Comparative Assessment of Phenolic Profiles, Cellular Antioxidant and Antiproliferative Activities in Ten Varieties of Sweet Potato (Ipomoea Batatas) Storage Roots
Abstract
:1. Introduction
2. Results
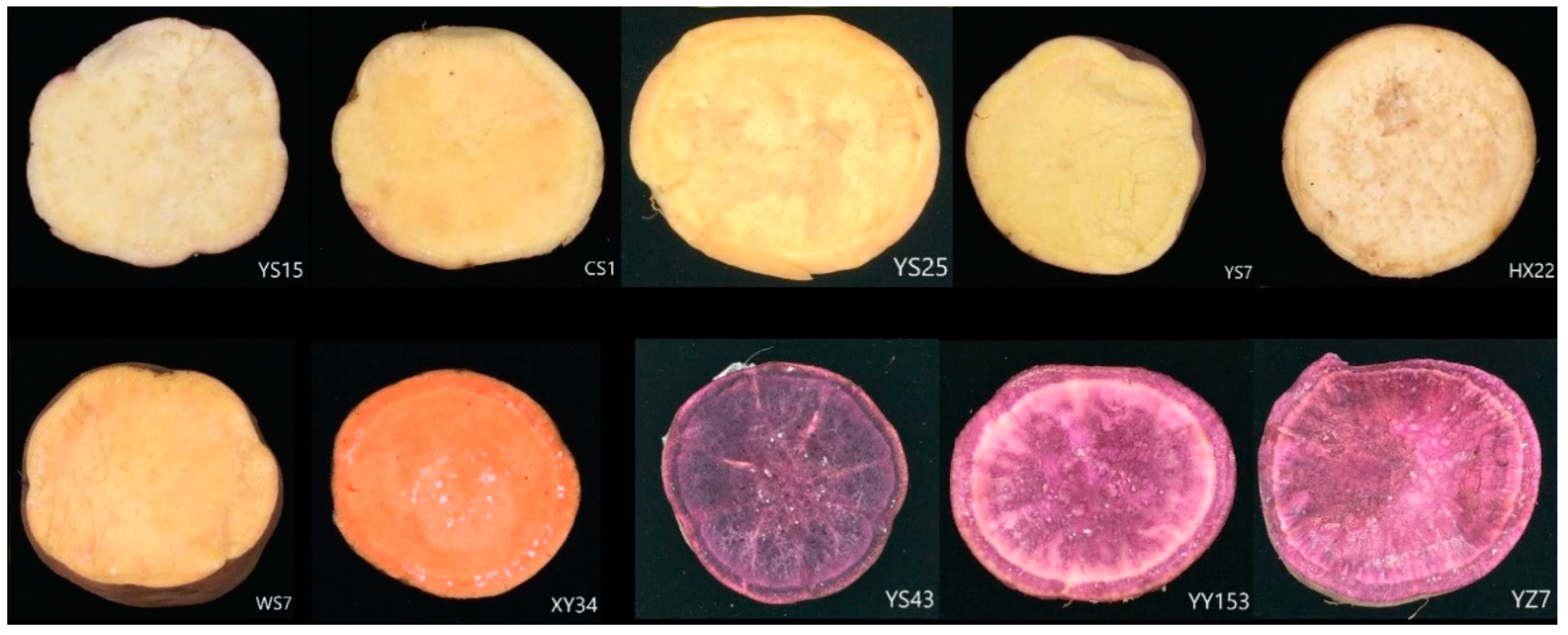
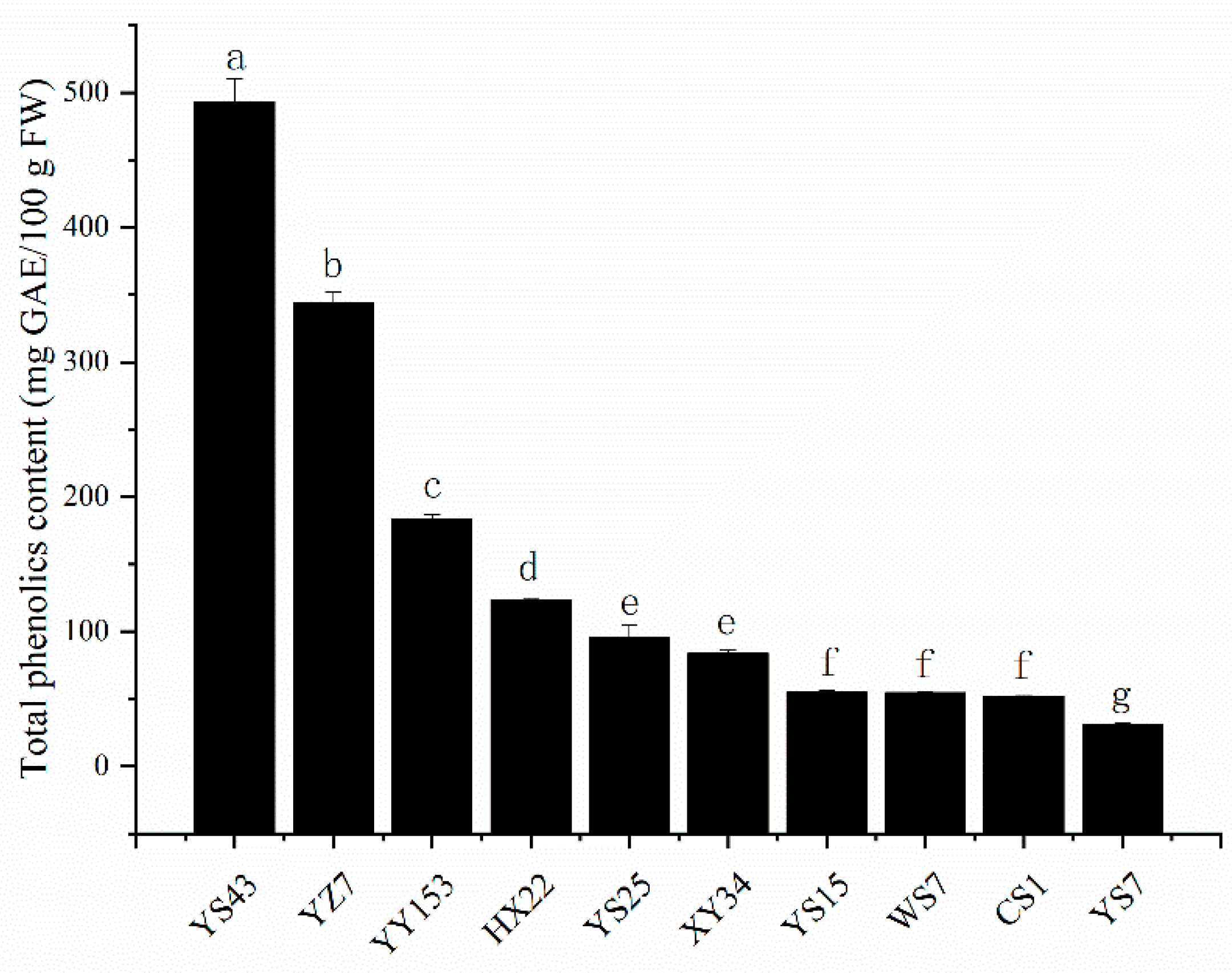
2.1. Total Phenolic Content in Different Varieties
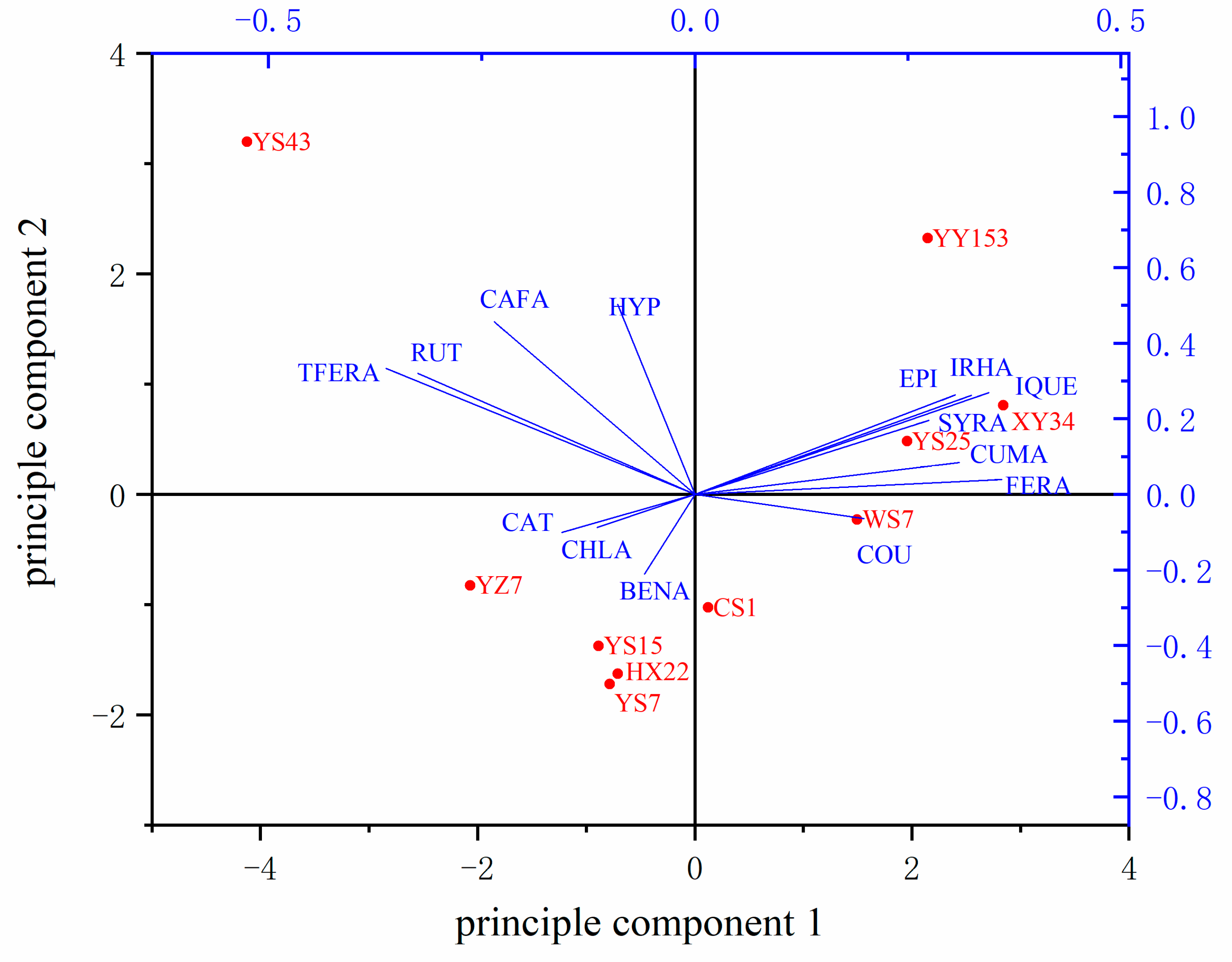
2.2. Phytochemical Composition of Ten Varieties
2.3. Total Antioxidant Activity
2.4. Cellular Antioxidant Activity
2.5. Antiproliferative Activity against Human Liver Cancer HepG2 Cell
2.6. Correlation Analysis among Phytochemicals and Antioxidant and Proliferative Activities
3. Discussion
3.1. Comparison of Total Phenolics Content in Different Varieties
3.2. Comparison of Phytochemical Composition and PCA Analysis of Ten Varieties
3.3. Comparison of Total Antioxidant Activity
3.4. Comparison of Cellular Antioxidant Activity
3.5. Comparison of Antiproliferative Activity against Human Liver Cancer HepG2 Cell
4. Materials and Methods
4.1. Sweet Potato Samples Preparation
4.2. Phenolic Extraction for Sweet Potato
4.3. Determination of Phenolics for Sweet Potato Extracts
4.4. Phenolic Profiles of Sweet Potato Extracts Analysis by HPLC-PAD
4.5. Total Antioxidant Activity Analysis for Sweet Potato Extracts
4.6. Cellular Antioxidant Activity Analysis for Sweet Potato Extracts
4.7. Determination of Antiproliferative and Cytotoxicity Activities of Sweet Potato Extracts
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Truong, V.D.; Yencho, G.C.; Lila, M.A. Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweetpotato storage and impacts on bioactive properties. Food. Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food. Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food. Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical characterization of five edible purple-reddish vegetables: Anthocyanins, flavonoids, and phenolic acid derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Lee, S.Y.; Yang, J.W.; Lee, J.S.; Oh, S.D.; Oh, S.; Lee, S.M.; Lim, M.H.; Park, S.K.; Jang, J.S.; et al. Comparative analysis of phytochemicals and polar metabolites from colored sweet potato (Ipomoea batatas l.) tubers. Food. Sci. Biotechnol. 2016, 25, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res. 2017, 15, 2751. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [Green Version]
- La Vecchia, C.; Decarli, A.; Serafini, M.; Parpinel, M.; Bellocco, R.; Galeone, C.; Bosetti, C.; Zucchetto, A.; Polesel, J.; Lagiou, P.; et al. Dietary total antioxidant capacity and colorectal cancer: A large case-control study in italy. Int. J. Cancer. 2013, 133, 1447–1451. [Google Scholar] [CrossRef]
- Del Rio, D.; Agnoli, C.; Pellegrini, N.; Krogh, V.; Brighenti, F.; Mazzeo, T.; Masala, G.; Bendinelli, B.; Berrino, F.; Sieri, S.; et al. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large italian cohort. J. Nutr. 2011, 141, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Brighenti, F.; Valtuena, S.; Pellegrini, N.; Ardigo, D.; Del Rio, D.; Salvatore, S.; Piatti, P.; Serafini, M.; Zavaroni, I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity c-reactive protein in adult italian subjects. Br. J. Nutr. 2005, 93, 619–625. [Google Scholar] [CrossRef]
- Huang, D.-J.; Chun-Der, L.; Hsien-Jung, C.; Yaw-Huei, L. Antioxidant and antiproliferative activities of sweet potato (Ipomoea batatas [L.] Lam Tainong 57′) constituents. Bot. Bull. Acad. Sin. 2004, 45, 179–186. [Google Scholar]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweet potato (Ipomoea batatas). J. Agric. Food. Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Sugata, M.; Lin, C.Y.; Shih, Y.C. Anti-inflammatory and anticancer activities of taiwanese purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts. Biomed. Res. Int. 2015, 2015, 768093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, S.; Naqvi, S.A.R.; Khan, Z.A.; Mansha, A.; Ahmad, M.; Zahoor, A.F.; Hussain, Z. Antioxidant, antimicrobial and antiproliferative activities of peel and pulp extracts of red and white varieties of Ipomoea batatas (L) Lam. Trop. J. Pharm. Res. 2017, 16, 2221. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (caa) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food. Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Rumbaoa, R.G.O.; Cornago, D.F.; Geronimo, I.M. Phenolic content and antioxidant capacity of philippine sweet potato (Ipomoea batatas) varieties. Food. Chem. 2009, 113, 1133–1138. [Google Scholar] [CrossRef]
- Lee, J.H.; Woo, K.S.; Lee, H.-U.; Nam, S.S.; Lee, B.W.; Lee, Y.-Y.; Lee, B.; Kim, H.-J. Intracellular reactive oxygen species (ros) removal and cytoprotection effects of sweet potatoes of various flesh colors and their polyphenols, including anthocyanin. Prev. Nutr. Food. Sci. 2019, 24, 293–298. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.-D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food. Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Rabah, I.O.; Hou, D.-X.; Komine, S.-I.; Fujii, M. Potential chemopreventive properties of extract from baked sweet potato (Ipomoea batatas Lam. Cv. Koganesengan). J. Agri. Food. Chem. 2004, 52, 7152–7157. [Google Scholar] [CrossRef]
