Phytochemical Fingerprinting and In Vitro Bioassays of the Ethnomedicinal Fern Tectaria coadunata (J. Smith) C. Christensen from Central Nepal
Abstract
1. Introduction
2. Results and Discussion
2.1. NMR Analysis of Tectaria coadunata Extracts
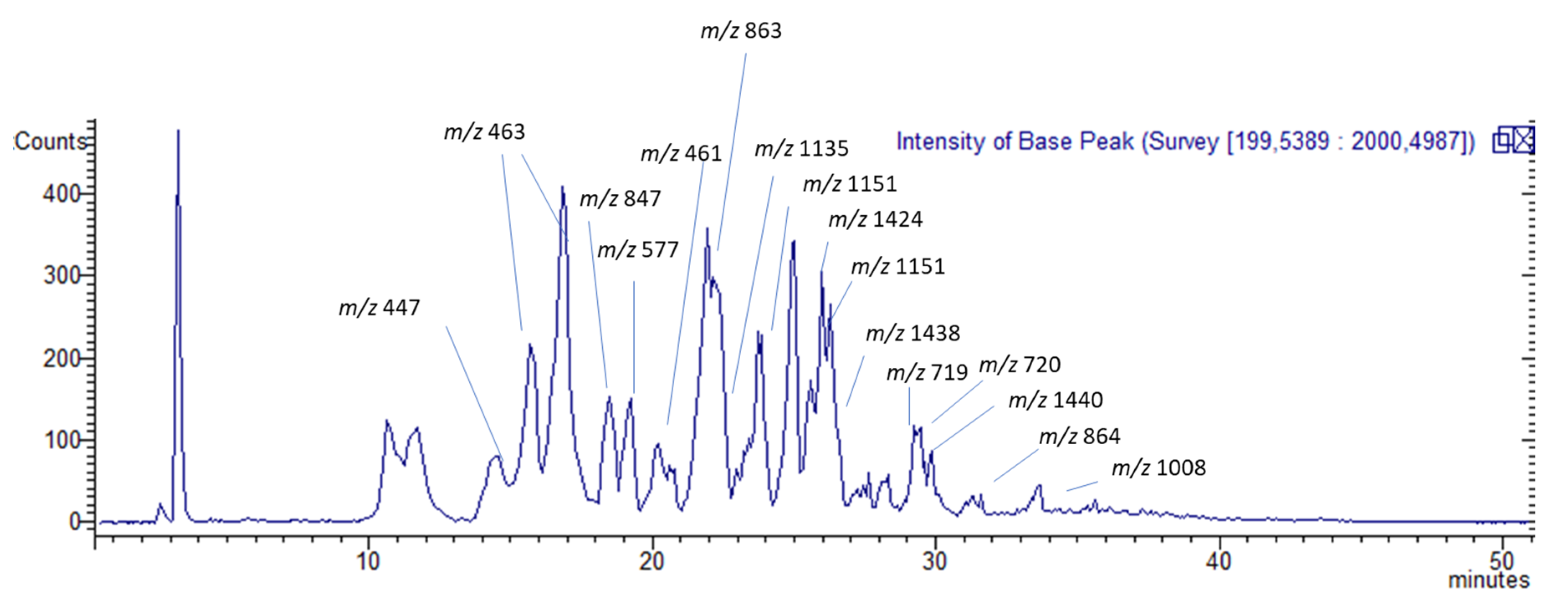
2.2. Quali-Quantitative Analysis
HPLC Coupled with Diode Array, Mass Spectrometry, and Fluorescence for the Analysis of Phenolic Constituents
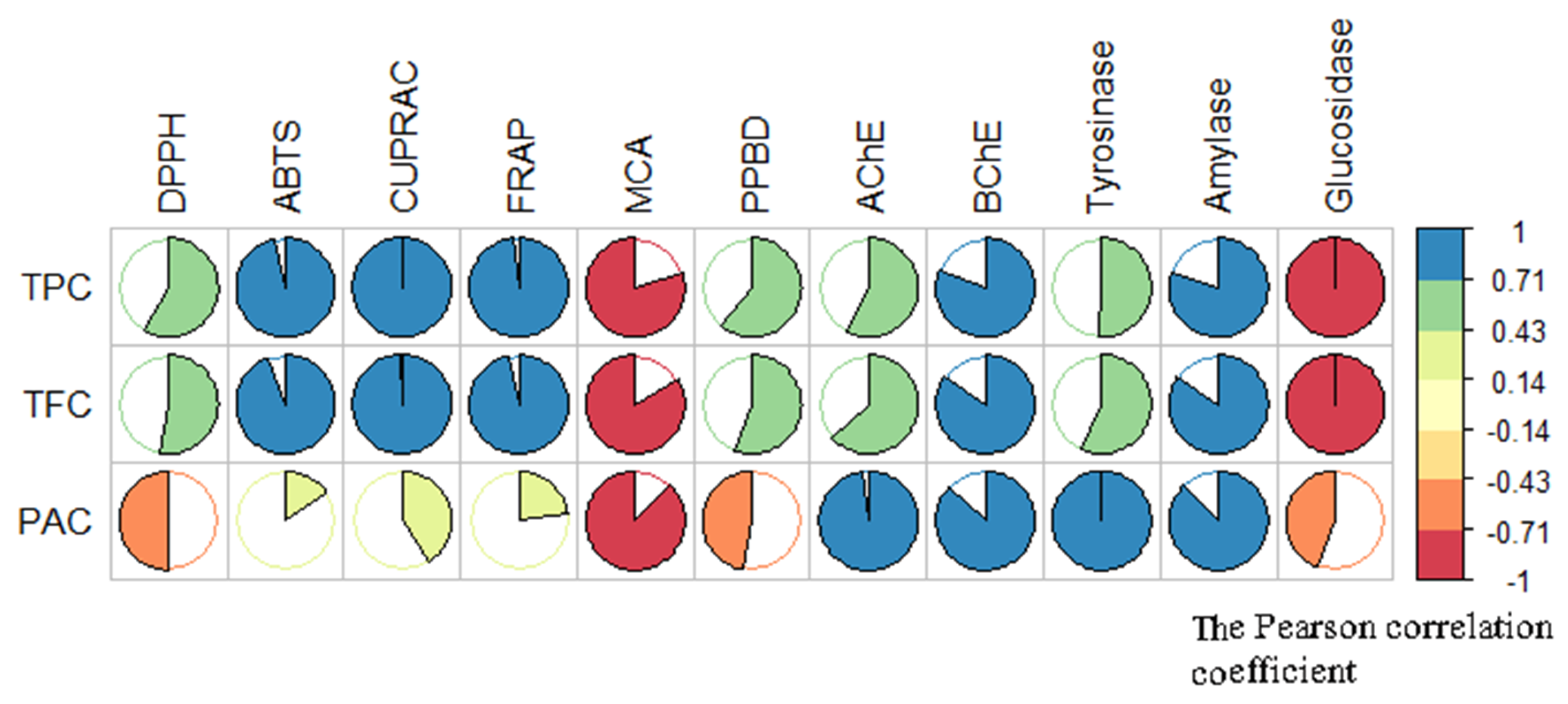
2.3. In Vitro Bioassays
2.3.1. Antioxidant Activity
2.3.2. Test of Inhibitory Effect Against Degenerative and Metabolic Enzymatic Activities: Cholinesterases, α-Amylase, α-Glucosidase, and Tyrosinase
2.3.3. Discussion of the Results of Acetyl and Butyril Cholinesterases Related to Phytochemical Composition of the T. coadunata Extracts
2.3.4. Discussion of the Results of Amylase and Glucosidase Inhibitory Activity Related to Phytochemical Composition of the T. coadunata Extracts
2.4. Cytotoxicity Tests
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
3.3. Isolation of Main Constituents
3.4. Quali-Quantitative Analysis: HPLC HILIC-DAD-FLD-ESI-MS
3.5. Total Phenolic Content, Antioxidant, and Enzyme Inhibitory Assays
3.6. Cytotoxicity Studies
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2:2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| CUPRAC | cupric-reducing antioxidant |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | ferric-reducing antioxidant power |
| FLD | fluorescence detector |
| ESI-LC-DAD-MS | electrospray ionization source–liquid chromatography–diode array detector–mass spectrophotometry |
| NMR | nuclear magnetic resonance |
| PAC | procyanidin |
| TC-MeOH | Tectaria coadunata methanolic extract |
| TC-EtOAc | T. coadunata ethyl acetate extract |
| TC-H2O | T. coadunata water extract |
| GC-MS | gas chromatography–mass spectrophotometry |
| AChE | acetylcholinesterase |
| BuChE | butyrylcholinesterase |
| AD | Alzheimer’s disease |
| TLC | thin layer chromatography |
| TDDS | turbo detection data scanning |
| HSQC-DEPT | heteronuclear single quantum coherence spectroscopy–distortionless enhancement by polarization transfer |
| HMBC | heteronuclear multiple quantum coherence |
| COSY | correlation spectroscopy |
References
