolysis 2015, 112, 88–93. [Google Scholar] [CrossRef]
- Bellan, A.B.; Hafner, S.; Hartdegen, V.A.; Klapötke, T.M. Polyurethanes based on 2, 2-Dinitropropane-1,3-diol and 2, 2-bis (azidomethyl) propane-1, 3-diol as potential energetic binders. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Chizari, M.; Bayat, Y. Synthesis and kinetic study of a PCL-GAP-PCL tri-block copolymer. Cent. Eur. J. Energy Mater. 2018, 15, 243–257. [Google Scholar] [CrossRef]
- Hafner, S.; Hartdegen, V.A.; Hofmayer, M.S.; Klapötke, T.M. Potential Energetic Plasticizers on the Basis of 2, 2-Dinitropropane-1, 3-diol and 2, 2-Bis (azidomethyl) propane-1, 3-diol. Propellants Explos. Pyrotech. 2016, 41, 806–813. [Google Scholar] [CrossRef]
- Bodaghi, A.; Shahidzadeh, M. Synthesis and Characterization of New PGN Based Reactive Oligomeric Plasticizers for Glycidyl Azide Polymer. Propellants Explos. Pyrotech. 2018, 43, 364–370. [Google Scholar] [CrossRef]
- Baghersad, M.H.; Habibi, A.; Heydari, A. Synthesis, characterization, thermal and computational studies of novel tetra–azido compounds as energetic plasticizers. J. Mol. Struct. 2017, 1130, 447–454. [Google Scholar] [CrossRef]
- Ikeda, T.; Nagao, I.; Moriyama, S.; Kim, J.D. Synthesis and characterization of glycidyl-polymer-based poly (ionic liquid) s: Highly designable polyelectrolytes with a poly (ethylene glycol) main chain. RSC Adv. 2015, 5, 87940–87947. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.; Jafari, N.; Shahidzadeh, M.; Zekri, N. Post Modification of Poly Glycidyl Azide with Ionic-Liquid-Based Reactive Plasticizer through Catalyst-Free Click Reaction. ChemistrySelect 2018, 3, 6617–6621. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.; Jafari, N.; Shahidzadeh, M.; Zekri, N. Reactive Plasticizers Covalently Linked to Glycidyl Azide Polymer via Catalyst-Free Huisgen Azide-Alkyne Cycloaddition. Propellants Explos. Pyrotech. 2018, 43, 893–897. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Zhang, W.; Xu, H.; Xie, W.; Du, J.; Liu, Y. Simulation and Experimental on the Solvation Interaction between the GAP Matrix and Insensitive Energetic Plasticizers in Solid Propellants. J. Phys. Chem. A 2016, 120, 765–770. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Wang, G. A promising azido nitrate ester plasticizer for propellant. Comput. Mater. Sci. 2015, 110, 71–76. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Agawane, N.T.; Talawar, M.B.; Khan, M.A.S. Examining the compatibility of energetic plasticizer DNDA-5 with energetic binders. J. Macromol. Sci. Part A 2019, 57, 46–54. [Google Scholar] [CrossRef]
- Ou, Y.; Zhao, Q.; Zhang, W.; Zhang, B.; Yan, S.; Jiao, Q. Fabrication of glycidyl azide polymer–hydroxyl terminated polyether semi-interpenetrating network via synchronous dual curing system. Mater. Lett. 2019, 237, 152–155. [Google Scholar] [CrossRef]
- Ma, M.; Shen, Y.; Kwon, Y.; Chung, C.; Kim, J.S. Reactive Energetic Plasticizers for Energetic Polyurethane Binders Prepared via Simultaneous Huisgen Azide-Alkyne Cycloaddition and Polyurethane Reaction. Propellants Explos. Pyrotech. 2016, 41, 746–756. [Google Scholar] [CrossRef]
- Betzler, F.M.; Hartdegen, V.A.; Klapötke, T.M.; Sproll, S.M. A new energetic binder: Glycidyl nitramine polymer. Cent. Eur. J. Energy Mater. 2016, 13, 289–300. [Google Scholar] [CrossRef]
- Shafeeuulla Khan, M.A.; Dey, A.; Athar, J.; Sikder, A.K. Calculation of enthalpies of formation and band gaps of polymeric binders. RSC Adv. 2014, 4, 32840–32846. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, B.; Li, X.; Luo, Y. A constitutive model for understanding the mechanical response of energetic polymers to the strain rate and temperature. Soft Mater. 2017, 15, 13–26. [Google Scholar] [CrossRef]
- Pei, J.F.; Zhao, F.Q.; Lu, H.L.; Song, X.D.; Zhou, R.; Yuan, Z.F.; Zhang, J.; Chen, J.B. Compatibility study of BAMO–GAP copolymer with some energetic materials. J. Therm. Anal. Calorim. 2016, 124, 1301–1307. [Google Scholar] [CrossRef]