- Everette, J.D.; Islam, S. Effect of extraction procedures, genotypes and screening methods to measure the antioxidant potential and phenolic content of orange-fleshed sweetpotatoes (Ipomoea batatas L.). Am. J. Food. Technol. 2012, 7, 50–61. [Google Scholar]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular antioxidant activity of common vegetables. J. Agric. Food. Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (vigna radiata). J. Agric. Food. Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; Liu, R.H. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food. Chem. 2015, 174, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.S.; Luo, S.J.; Li, T.; Liu, C.M.; Zhang, G.W.; Chen, J.; Zeng, Z.C.; Liu, R.H. Phytochemical profiles and antioxidant activity of brown rice varieties. Food. Chem. 2017, 227, 432–443. [Google Scholar] [CrossRef]
- Huang, D.J.; Ou, B.X.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (orac) using a multichannel liquid handling system coupled with a microplate flourescence reader in 96-well format. J. Agric. Food. Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food. Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, C.; Chen, L.; Abbasi, A.M.; Guo, X.; Liu, R.H. Comparative study of phenolic profiles, antioxidant and antiproliferative activities in different vegetative parts of ramie (Boehmeria nivea L.). Molecules 2019, 24, 1551. [Google Scholar] [CrossRef] [Green Version]
- Felice, D.L.; Sun, J.; Liu, R.H. A modified methylene blue assay for accurate cell counting. J. Funct. Foods. 2009, 1, 109–118. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology: Oxidants and Antioxidants Part A; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Yoon, H.; Liu, R.H. Effect of 2α-hydroxyursolic acid on nf-κb activation induced by tnf-α in human breast cancer mcf-7 cells. J. Agric. Food. Chem. 2008, 56, 8412–8417. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Identified Compound | YS7 | HX22 | YS43 | WS7 | YS25 | YZ7 | YY153 | CS1 | XY34 | YS15 |