- Burlakoti, C.; Kunwar, R.M. Folk Herbal Medicines of Mahakali Watershed Area, Nepal. Med. Plants Nepal An Anthol. Contemp. Res. 2008, 187–193. [Google Scholar]
- Bhattarai, K.R.; Vetaas, O.R.; Grytnes, J.A.; Journal, S.; Mar, N.; Bhattarail, K.R.; Vetaas, O.R.; Grytnes, J.A. Fern Species Richness along a Central Himalayan Elevational Gradient, Nepal Fern species richness along a central Wg0T 111|Himalayan elevational gradient, Nepal. J. Biogeogr. 2018, 31, 389–400. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Raven, P.; Hong, D.Y. Pteridophytes. In Flora of China, 1st ed.; Science Press, Beijing, and Missouri Botanical Garden Press: St. Louis, MO, USA, 2013; Volume 2–3. [Google Scholar]
- Joshi, K.; Joshi, R.; Joshi, A.R. Indigenous knowledge and uses of medicinal plants in Macchegaun, Nepal. Indian, J. Tradit. Knowl. 2011, 10, 281–286. [Google Scholar]
- Subba, B.; Srivastav, C.; Kandel, R.C. Scientific validation of medicinal plants used by Yakkha community of Chanuwa VDC, Dhankuta, Nepal. Springerplus 2016, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dubal, K.; Kale, M. Studies on bioactive compounds of Tectaria coadunata(Wall. Ex Hook. & Grev.). J. Pharm. Clin. Res. 2019, 4–6. [Google Scholar]
- Pawar, S.G.; Kamble, S.Y.; Patil, S.R.; Sawant, P.S.; Singh, E.A. Preliminary phytochemical investigations of three species of traditional medicinal plants of Tribal regions of Maharashtra (India). Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 742–745. [Google Scholar]
- Sut, S.; Dall’Acqua, S.; Uysal, S.; Zengin, G.; Aktumsek, A.; Picot-Allain, C.; Mahomoodally, F. LC-MS, NMR fingerprint of Potentilla argentea and Potentilla recta extracts and their in vitro biopharmaceutical assessment. Ind. Crops Prod. 2019, 131, 125–133. [Google Scholar] [CrossRef]
- Sut, S.; Dall’Acqua, S.; Zengin, G.; Senkardes, I.; Bulut, G.; Cvetanović, A.; Stupar, A.; Mandić, A.; Picot-Allain, C.; Dogan, A.; et al. Influence of different extraction techniques on the chemical profile and biological properties of Anthemis cotula L.: Multifunctional aspects for potential pharmaceutical applications. J. Pharm. Biomed. Anal. 2019, 173, 75–85. [Google Scholar] [CrossRef]
- Ezaki-Furuichi, E.; Hayashi, K.; Nonaka, G.I.; Nishioka, I. Isolation and Structures of Procyanidins (Condensed Tannins) from Rhaphiolepis umbellata. Agric. Biol. Chem. 1986, 50, 2061–2067. [Google Scholar] [CrossRef]