- He, Y.; Liang, Y.; Wang, D. The highly sensitive and facile colorimetric detection of the glycidyl azide polymer based on propargylamine functionalized gold nanoparticles using click chemistry. Chem. Commun. 2015, 51, 12092–12094. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, H.; Zhu, N.; Lin, Y.; Yu, P.; Mao, L. A simple assay for direct colorimetric visualization of trinitrotoluene at picomolar levels using gold nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 8601–8604. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Mukherji, S. Gold nanoparticle coated U-bend fibre optic probe for localized surface plasmon resonance based detection of explosive vapours. Sens. Actuators B 2014, 192, 804–811. [Google Scholar] [CrossRef]
- Üzer, A.; Can, Z.; Akin, I.; Erçaǧ, E.; Apak, R. 4-Aminothiophenol Functionalized Gold Nanoparticle-Based Colorimetric Sensor for the Determination of Nitramine Energetic Materials. Anal. Chem. 2014, 86, 351–356. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Shu, Y.J.; Liu, N.; Shu, Y.; Wang, K.; Wu, Z.K.; Wang, X.C.; Ding, X.Y. Theoretical simulations on the glass transition temperatures and mechanical properties of modified glycidyl azide polymer. Comput. Mater. Sci. 2017, 139, 132–139. [Google Scholar] [CrossRef]
- Xu, H.X.; Zhou, W.J.; Zhao, F.Q.; Pang, W.Q.; Sun, Z.H.; Xu, S.Y.; Wang, G.Q. Rheological and interfacial property of nano-Al with HTPB, GAP and PET. Adv. Mater. Res. 2014, 924, 170–175. [Google Scholar] [CrossRef]
- Han, X.; Wang, T.F.; Lin, Z.K.; Han, D.L.; Li, S.F.; Zhao, F.Q.; Zhang, L.Y. RDX/AP-CMDB propellants containing fullerenes and carbon black additives. Def. Sci. J. 2009, 59, 284–293. [Google Scholar] [CrossRef]
- Jin, B.; Peng, R.; Zhao, F.; Yi, J.; Xu, S.; Wang, S.; Chu, S. Combustion effects of nitrofulleropyrrolidine on RDXCMDB propellants. Propellants Explos. Pyrotech. 2014, 39, 874–880. [Google Scholar] [CrossRef]
- Huang, T.; Jin, B.; Peng, R.F.; Chen, C.D.; Zheng, R.Z.; He, Y.; Chu, S.J. Synthesis and characterization of [60]fullerene-glycidyl azide polymer and its thermal decomposition. Polymers 2015, 7, 896–908. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, T.; Ge, Z.; Luo, Y. Fabrication and thermal decomposition of glycidyl azide polymer modified nitrocellulose double base propellants. Sci. China Chem. 2016, 59, 472–477. [Google Scholar] [CrossRef]
- Wu, Y.; Ge, Z.; Luo, Y. Properties and application of a novel type of glycidyl azide polymer modified double-base spherical powders. J. Therm. Anal. Calorim. 2016, 124, 107–115. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, Z.; Luo, Y.; Ge, Z.; Du, F.; Chen, S.; Sun, J. Fabrication and properties of glycidyl azide polymer-modified nitrocellulose spherical powders. J. Therm. Anal. Calorim. 2017, 129, 1555–1562. [Google Scholar] [CrossRef]
- Jensen, T.L.; Moxnes, J.F.; Kjønstad, E.F.; Unneberg, E. A study of the detonation properties, propellant impulses, impact sensitivities and synthesis of nitrated ANTA and NTO derivatives. Cent. Eur. J. Energy Mater. 2016, 13, 445–467. [Google Scholar] [CrossRef]
- Fischer, N.; Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Pushing the limits of energetic materials—The synthesis and characterization of dihydroxylammonium 5, 5′-bistetrazole-1, 1′-diolate. J. Mater. Chem. 2012, 22, 20418–20422. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, W.; Qi, X.; Liu, Y.; Tang, Q.; Song, K.; Zhang, W. Comparison of the interfacial bonding interaction between GAP matrix and ionic/non-ionic explosive: Computation simulation and experimental study. Appl. Surf. Sci. 2019, 497, 143813. [Google Scholar] [CrossRef]
- Yu, C.; Yang, L.; Chen, H.; Qin, Y.; Wang, T.; Sun, W.; Wang, C. Microscale investigations of mechanical responses of TKX-50 based polymer bonded explosives using MD simulations. Comput. Mater. Sci. 2020, 172, 109287. [Google Scholar] [CrossRef]