|---|---|---|---|---|---|---|---|---|---|---|
| chlorogenic acid | ND | 20.11 ± 1.68a | 5.34 ± 0.32b | 0.51 ± 0.01c | 0.67 ± 0.01c | ND | ND | ND | 1.37 ± 0.09c | ND |
| caffeic acid | ND | 0.56 ± 0.002cd | 4.57 ± 1.18a | 0.96 ± 0.08bc | 0.59 ± 0.05cd | 1.39 ± 0.001b | 0.93 ± 0.01bc | 0.13 ± 0.01d | 1.55 ± 0.01b | 0.10 ± 0.01d |
| syringic acid | ND | ND | ND | 0.73 ± 0.11a | 0.74 ± 0.13a | ND | 0.67 ± 0.02a | ND | ND | ND |
| cumaric acid | ND | ND | ND | 1.48 ± 0.08a | 1.46 ± 0.02a | ND | ND | ND | 1.46 ± 0.01a | ND |
| ferulic acid | 19.13 ± 0.24d | 8.25 ± 0.05g | ND | 18.62 ± 0.01e | 10.86 ± 0.45f | ND | 26.03 ± 0.28b | 23.98 ± 0.04c | 27.97 ± 0.13a | 1.31 ± 0.003h |
| trans-ferulic acid | ND | ND | 1.34 ± 0.03a | ND | ND | 0.58 ± 0.02b | ND | ND | ND | ND |
| isoquercetin | ND | ND | ND | ND | 0.43 ± 0.03b | ND | 0.41 ± 0.01c | ND | 0.60 ± 0.01a | ND |
| benzoic acid | 12.37 ± 2.60 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| isorhamnetin | ND | ND | ND | ND | 2.13 ± 0.19b | ND | 2.25 ± 0.02a | 0.81 ± 0.03c | 0.87 ± 0.04c | ND |
| hyperoside | ND | ND | 417.6 ± 14.1b | 7.94 ± 0.97e | ND | 14.78 ± 1.75cd | 471.3 ± 2.5a | 16.34 ± 1.10c | 11.13 ± 1.24cde | 9.26 ± 0.89de |
| catechin | ND | ND | ND | ND | ND | 2.15 ± 0.17a | ND | ND | ND | ND |
| epicatechin | ND | ND | ND | 0.17 ± 0.001c | ND | ND | 0.30 ± 0.004b | ND | 0.35 ± 0.02a | ND |
| coumarin | ND | 0.26 ± 0.01b | ND | 0.23 ± 0.001b | 0.03 ± 0.01d | 0.04 ± 0.004c | ND | ND | 0.34 ± 0.02a | ND |
| rutin hydrate | 10.53 ± 2.51b | ND | 33.38 ± 3.09a | ND | ND | ND | ND | ND | ND | ND |
| Total Antioxidant Activities (mmol TE/100 g FW) | Cellular Antioxidant Activities (μmol QE/100 g FW) | Contribution (%) | Cyctoxicity (mg/mL) | |
|---|---|---|---|---|