- Zygmunt, A.; Cieślak, K.; Gołofit, T. Magnez-Istotny składnik mieszanin wysokoenergetycznych. J. Elem. 2014, 19, 617–626. [Google Scholar] [CrossRef]
- An, C.; Li, F.; Wang, J.; Guo, X. Surface coating of nitroamine explosives and its effects on the performance of composite modified double-base propellants. J. Propuls. Power 2012, 28, 444–448. [Google Scholar] [CrossRef]
- Chen, T.; Gou, B.; Hao, G.Z.; Gao, H.; Xiao, L.; Ke, X.; Guo, S.; Jiang, W. Preparation, characterization of RDX/GAP nanocomposites, and study on the thermal decomposition behavior. J. Energy Mater. 2019, 37, 80–89. [Google Scholar] [CrossRef]
- Ye, B.; An, C.; Wang, J.; Li, H.; Ji, W.; Gao, K. Preparation and characterization of RDX-based composite with glycidyl azide polymers and nitrocellulose. J. Propuls. Power 2016, 32, 1035–1039. [Google Scholar] [CrossRef]
- Li, G.P.; Liu, Y.Z.; Liu, M.H.; Chai, C.P.; Luo, Y.J. Preparation and Characterization of Hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine/Ammonium Perchlorate Intermolecular Explosives. Propellants Explos. Pyrotech. 2016, 41, 641–644. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Zhang, R.; Shen, L.; Liu, Y.; Luo, Y. Synthesis and properties of RDX/GAP nano-composite energetic materials. Colloid Polym. Sci. 2015, 293, 2269–2279. [Google Scholar] [CrossRef]
- Futko, S.I.; Koznacheev, I.A.; Ermolaeva, E.M. Influence of the Structure of a Solid-Fuel Mixture on the Thermal Efficiency of the Combustion Chamber of an Engine System. J. Eng. Phys. Thermophys. 2014, 87, 1313–1321. [Google Scholar] [CrossRef]
- Jensen, T.L.; Unneberg, E.; Kristensen, T.E. Smokeless GAP-RDX Composite Rocket Propellants Containing Diaminodinitroethylene (FOX-7). Propellants Explos. Pyrotech. 2017, 42, 381–385. [Google Scholar] [CrossRef]
- Abd-Elghany, M.; Elbeih, A.; Klapötke, T.M. Thermo-analytical study of 2, 2, 2-trinitroethyl-formate as a new oxidizer and its propellant based on a GAP matrix in comparison with ammonium dinitramide. J. Anal. Appl. Pyrolysis 2018, 133, 30–38. [Google Scholar] [CrossRef]
- Palla Papavlu, A.; Urech, L.; Lippert, T.; Phipps, C.; Hermann, J.; Wokaun, A. Fs Laser-Induced Plasmas from Energetic Polymers: Towards Micro-Laser Plasma Thruster Application. Plasma Process. Polym. 2016, 13, 611–622. [Google Scholar] [CrossRef]
- Jiao, L.; Truscott, B.S.; Liu, H.; Ashfold, M.N.R.; Ma, H. Imaging spectroscopy of polymer ablation plasmas for laser propulsion applications. J. Appl. Phys. 2017, 121. [Google Scholar] [CrossRef]
- Gołofit, T.; Zys̈k, K. Thermal decomposition properties and compatibility of CL-20 with binders HTPB, PBAN, GAP and polyNIMMO. J. Therm. Anal. Calorim. 2015, 119, 1931–1939. [Google Scholar] [CrossRef]
- Nair, U.R.; Sivabalan, R.; Gore, G.M.; Geetha, M.; Asthana, S.N.; Singh, H. Hexanitrohexaazaisowurtzitane (CL-20) and CL-20-based formulations (review). Combust. Explos. Shock Waves 2005, 41, 121–132. [Google Scholar] [CrossRef]
- Huang, H.; Shi, Y.; Yang, J.; Li, B. Compatibility Study of Dihydroxylammonium 5,5′-Bistetrazole-1,1′-diolate (TKX-50) with Some Energetic Materials and Inert Materials. J. Energy Mater. 2015, 33, 66–72. [Google Scholar] [CrossRef]
- Guo, C.; Wang, D.; Gao, B.; Wang, J.; Luo, B.; Yang, G.; Nie, F. Solid-solid phase transition study of ε-CL-20/binder composites. RSC Adv. 2016, 6, 859–865. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; An, C.; Li, H.; Wen, X.; Yu, B. GAP/CL-20-Based Compound Explosive: A New Booster Formulation Used in a Small-Sized Initiation Network. J. Energy Mater. 2017, 35, 53–62. [Google Scholar] [CrossRef]
- Bayat, Y.; Soleyman, R.; Zarandi, M. Synthesis and characterization of novel 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane (2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazatetracyclo dodecane based nanopolymer-bonded explosives by microemulsion. J. Mol. Liq. 2015, 206, 190–194. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, B.; Guo, C.; Gao, B.; Wang, J.; Yang, G.; Huang, H.; Nie, F. Formulation and performance of functional sub-micro CL-20-based energetic polymer composite ink for direct-write assembly. RSC Adv. 2016, 6, 112325–112331. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Burzhava, A.V.; Sheremetev, A.B.; Aleksandrova, N.S. Thermal and combustion properties of 3, 4-bis(3-nitrofurazan-4-yl)furoxan (DNTF). Propellants Explos. Pyrotech. 2012, 37, 575–580. [Google Scholar] [CrossRef]
- An, C.; Wen, X.; Wang, J.; Wu, B. GAP/DNTF based PBX explosives: A novel formula used in small sized explosive circuits. Cent. Eur. J. Energy Mater. 2016, 13, 397–410. [Google Scholar] [CrossRef]
- Qian, W.; Shu, Y.; Ma, Q.; Li, H.; Wang, S.; Chen, X. The reinforcement of the TNT system by a newlydesigned GAP-based polyurethane-urea: A molecular simulation investigation. Cent. Eur. J. Energy Mater. 2016, 13, 411–426. [Google Scholar] [CrossRef]
- Manner, V.W.; Yeager, J.D.; Patterson, B.M.; Walters, D.J.; Stull, J.A.; Cordes, N.L.; Luscher, D.J.; Henderson, K.C.; Schmalzer, A.M.; Tappan, B.C. In situ imaging during compression of plastic bonded explosives for damage modeling. Materials 2017, 10, 638. [Google Scholar] [CrossRef]
- Hussein, A.K.; Elbeih, A.; Zeman, S. The effect of glycidyl azide polymer on the stability and explosive properties of different interesting nitramines. RSC Adv. 2018, 8, 17272–17278. [Google Scholar] [CrossRef]
- Elbeih, A.; Zeman, S.; Jungová, M.; Vávra, P. Attractive nitramines and related PBXs. Propellants Explos. Pyrotech. 2013, 38, 379–385. [Google Scholar] [CrossRef]
- Elbeih, A.; Zeman, S.; Jungova, M.; Vávra, P.; Akstein, Z. Effect of different polymeric matrices on some properties of plastic bonded explosives. Propellants Explos. Pyrotech. 2012, 37, 676–684. [Google Scholar] [CrossRef]
- Elbeih, A.; Wafy, T.Z.; Elshenawy, T. Performance and detonation characteristics of polyurethane matrix bonded attractive nitramines. Cent. Eur. J. Energy Mater. 2017, 14, 77–89. [Google Scholar] [CrossRef]
- Hussein, A.K.; Zeman, S.; Elbeih, A. Thermo-analytical study of glycidyl azide polymer and its effect on different cyclic nitramines. Thermochim. Acta 2018, 660, 110–123. [Google Scholar] [CrossRef]
- Shin, W.G.; Han, D.; Park, Y.; Hyun, H.S.; Sung, H.G.; Sohn, Y. Combustion of boron particles coated with an energetic polymer material. Korean J. Chem. Eng. 2016, 33, 3016–3020. [Google Scholar] [CrossRef]
- Song, S.; Ko, Y.G.; Lee, H.L.; Wi, D.W.; Ree, B.J.; Li, Y.L.; Michinobu, T.M.; Ree, M.R. High-performance triazole-containing brush polymers via azide-alkyne click chemistry: A new functional polymer platform for electrical memory devices. NPG Asia Mater. 2015, 7, e228. [Google Scholar] [CrossRef]
- Ikeda, T. Copper-Free Synthesis of Glycidyl Triazolyl Polymers. Macromol. Chem. Phys. 2018, 219, 1800147. [Google Scholar] [CrossRef]
- O’Connor, A.; Marsat, J.N.; Mitrugno, A.; Flahive, T.; Moran, N.; Brayden, D.; Devocelle, M. Poly (ethylene glycol)-based backbones with high peptide loading capacities. Molecules 2014, 19, 17559–17577. [Google Scholar] [CrossRef]
- Lemos, M.F.; Bohn, M.A. DMA of polyester-based polyurethane elastomers for composite rocket propellants containing different energetic plasticizers. J. Therm. Anal. Calorim. 2018, 131, 595–600. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Porter, M.M.; Brady, J.E.; Levine, R.M. Polymer packaging of I2 producing pyrotechnic biocides. J. Energy Mater. 2018, 36, 493–501. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, J.; He, G.; Huang, C.; Yang, Z.; Liu, S.; Gong, F. Enhanced water resistance and energy performance of core–shell aluminum nanoparticles via in situ grafting of energetic glycidyl azide polymer. J. Mater. Sci. 2018, 53, 12091–12102. [Google Scholar] [CrossRef]