| Varieties | ORAC | PBS Wash | PBS Wash/ORAC | CC10 |
| YS7 | 0.45 ± 0.07d | 3.34 ± 0.48b | 0.74 | 0.698 |
| HX22 | 1.73 ± 0.03cd | 30.15 ± 5.87b | 1.74 | 1.993 |
| YS43 | 7.38 ± 0.98a | 120.68 ± 36.9a | 1.64 | 1.662 |
| WS7 | 1.17 ± 0.18d | 38.47 ± 8.57b | 3.29 | 1.977 |
| YS25 | 1.90 ± 0.28cd | 23.14 ± 3.86b | 1.22 | 2.013 |
| YZ7 | 3.39 ± 0.36b | 50.73 ± 7.56b | 1.50 | 2.478 |
| YY153 | 2.38 ± 0.18bc | 35.22 ± 6.97b | 1.48 | 5.304 |
| CS1 | 0.40 ± 0.03d | 0.46 ± 0.08b | 0.12 | ND |
| XY34 | 1.19 ± 0.15d | 181.15 ± 18.24a | 15.22 | ND |
| YS15 | 0.52 ± 0.04d | ND | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Pan, Z.; Yang, C.; Jia, Z.; Guo, X. Comparative Assessment of Phenolic Profiles, Cellular Antioxidant and Antiproliferative Activities in Ten Varieties of Sweet Potato (Ipomoea Batatas) Storage Roots. Molecules 2019, 24, 4476. https://doi.org/10.3390/molecules24244476
Sun Y, Pan Z, Yang C, Jia Z, Guo X. Comparative Assessment of Phenolic Profiles, Cellular Antioxidant and Antiproliferative Activities in Ten Varieties of Sweet Potato (Ipomoea Batatas) Storage Roots. Molecules. 2019; 24(24):4476. https://doi.org/10.3390/molecules24244476
Chicago/Turabian StyleSun, Yiming, Zhijun Pan, Chunxian Yang, Zhenzhen Jia, and Xinbo Guo. 2019. "Comparative Assessment of Phenolic Profiles, Cellular Antioxidant and Antiproliferative Activities in Ten Varieties of Sweet Potato (Ipomoea Batatas) Storage Roots" Molecules 24, no. 24: 4476. https://doi.org/10.3390/molecules24244476
APA StyleSun, Y., Pan, Z., Yang, C., Jia, Z., & Guo, X. (2019). Comparative Assessment of Phenolic Profiles, Cellular Antioxidant and Antiproliferative Activities in Ten Varieties of Sweet Potato (Ipomoea Batatas) Storage Roots. Molecules, 24(24), 4476. https://doi.org/10.3390/molecules24244476