- Liu, J.; Gan, Z. Hydrophilic block azidation of PCL-b-PEO block copolymers from epichlorohydrin. Macromol. Biosci. 2014, 14, 699–708. [Google Scholar] [CrossRef] [PubMed]
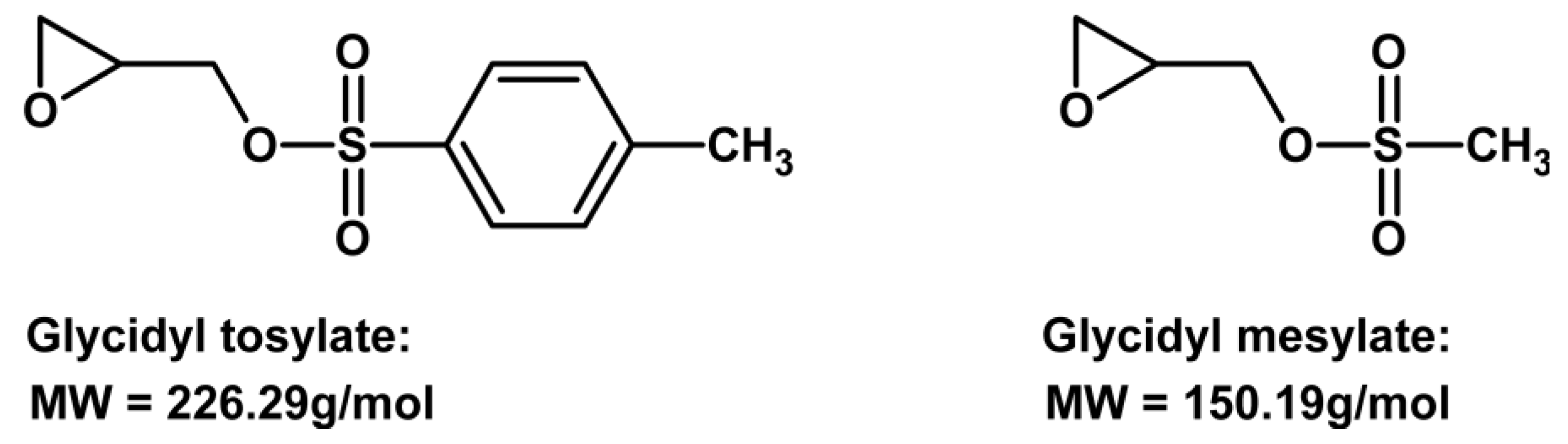
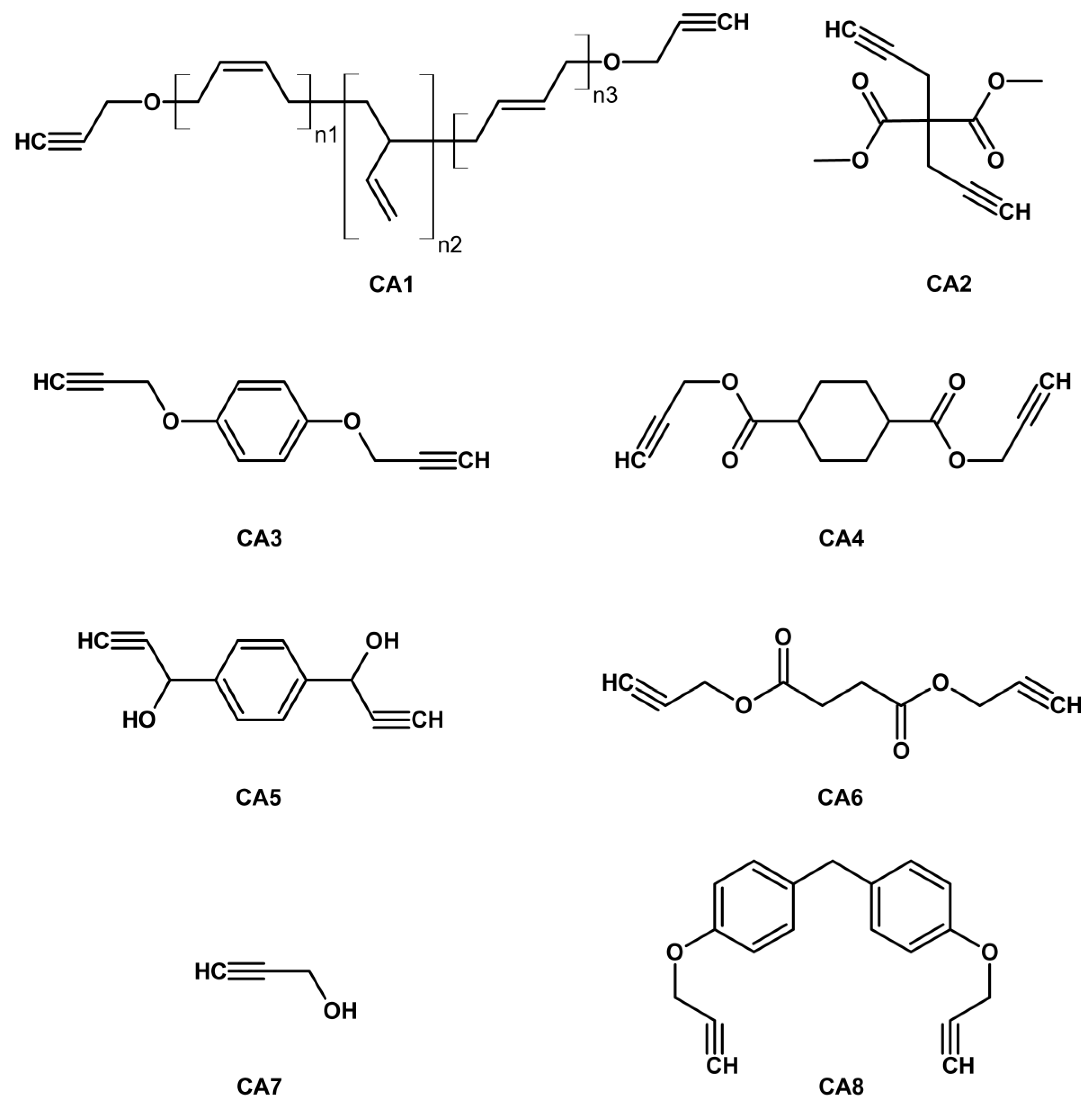
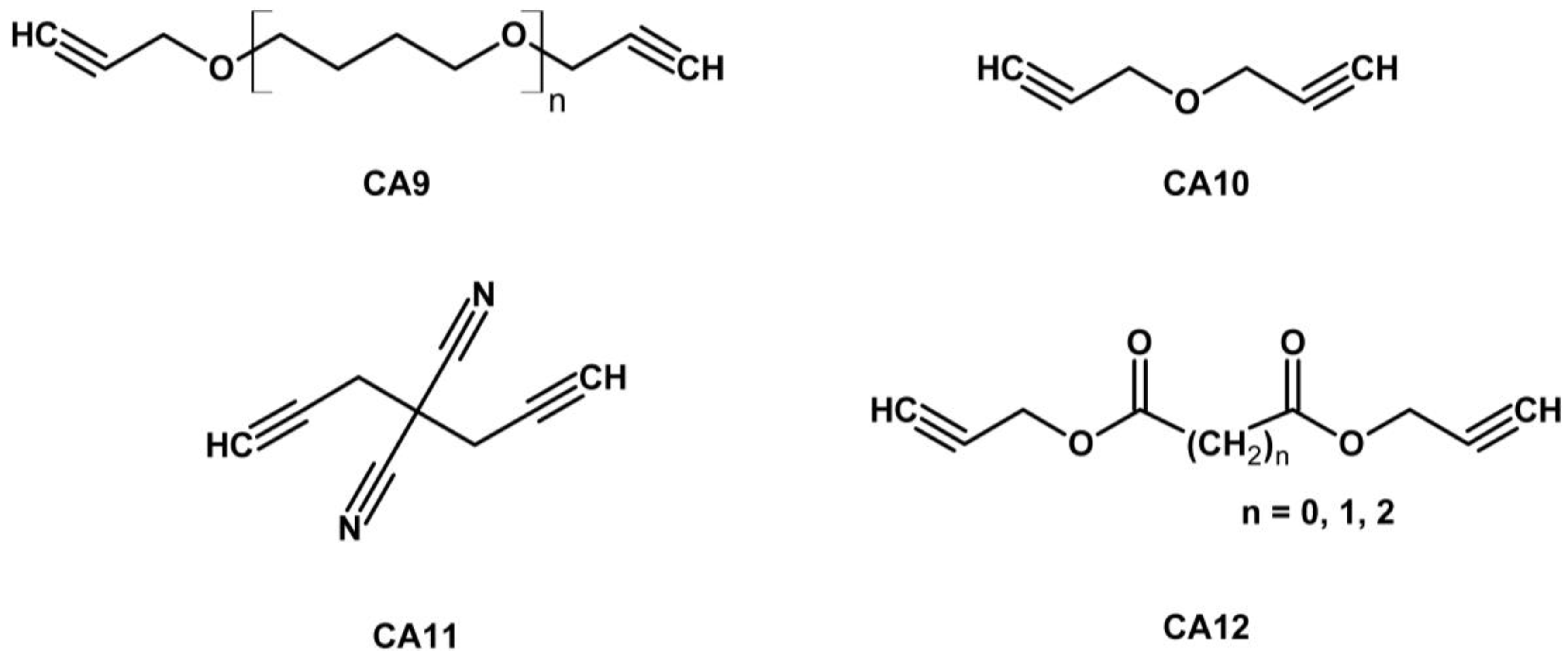
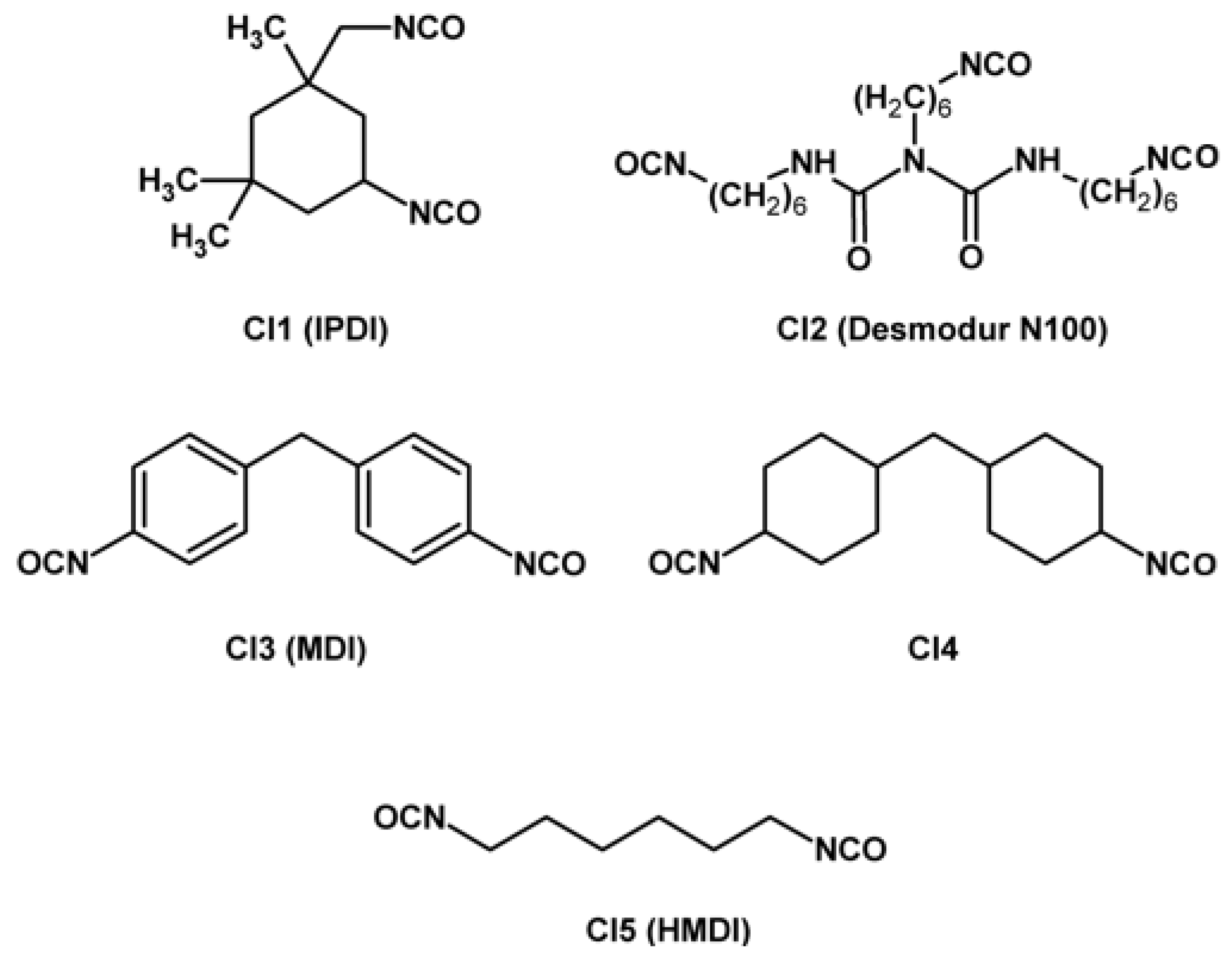
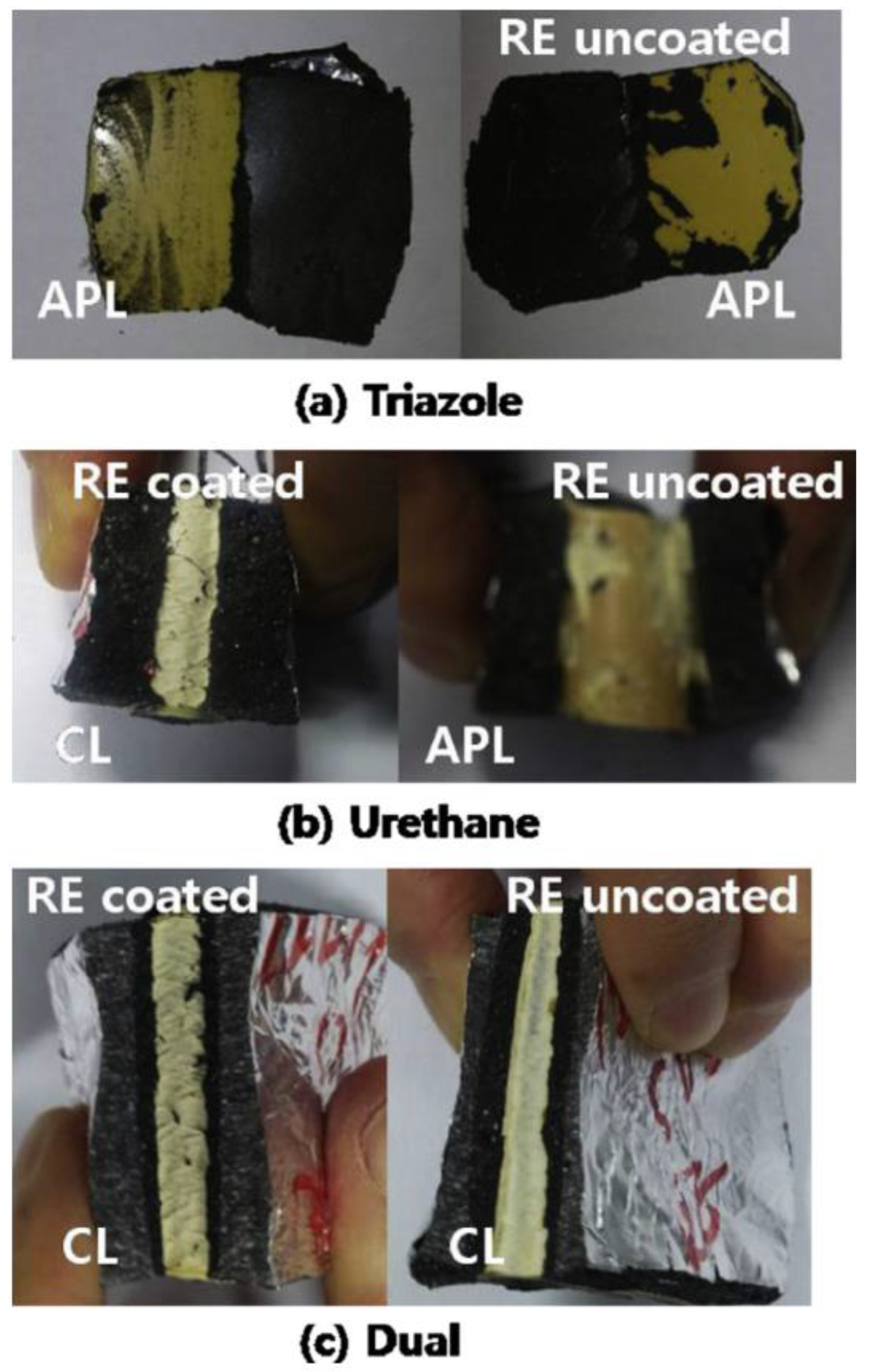
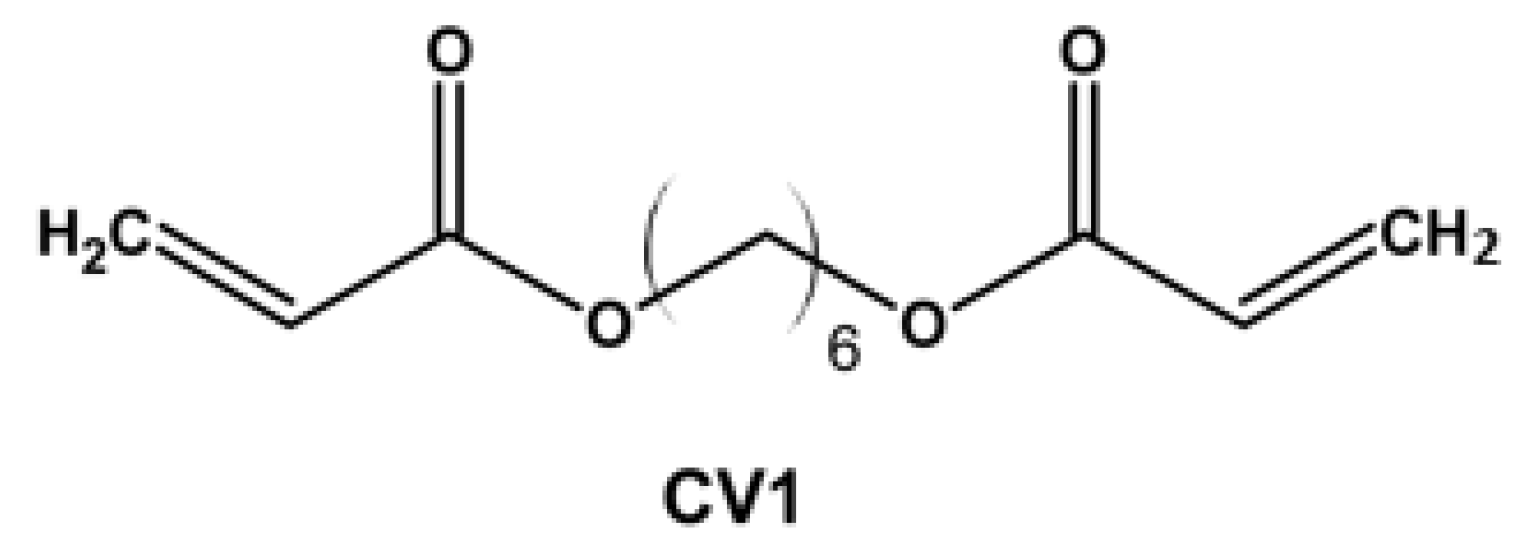
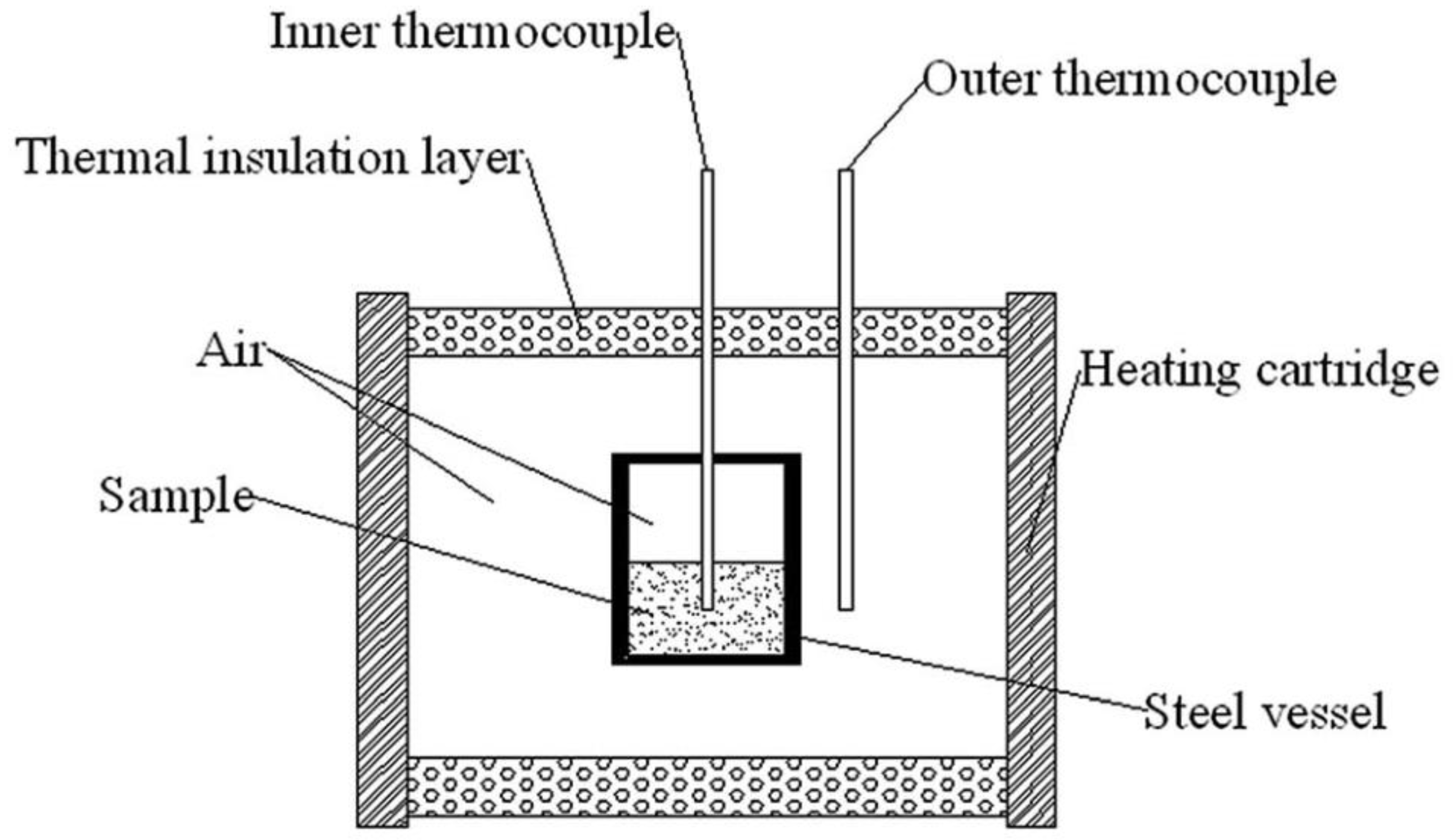
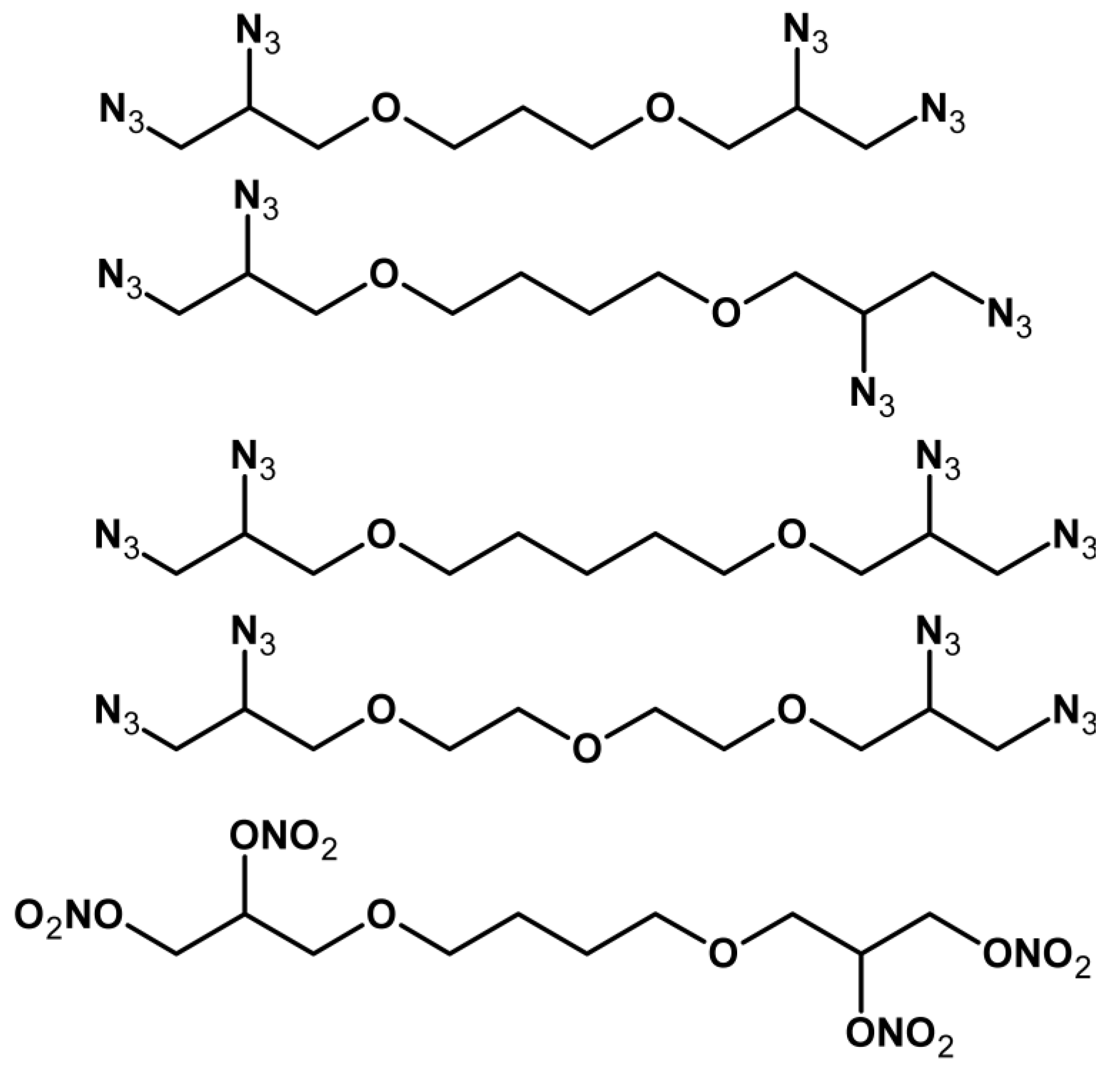
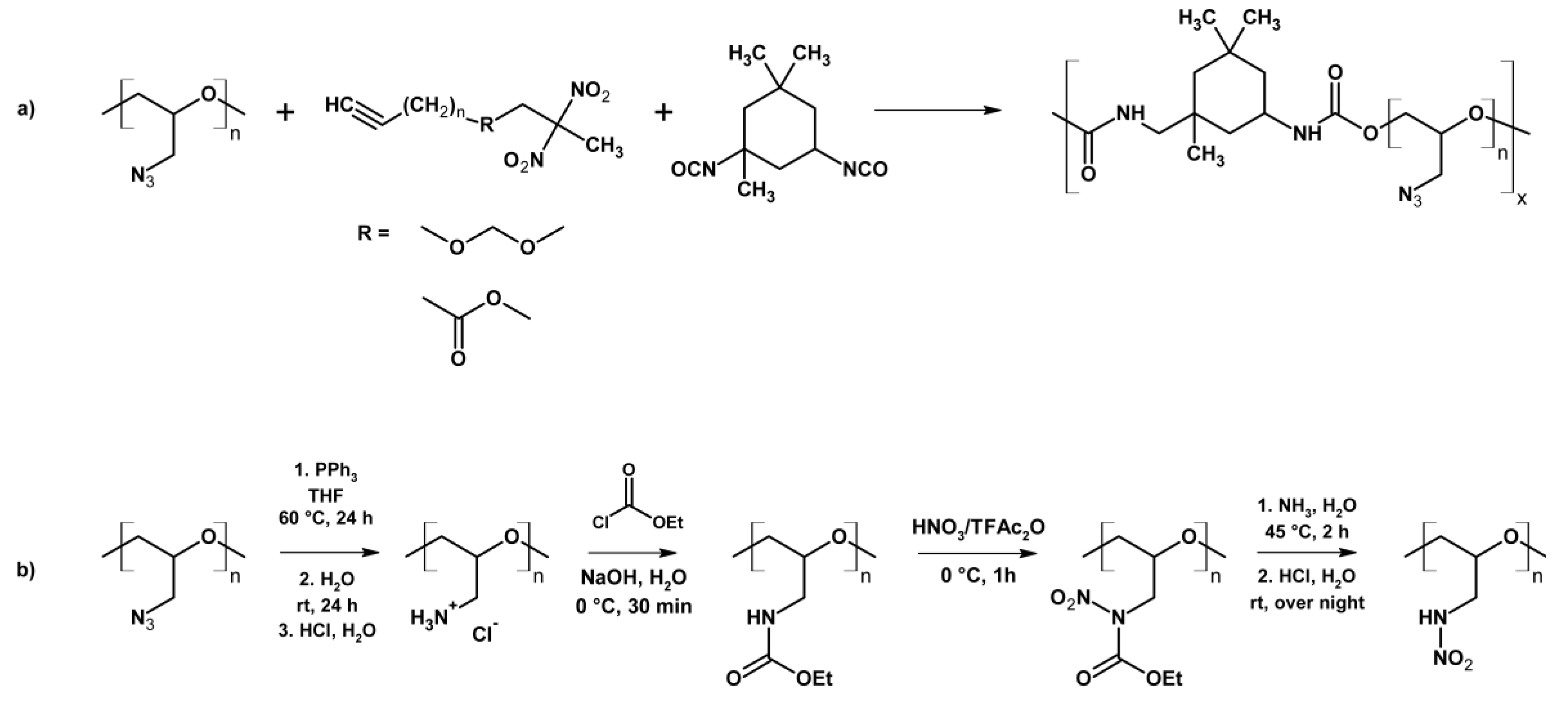
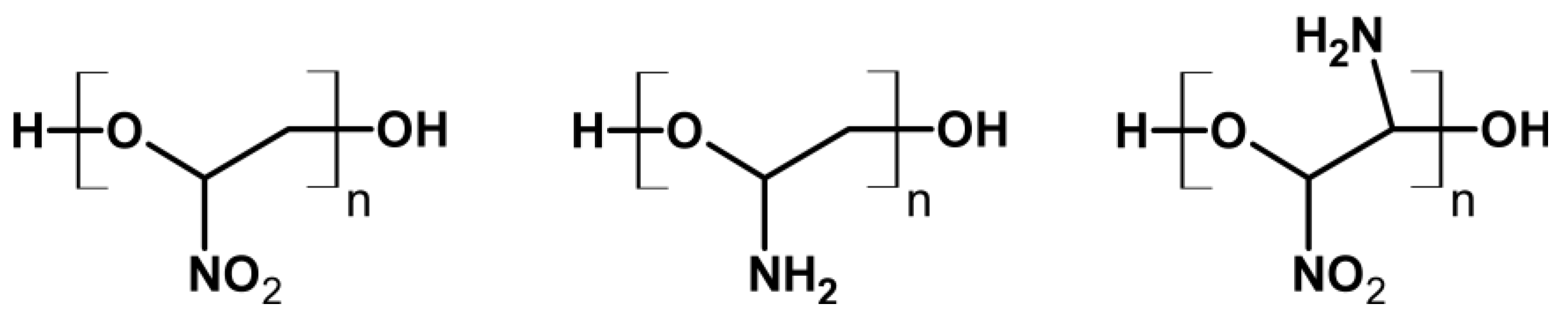
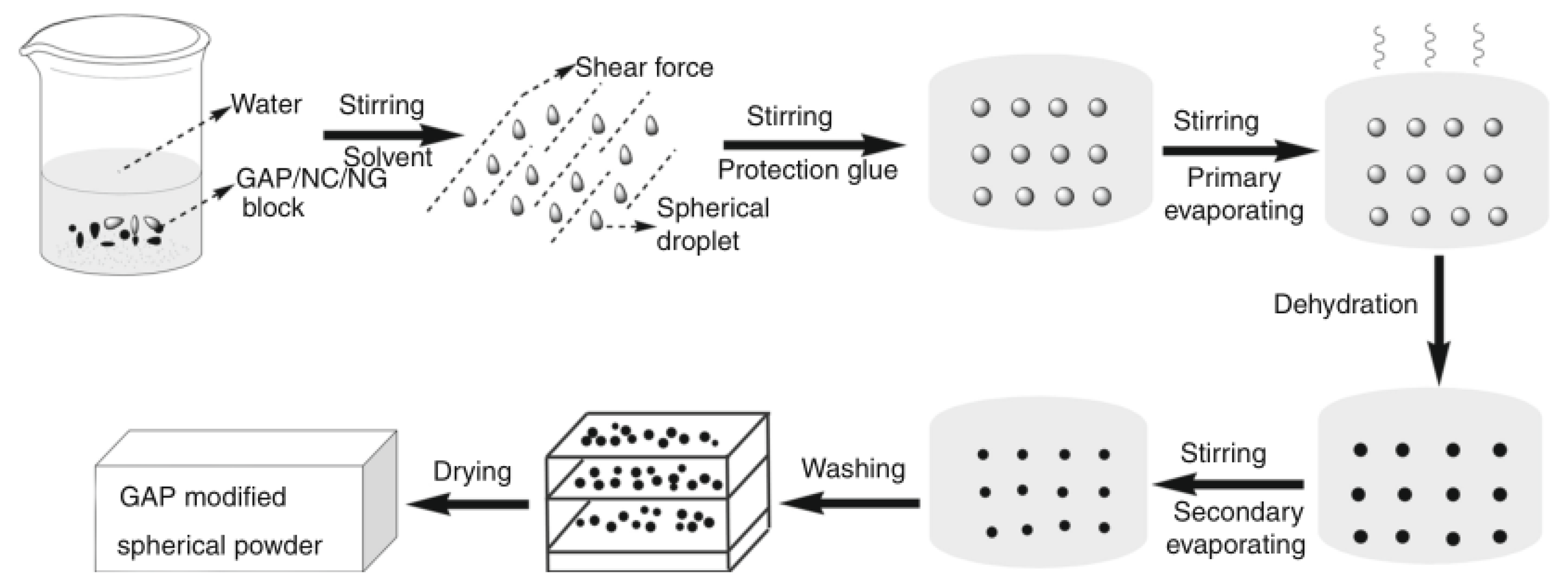
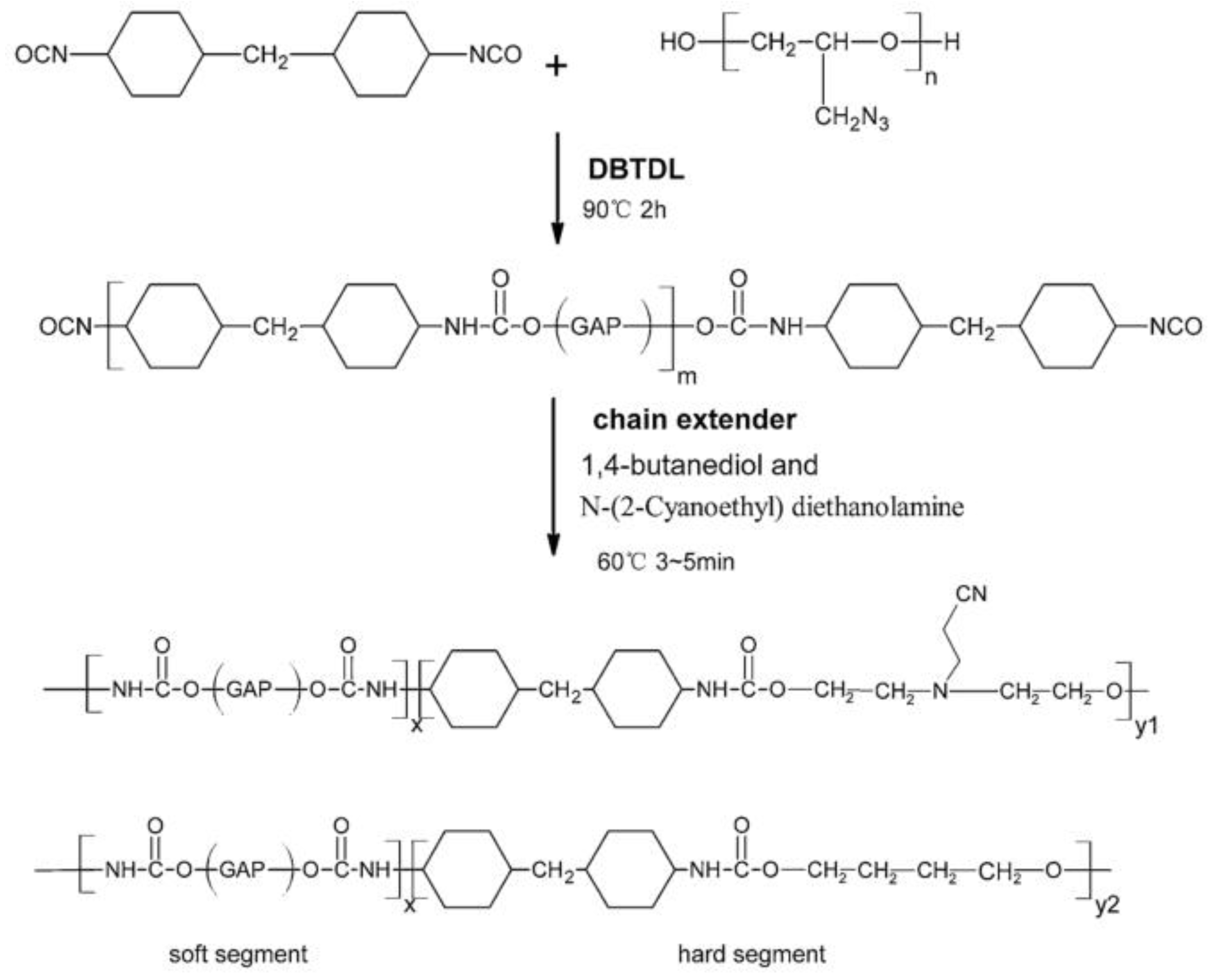
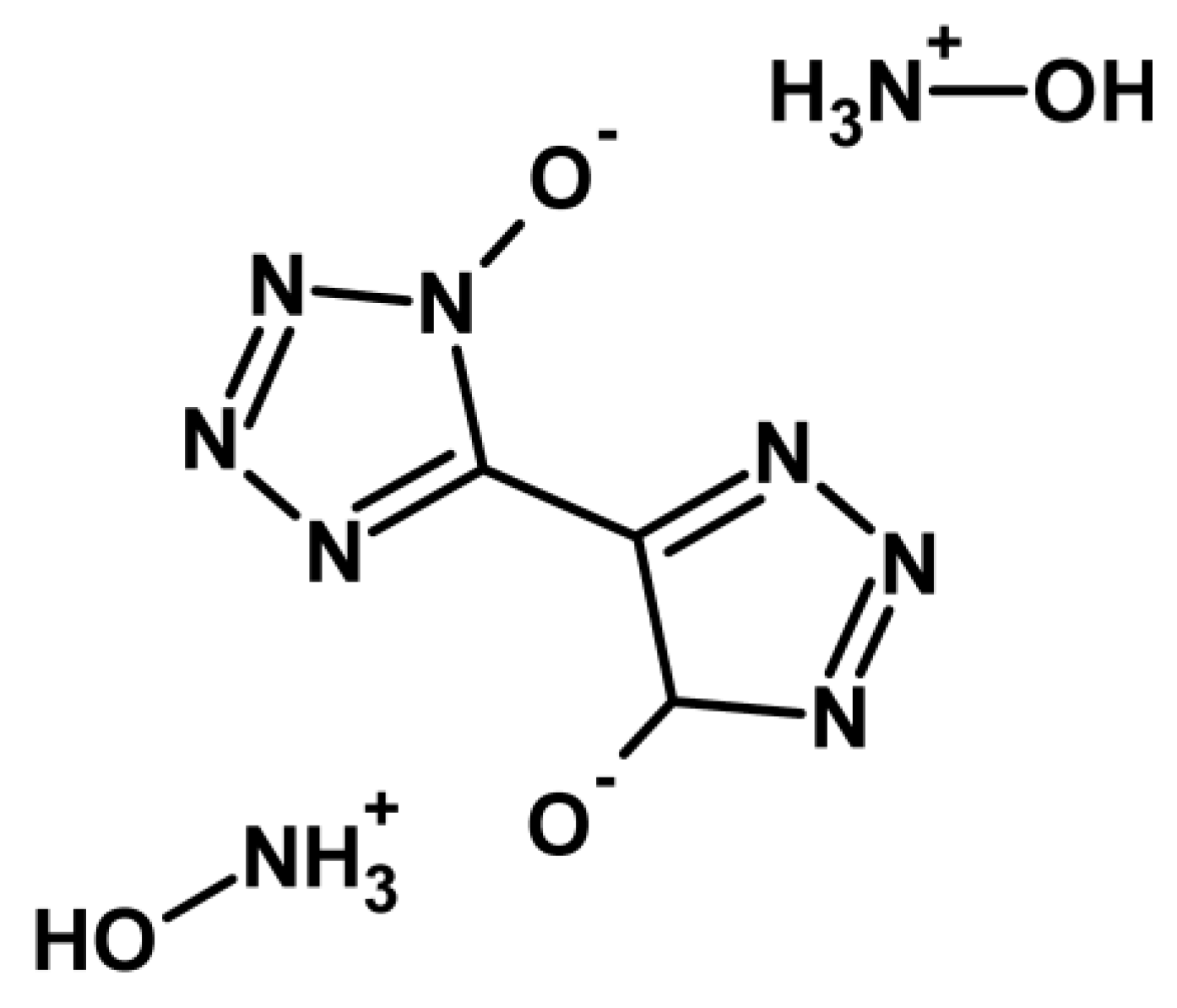
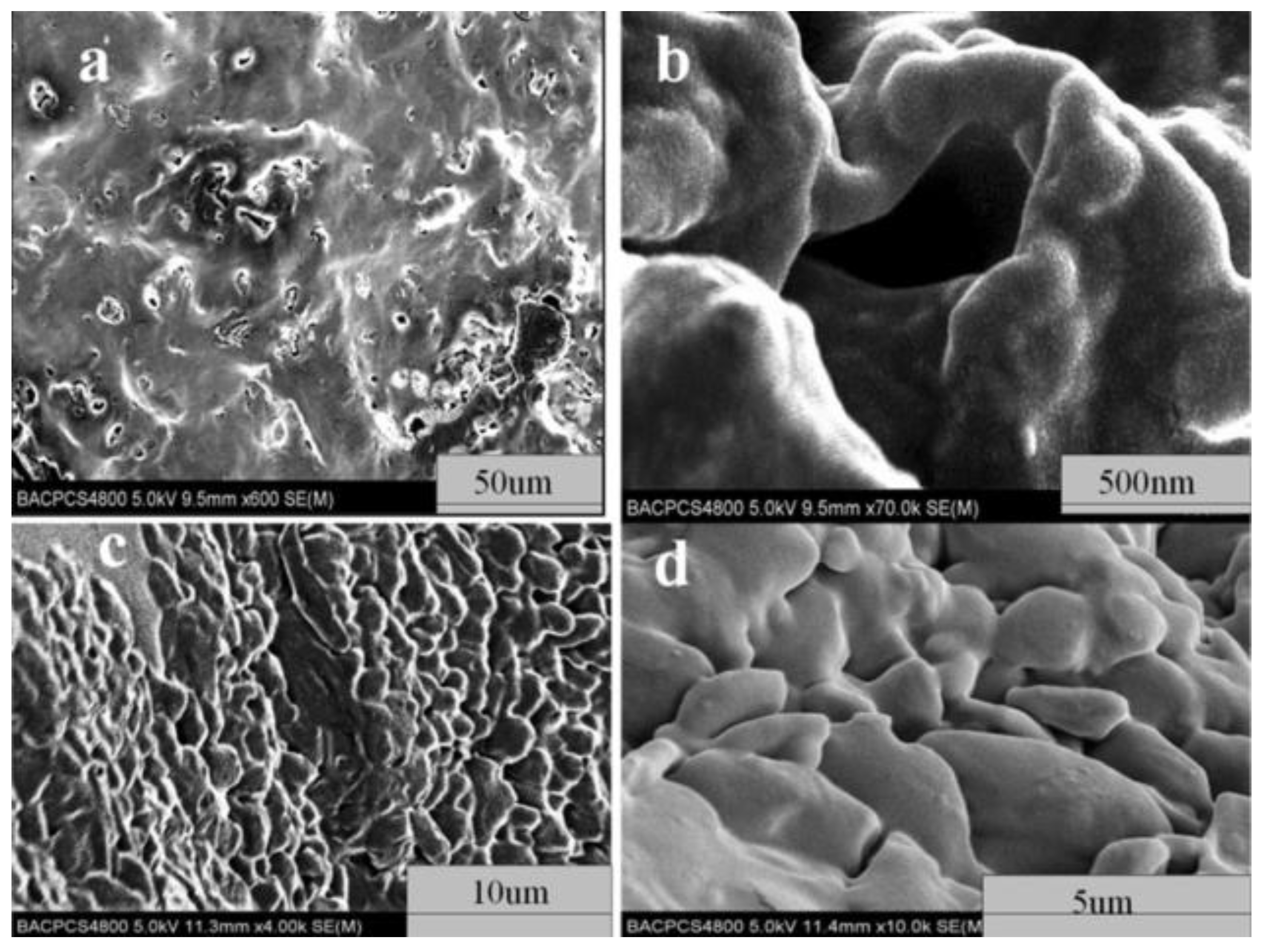
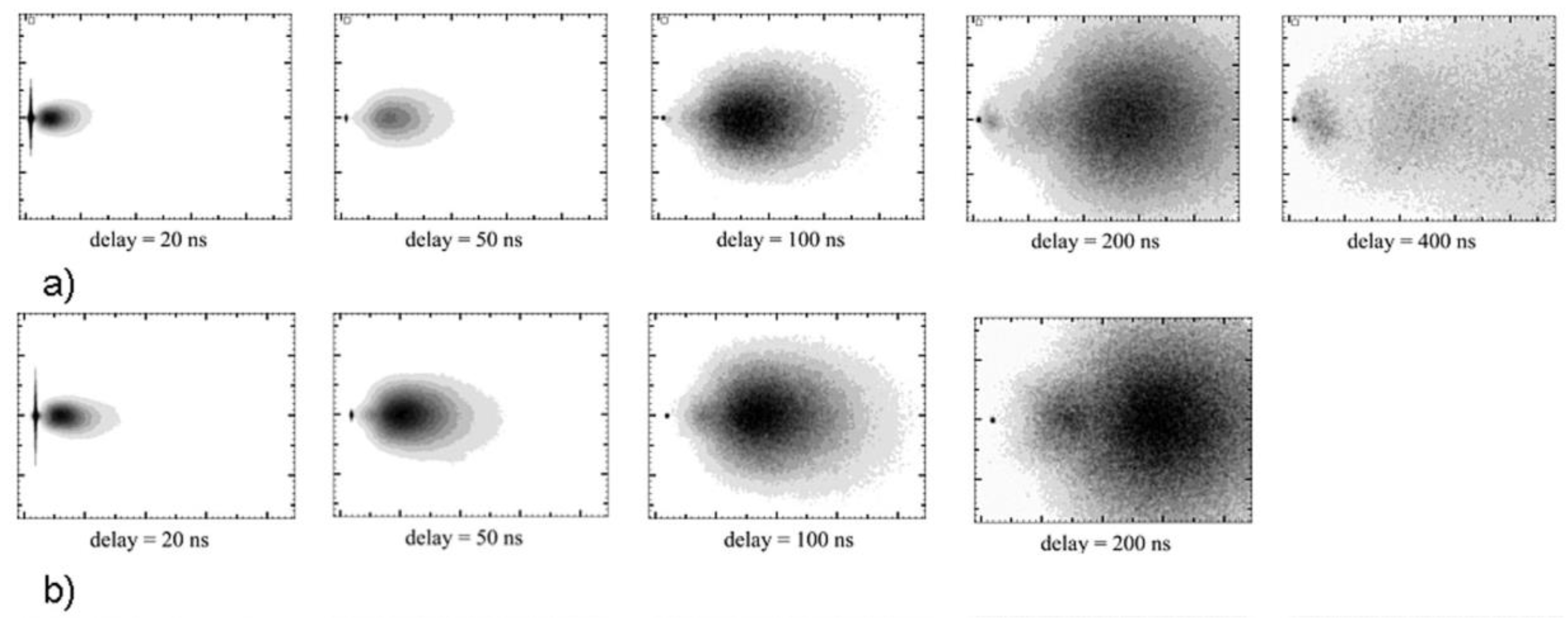

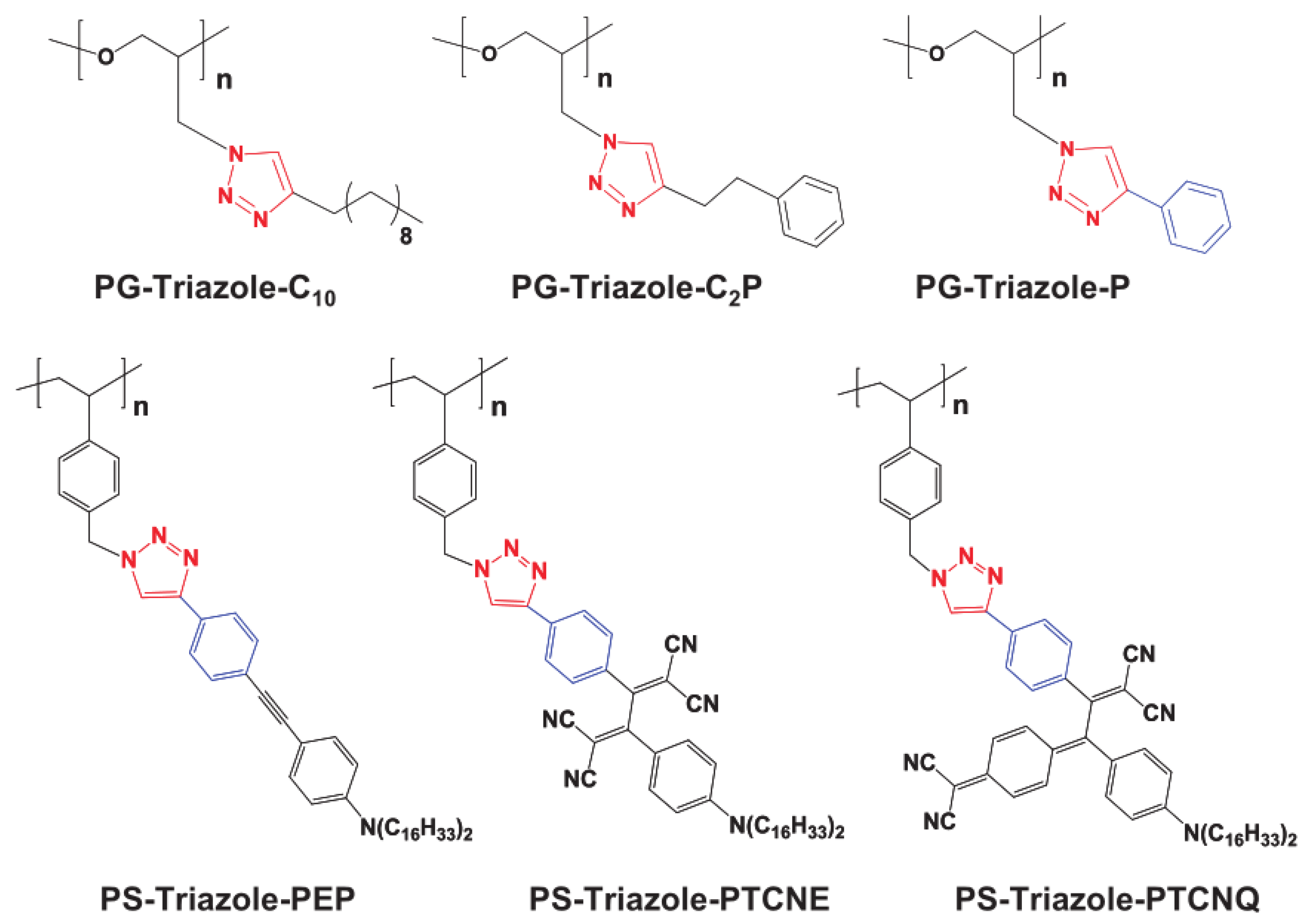
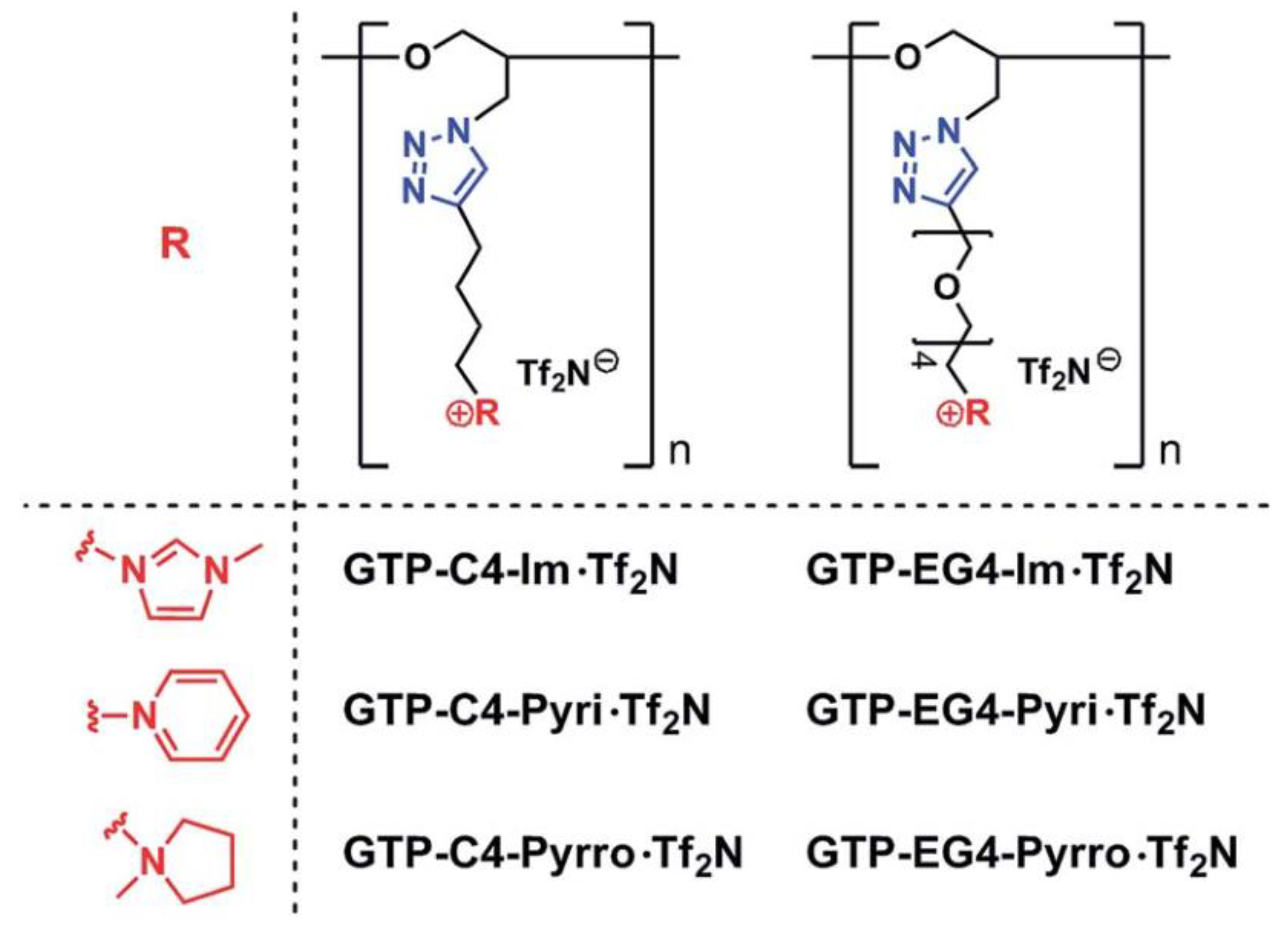
| Property [Units] | Value |
|---|---|
| Viscosity at 25 °C [Pa·s] | 12 a,b |
| Density [g/cm3] | 1.3 |
| Hydroxyl equivalent weight [g/mol] | 2000 c |
| Functionality [-OH groups per molecule] | 2.5 ÷ 3 c |
| Solubility | Most organic solvents, but not water, lower alcohols or aliphatic hydrocarbons |
| Impact sensitivity test (Bureau of Explosives) | 0/10 at 9.04 J d neat |
| Friction sensitivity test (Bureau of Explosives) | 0/10 at 444.8 N e |
| Decomposition Temperature of CL-20 (TA) | Heating Rate | Experimental Pan | Decomposition Temperature of Mixture (TAB) | Compatibility (Criterion: Value of TAB-TA) |
|---|---|---|---|---|
| 500 K a | 2 K/min | w/pinhole | 474 K | <−20K, incompatible |
| 499 K | 2 K/min | Hermetic | 474 K | <−20K, incompatible |
| 505 K | 10 K/min | w/pinhole | 486 K | −19 K, possibly incompatible |
| 529 K | 10 K/min | Hermetic | 491 K | <−20 K, incompatible |
| Components a | Heating Rate [K/min] | Type of Experimental Pan | Δt b [k] | Authors’ Compatibility Evaluation | Ref. | |
|---|---|---|---|---|---|---|
| CL-20 | poly(NIMMO) | 2 | w/pinhole | −26 | incompatible | [102] |
| CL-20 | PBAN | −30 | incompatible | |||
| CL-20 | HTPB | −20 | possibly incompatible | |||
| CL-20 | GAP | −31 | incompatible | |||
| TKX-50 | 2,4-Dinitroanisole | 10 | open, N2 atmosphere | −2.03 | A–B (compatible/slightly sensitised) | [104] |
| TKX-50 | TNT | −11.47 | C (sensitised) | |||
| RDX | TKX-50 | −17.64 | D (hazardous) | |||
| TKX-50 | HMX | −3.89 | B (slightly sensitised) | |||
| CL-20 | TKX-50 | −8.17 | C (sensitised) | |||
| TKX-50 | Centralite | −10.82 | C (sensitised) | |||
| NC | TKX-50 | −9.85 | C (sensitised) | |||
| NC+NG c | TKX-50 | −16.9 | D (hazardous) | |||
| TKX-50 | Ammonium chlorate(VII) | −8.77 | C (sensitised) | |||
| Hexanitro-ethane | TKX-50 | +8.04 | A (compatible) | |||
| TKX-50 | Al | −8.01 | C (sensitised) | |||
| TKX-50 | B | −17.61 | D (hazardous) | |||
| TKX-50 | GAP | −6.16 | C (sensitised) | |||
| TKX-50 | HTPB | −68.4 | D (hazardous) | |||
| CL-20 d | Polyethylene | +3.9 | - | [105] | ||
| CL-20 d | GAP | −8.4 | - | |||
| CL-20 d | Desmodur N100 | −31.8 | - | |||
| CL-20 d | Polyvinylpyrrolidone | −0.6 | - | |||
| CL-20 d | Polyethylenimine | +2.9 | - | |||
| Explosive | BCHMX | β-HMX | RDX | ε-CL20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binder | - | HTPB | GAP | - | HTPB | GAP | - | HTPB | GAP | - | HTPB | GAP |
| Density [g/cm3] | 1.79 | 1.56 | 1.62 | 1.90 | 1.57 | 1.64 | 1.76 | 1.52 | 1.59 | 1.98 | 1.63 | 1.73 |
| Detonation velocity [m/s] | 8650 | 7746 | 8292 | 9100 | 7812 | 8384 | 8750 | 7526 | 8074 | 9473 | 8167 | 8676 |
| Detonation pressure [GPa] | 33.9 | 21.2 | 28.6 | 38.0 | 21.3 | 28.4 | 32.1 | 20.1 | 26.2 | 41.7 | 23.7 | 32.1 |
| Heat of explosion [kJ/kg] | 6447 | 5744 | 6658 | 6075 | 5453 | 6297 | 6085 | 5453 | 6152 | 6455 | 5786 | 6559 |
| Heat of combustion [J/g] | 9124 | 13,798 | 11,789 | 9485 | 14,118 | 11,893 | 9522 | 14,162 | 12,011 | 8311 | 13,255 | 10,922 |
| Impact sensitivity [J] | 3.2 | 9.6 | 7.7 | 6.4 | 15.2 | 11.2 | 5.6 | 14.6 | 11.5 | 4.1 | 10.8 | 8.4 |
| Friction sensitivity [N] | 88 | 322 | 294 | 95 | >360 | 338 | 120 | >360 | >360 | 69 | 214 | 247 |
| Main Component | Curing Agent | Young’s Modulus MPa | Tensile Strength MPa | Elongation at Break % | Notes/Ratios of Reagents | Ref. |
|---|---|---|---|---|---|---|
| GAP | CA1 | 5.36 | 2.53 | 47.6 | N3/alkyne 2:1 | [17] |
| 0.65 | 0.33 | 81.6 | N3/alkyne 3:1 | |||
| GAP | CA2 | 174.1 | 13.1 | ~28 | CA2/GAP 5:1 | [18] |
| 9.1 | 4.5 | 81.7 | CA2/GAP 3:1 | |||
| GAP | CA3 | 0.07 | 0.08 | 147 | GAP/CA3 0.9:1 | [19] |
| 0.16 | 0.11 | 73.2 | GAP/CA3 1:1 | |||
| 0.25 | 0.12 | 48.3 | GAP/CA3 1.1:1 | |||
| 0.18 | 47.78 | GAP/CA3 1.2:1 | ||||
| 0.53 | 0.19 | 38.5 | GAP/CA3 1.3:1 | |||
| 0.72 | 0.20 | 27 | GAP/CA3 1.4:1 | |||
| 4.17 | 1.04 | 28.11 | GAP/CA3 2.5:1 | |||
| GAP | CI2 | 0.21 | 0.25 | 148.5 | not given | [20] |
| CA3 | 8.22 | 3.58 | 47.78 | |||
| 2-nitroderivative of CA3 | 5.72 | 1.83 | 35.84 | |||
| GAP | CA4 | 1.52 | 0.21 | 47.6 | alkyne/azide 1.2:1 | [22] |
| 3.60 | 0.41 | 25.4 | alkyne/azide 2:1 | |||
| PAMMO | 0.89 | 0.41 | 66.4 | alkyne/azide 1.2:1 | ||
| 2.56 | 0.67 | 37 | alkyne/azide 2:1 | |||
| Acyl-GAP | CA2 | - | 2.69 | 86 | 10% CA2 | [23] |
| - | 1.28 | 464 | Acyl-GAP + CA2/HTPB + CI1 0:1 | |||
| - | 2.69 | 86 | Acyl-GAP + CA2/HTPB + CI1 1:0 | |||
| - | 5.26 | 318 | Acyl-GAP + CA2/HTPB + CI1 1:1 | |||
| MWCNT-HTPB + MWCNT-Acyl-GAP | CI1, CI2 | - | 8.17 | 312 | NCO/OH = 0.8; 50% Acyl-GAP | [24] |
| HTPB+GAP | - | 5.89 | 359 | NCO/OH = 1; 50% GAP | [25] | |
| GAP | CA9 | 3.23 | 0.80 | 28.3 | Azide/alkyne 1:2.5 | [26] |
| CA10 | 7.44 | 1.77 | 36.3 | Azide/alkyne 1:2.5 + 1% MWCNT | ||
| CA11 | 6.33 | 1.41 | 19.7 | Azide/alkyne 1:2.5 + 1% MWCNT-COOH | ||
| GAP | dual isocyanate/CA6 | 1.68 | - | - | NCO/OH 1.1:1; GAP/dipolarophile 100:1 (w/w) | [27] |
| 3.29 | - | - | NCO/OH 1.1:1; GAP/dipolarophile 100:1,5 (w/w) | |||
| 4.69 | - | - | NCO/OH 1.1:1; GAP/dipolarophile 100:2 (w/w) | |||
| 7.64 | - | - | NCO/OH 1.1:1; GAP/dipolarophile 100:5 (w/w) | |||
| dual isocyanate/CA5 | 3.07 | - | - | NCO/OH 1.1:1; GAP/dipolarophile 100:1.5 (w/w) | ||
| 5.42 | - | - | NCO/OH 1.1:1; GAP/dipolarophile 100:2 (w/w) | |||
| 7.6 | - | - | NCO/OH 1.1:1; GAP/dipolarophile 100:5 (w/w) | |||
| GAP | CI2 | 0.18 | 0.26 | 161 | NCO/OH 1:0.8 | [28] |
| 0.47 | 0.5 | 122 | NCO/OH 1:1 | |||
| 1.09 | 0.7 | 69 | NCO/OH 1:1.2 | |||
| CI1, CI2 (1:1) | 0.082 | 0.23 | 285 | NCO/OH 1:1 | ||
| 0.315 | 0.52 | 192 | NCO/OH 1:1.2 | |||
| 0.6 | 0.76 | 147 | NCO/OH 1:1.4 | |||
| CA3 | 0.55 | 0.34 | 68 | CA3/GAP: 1:1 | ||
| 1.24 | 0.57 | 48 | CA3/GAP: 1.3:1 | |||
| CA3, CI1 | 0.71 | 0.6 | 95 | CA3/GAP: 0.5:1; NCO/OH 1:1 | ||
| 1.28 | 0.85 | 72 | CA3/GAP: 0.7:1; NCO/OH 1:1 | |||
| CA7, CI1 | 0.57 | 0.79 | 157 | CA7/GAP: 0.5:1; NCO/OH 1:1 | ||
| 1.11 | 1.2 | 117 | CA7/GAP: 0.7:1; NCO/OH 1:1 | |||
| GAP/Al/NH4ClO4/HMX/Bu-NENA | - | 1.48 | 0.26 | 45.6 | Blank sample | [36] |
| 1.86 | 0.29 | 37.9 | BindAgent1 0.1% | |||
| 2.17 | 0.35 | 36.7 | BindAgent1 0.2% | |||
| 2.26 | 0.36 | 33.1 | BindAgent1 0.3% | |||
| 1.57 | 0.3 | 35.2 | BindAgent2 0.1% | |||
| 1.59 | 0.36 | 33.8 | BindAgent2 0.2% | |||
| 1.8 | 0.41 | 32.7 | BindAgent2 0.3% | |||
| 1.85 | 0.32 | 45.2 | BindAgent3 0.1% | |||
| 2.07 | 0.39 | 44.2 | BindAgent3 0.2% | |||
| 2.1 | 0.39 | 43.1 | BindAgent3 0.3% | |||
| GAP | toluene diisocyanate | - | 1.6 | 1041 | R = NCO/OH. R = 1.6 | [37] |
| FGAP | CI2 | - | 1.5 | 81.6 | NCO/OH = 1 | [47] |
| GAP | - | 0.66 | 51.1 | |||
| GAP | CI1 | - | 2.4 | 101 | NCO/OH = 1 | [50] |
| FGAP | - | 5.52 | 162.8 | |||
| ETPE+NC | - | - | 7.5 | 490 | NC 0% | [52] |
| - | 8.9 | 485.6 | NC 10% | |||
| - | 7.8 | 110.5 | NC 20% | |||
| - | 8.7 | 96.8 | NC 30% | |||
| - | 13.3 | 45.1 | NC 40% | |||
| - | 18.2 | 28.9 | NC 50% | |||
| PBAMO/GAP | toluene diisocyanate + 1,4-butanediol | - | 2.55 | 217 | TDI+BDO = 30% | [53] |
| GAP/P(EO-co-THF) | CI1, CI2 NCO/OH = 1,2:1 | - | 0.662 | 212.4 | Flexible polyether: 0%( w/w) | [54] |
| - | 0.694 | 237.4 | Flexible polyether: 5% (w/w) | |||
| - | 0.824 | 279.6 | Flexible polyether: 10% (w/w) | |||
| - | 0.857 | 280.7 | Flexible polyether: 15% (w/w) | |||
| - | 0.933 | 284.1 | Flexible polyether: 20% (w/w) | |||
| - | 0.953 | 289.9 | Flexible polyether: 25% (w/w) | |||
| - | 0.986 | 296.1 | Flexible polyether: 30% (w/w) | |||
| GAP/PAO | CI1, CI2 NCO/OH = 1,2:1 | - | 0.662 | 212.4 | Flexible polyether: 0% (w/w) | |
| - | 0.753 | 256.7 | Flexible polyether: 5% (w/w) | |||
| - | 0.885 | 260.4 | Flexible polyether: 10% (w/w) | |||
| - | 1.633 | 271.4 | Flexible polyether: 15% (w/w) | |||
| - | 1.917 | 276.1 | Flexible polyether: 20% (w/w) | |||
| - | 2.528 | 330.1 | Flexible polyether: 25% (w/w) | |||
| - | 3.512 | 435.4 | Flexible polyether: 30% (w/w) | |||
| GAP-based PU | CI1 | 0.12 | 0.21 | 286 | Alkyne/azide = 0/1; 2.27 mmol CI1 for 1.62 mmol GAP | [70] |
| DNPMP (n=1)/GAP-based PU | 0.13 | 0.27 | 293 | Alkyne/azide = 0.1/1 | ||
| 0.06 | 0.43 | 505 | Alkyne/azide = 0.3/1 | |||
| 1.05 | 1.71 | 390 | Alkyne/azide = 0.5/1 | |||
| DNPMB (n=2)/GAP-based PU | 0.09 | 0.3 | 402 | Alkyne/azide = 0.1/1 | ||
| 0.08 | 0.41 | 539 | Alkyne/azide = 0.3/1 | |||
| 0.34 | 1.21 | 461 | Alkyne/azide = 0.5/1 | |||
| PDNP (n=1)/GAP-based PU | 0.1 | 0.32 | 425 | Alkyne/azide = 0.1/1 | ||
| 0.15 | 0.55 | 638 | Alkyne/azide = 0.3/1 | |||
| 6.06 | 2.9 | 687 | Alkyne/azide = 0.5/1 | |||
| BDNP (n=2)/GAP-based PU | 0.09 | 0.28 | 421 | Alkyne/azide = 0.1/1 | ||
| 0.11 | 0.57 | 574 | Alkyne/azide = 0.3/1 | |||
| 3.2 | 1.67 | 684 | Alkyne/azide = 0.5/1 | |||
| GAP doped NC and NG propellants | - | - | 44.97 | 7.314 | 30% GAP/NC | [84] |
| - | 44.93 | 7.311 | 30% GAP/NC | |||
| - | 44.82 | 7.727 | 30% GAP/NC | |||
| - | 37.71 | 37.4 | 40% GAP/NC | |||
| - | 37.37 | 36.94 | 40% GAP/NC | |||
| - | 38.42 | 36.57 | 40% GAP/NC | |||
| - | 29.92 | 75.78 | 50% GAP/NC | |||
| - | 30.16 | 74.37 | 50% GAP/NC | |||
| - | 29.45 | 74.9 | 50% GAP/NC | |||
| - | 31.38 | 35.11 | 40% GAP/NG | |||
| - | 31.05 | 35.17 | 40% GAP/NG | |||
| - | 31.61 | 35.29 | 40% GAP/NG | |||
| GAP doped propellants | CI2 | 5.26 | 0.72 | 26.3 | 0% GAP/−40 °C | [85] |
| CI3 | 7.18 | 1.18 | 30.2 | 10% GAP/−40 °C | ||
| CI4 | 7.17 | 1.16 | 29.8 | 20% GAP/−40 °C | ||
| CI5 | 3.02 | 0.41 | 34.9 | 0% GAP/+20 °C | ||
| CI6 | 3.81 | 0.54 | 52.5 | 10% GAP/+20 °C | ||
| CI7 | 3.71 | 0.55 | 52.1 | 20% GAP/+20 °C | ||
| CI8 | 2.14 | 0.4 | 39.5 | 0% GAP/+50 °C | ||
| CI9 | 2.23 | 0.5 | 52.4 | 10% GAP/+50 °C | ||
| CI10 | 2.2 | 0.45 | 52.6 | 20% GAP/+50 °C | ||
| GAP (21%) doped propellants | CI2 | 4.71 | 0.29 | 18.9 | Propellant 1 | [87] |
| 4.53 | 0.26 | 14.7 | Propellant 2 | |||
| 2.25 | 0.22 | 24.4 | Propellant 3 |
| Investigated System [details] | Sensitivity/Other Properties | Ref. |
|---|---|---|
| GAP [commercial] | Impact: >9.04 J Friction: >444.8 N | [7] |
| MWCNT-HTPB + MWCNT-Acyl-GAP [cured with CI1 and CI2, NCO/OH = 0,8; 50% Acyl-GAP] | Impact: >40 J Friction: >360 N Electrostatic discharge: >5 J | [24] |
| Propellants based on MWCNT-HTPB [cured with CI1 and CI2] | Impact: 3–4 J Friction: 40–50 N | |
| Propellants based on MWCNT-HTPB + MWCNT-Acyl-GAP [cured with CI1 and CI2, NCO/OH = 0.8; 50% Acyl-GAP] | Impact: 4,5 J Friction: 210 N | |
| GAP-co-azidoTHF | Impact: 0% Friction: 0% Electrostatic discharge: 0.181 J (E50) | [43] |
| GAP [cured with CI4] | Impact: >40 J Friction: >360 N | [51] |
| Poly (2,2,2-trifluoro-ethoxymethyl epoxy-r-glycidyl azide) [cured with C1, NCO/OH = 1] | Impact: >129 cm (H50) | [52] |
| GAP [cured with C1, NCO/OH = 1] | Impact: 42.2 cm (H50) | |
| GAP [cured with CI3] | Impact: 7 J Friction: >360 N | [71] |
| BAMP [cured with diisocyanatoethane] | Impact: 40 J Friction: >360 N Electrostatic discharge: 1.5 J | |
| BAMP [cured with CI3] | Impact: 40 J Friction: >360 N | |
| DNPD [cured with diisocyanatoethane] | Impact: 40 J Friction: >360 N | |
| DNPD [cured with CI3] | Impact: 40 J Friction: >360 N Electrostatic discharge: 1.5 J | |
| Esters of BAMP | Impact: >40 J Friction: >360 N | [61,63] |
| Esters of DNPD | Impact: >40 J Friction: >360 N | |
| Bis (2,3-diazidopropoxy) alkanes GlyN dimer | Friction: >360 N | [71] |
| GNAP | Impact: 40 J Friction: >360 N VDET: 7165 m/s (theoretical) | [71] |
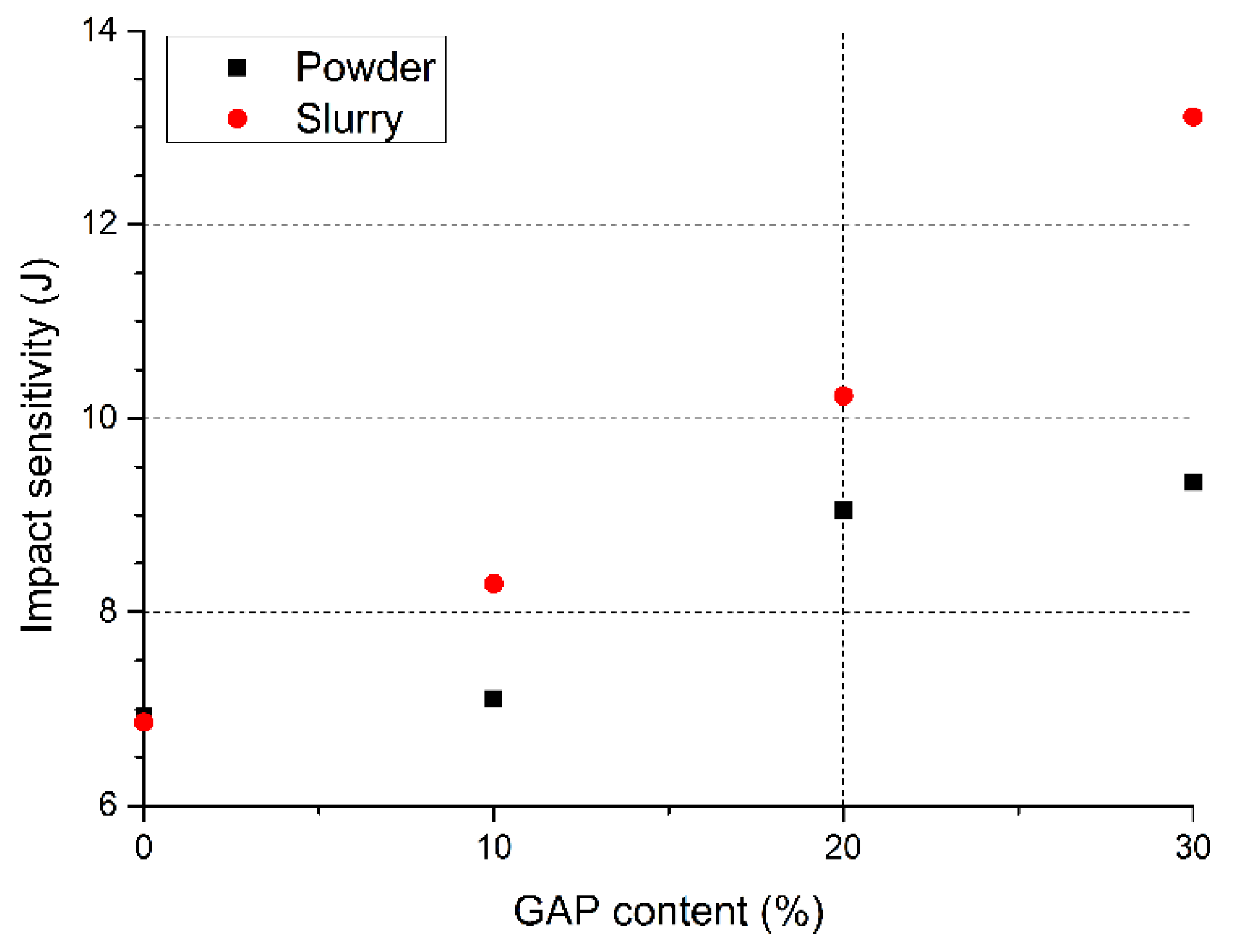
| NC/NG modified with GAP [0% GAP, powder mixture] | Impact: 6.92 J (E50) | [85] |
| NC/NG modified with GAP [10% GAP, powder mixture] | Impact: 7.1 J (E50) | |
| NC/NG modified with GAP [20% GAP, powder mixture] | Impact: 9.05 J (E50) | |
| NC/NG modified with GAP [30% GAP, powder mixture] | Impact: 9.34 J (E50) | |
| NC/NG/GAP-based propellant [0% GAP in powder mixture, 4.0–5.2% “free” GAP in propellant slurry] | Impact: 6.86 J (E50) | [94] |
| NC/NG/GAP-based propellant [10% GAP in powder mixture, 4.0–5.2% “free” GAP in propellant slurry] | Impact: 8.29 J (E50) | |
| NC/NG/GAP-based propellant [20% GAP in powder mixture, 4.0–5.2% “free” GAP in propellant slurry] | Impact: 10.23 J (E50) | |
| NC/NG/GAP-based propellant [30% GAP in powder mixture, 4.0–5.2% “free” GAP in propellant slurry] | Impact: 13.11 J (E50) | |
| RDX (95) coated with NC (3) and GAP (2) | Impact: 40.74 cm (H50) | |
| RDX | Impact: 23.40 cm (H50) | |
| RDX [pure] | Impact: 12.8 cm (H50) | [96] |
| RDX/GAP [blend cured with CI5, NCO/OH = 1.5, RDX = 40% w/w] | Impact: 15.59 cm (H50) | |
| RDX/GAP [nanocomposite cured with CI5, NCO/OH = 1.5, RDX = 40% w/w] | Impact: 30.2 cm (H50) | |
| εCL-20/GAP [82% Cl-20, 14% GAP (Mn = 3380, with a hydroxyl value of 0.646 mol/g), 1.5% toluene diisocyanate, 2,5% others, cured with toluene diisocyanate] | Impact: 38.2 cm (H50) Shock sensitivity: 7.74 mm (card gap) VDET: 7364 m/s Critical diameter: <0.6 mm | [107] |
| CL-20 [pure] | Impact: 15.9 cm (H50) Shock sensitivity: 42.11 mm (card gap) VDET: 9500 m/s | |
| CL-20/GAP [85% CL-20, cured with CI2. GAP Mn = 4000, with a hydroxyl value of 1.412 mmol/g] | Impact: 37.2 cm (H50) Critical diameter: <0.4 mm | [109] |
| CL-20 | Impact: 21.1 cm (H50) | |
| GAP/DNTF [DNTF 85%, GAP (Mn ≈ 3300) 11%, toluene diisocyanate 1.6%, others 2.4%] | Impact: 38.3 cm (H50) Friction: 0% VDET: 7362 m/s Critical diameter: <0.5 mm | [111] |
| DNTF (2–3 μm) | Impact: 25.6 cm (H50) Friction: 13% | |
| DNTF (15–20 μm) | Impact: 25.0 cm (H50) Friction: 12% | |
| HMX | Impact: 25.7 cm (H50) Friction: 100% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, T.; Stolarczyk, A.; Wawrzkiewicz-Jalowiecka, A.; Pawlus, K.; Miszczyszyn, K. Glycidyl Azide Polymer and its Derivatives-Versatile Binders for Explosives and Pyrotechnics: Tutorial Review of Recent Progress. Molecules 2019, 24, 4475. https://doi.org/10.3390/molecules24244475
Jarosz T, Stolarczyk A, Wawrzkiewicz-Jalowiecka A, Pawlus K, Miszczyszyn K. Glycidyl Azide Polymer and its Derivatives-Versatile Binders for Explosives and Pyrotechnics: Tutorial Review of Recent Progress. Molecules. 2019; 24(24):4475. https://doi.org/10.3390/molecules24244475
Chicago/Turabian StyleJarosz, Tomasz, Agnieszka Stolarczyk, Agata Wawrzkiewicz-Jalowiecka, Klaudia Pawlus, and Karolina Miszczyszyn. 2019. "Glycidyl Azide Polymer and its Derivatives-Versatile Binders for Explosives and Pyrotechnics: Tutorial Review of Recent Progress" Molecules 24, no. 24: 4475. https://doi.org/10.3390/molecules24244475
APA StyleJarosz, T., Stolarczyk, A., Wawrzkiewicz-Jalowiecka, A., Pawlus, K., & Miszczyszyn, K. (2019). Glycidyl Azide Polymer and its Derivatives-Versatile Binders for Explosives and Pyrotechnics: Tutorial Review of Recent Progress. Molecules, 24(24), 4475. https://doi.org/10.3390/molecules24244475