- Mitchell, A.; Robertson, D.; Koh, E. Optimizing the Extraction of Procyanidins Oligomers through Decamer. Nutr. Food Sci. Int. J. 2017, 4, 1–7. [Google Scholar] [CrossRef][Green Version]
- Wang, C.M.; Hsu, Y.M.; Jhan, Y.L.; Tsai, S.J.; Lin, S.X.; Su, C.H.; Chou, C.H. Structure elucidation of procyanidins isolated from Rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules 2015, 20, 12787–12803. [Google Scholar] [CrossRef] [PubMed]
- Ugartondo, V.; Mitjans, M.; Touriño, S.; Torres, J.L.; Vinardell, M.P. Comparative antioxidant and cytotoxic effect of procyanidin fractions from grape and pine. Chem. Res. Toxicol. 2007, 20, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Ricardo da Silva, J.M.; Cheynier, V.; Souquet, J.M.; Moutounet, M.; Cabanis, J.C.; Bourzeix, M. Interaction of grape seedprocyanidins with various proteins in relation to wine fining. J. Sci. Food Agric. 1991, 57, 111–125. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; Verstraeten, S.V.; Fraga, C.G.; Oteiza, P.I. The interaction of flavonoids with membranes: Potential determinant of flavonoid antioxidant effects. Free Radic. Res. 2004, 38, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Lois, S.; Torres, J.L.; Medina, I. Inhibition of hemoglobin- and iron-promoted oxidation in fish microsomes by natural phenolics. J. Agric. Food Chem. 2006, 54, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Lin, J.T.; Lin, H.L.; Liao, P.L.; Wu, P.J.; Yang, D.J. Phenolic compositions and antioxidant properties of leaves of eight persimmon varieties harvested in different periods. Food Chem. 2019, 289, 74–83. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, L.; Li, W.; Zhang, S.; Luo, L.; Wang, J.; Sun, B. In vitro evaluation of the anti-digestion and antioxidant effects of grape seed procyanidins according to their degrees of polymerization. J. Funct. Foods 2018, 49, 85–95. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforsch. C. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord-Dr. 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Kumar, A.; Panda, G. Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018). Bioorganic Med. Chem. 2019, 27, 895–930. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wu, G.; Kang, D.; Zhou, Z.; Song, Y.; Liu, X.; Zhan, P. Contemporary medicinal-chemistry strategies for the discovery of selective butyrylcholinesterase inhibitors. Drug Discov. Today 2019, 24, 629–635. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Ji, H.F.; Zhang, H.Y. Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer’s disease. J. Mol. Struct. 2006, 767, 3–9. [Google Scholar] [CrossRef]
- Wang, J.; Ferruzzi, M.G.; Ho, L.; Blount, J.; Janle, E.M.; Gong, B.; Pan, Y.; Nagana Gowda, G.A.; Raftery, D.; Arrieta-Cruz, I.; et al. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J. Neurosci. 2012, 32, 5144–5150. [Google Scholar] [CrossRef]
- Faria, A.; Mateus, N.; Calhau, C. Flavonoid transport across blood-brain barrier: Implication for their direct neuroprotective actions. Nutr. Aging 2012, 1, 89–97. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De Freitas, V.; Mateus, N.; Calhau, C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell. Mol. Biol. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.O.; Romero, I.; Weksler, B.; De Freitas, V.; Mateus, N.; Calhau, C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011, 2, 39–44. [Google Scholar] [CrossRef]
- Dai, T.; Chen, J.; Li, Q.; Li, P.; Hu, P.; Liu, C.; Li, T. International Journal of Biological Macromolecules Investigation the interaction between procyanidin dimer and α-amylase : Spectroscopic analyses and molecular docking simulation. Int. J. Biol. Macromol. 2018, 113, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Kushibiki, N.; Inagaki, Y.; Kurokawa, M.; Kawabata, J. Astilbe thunbergii reduces postprandial hyperglycemia in a type 2 diabetes rat model via pancreatic alpha-amylase inhibition by highly condensed procyanidins. Biosci. Biotechnol. Biochem. 2017, 81, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Touriño, S.; Selga, A.; Jiménez, A.; Juliá, L.; Lozano, C.; Lizárraga, D.; Cascante, M.; Torres, J.L. Procyanidin fractions from pine (Pinus pinaster) bark: Radical scavenging power in solution, antioxidant activity in emulsion, and Antiproliferative effect in melanoma cells. J. Agric. Food Chem. 2005, 53, 4728–4735. [Google Scholar] [CrossRef]
- Lee, Y. Cancer chemopreventive potential of procyanidin. Toxicol. Res. 2017, 33, 273–282. [Google Scholar] [CrossRef]
- Taparia, S.; Khanna, A. Effect of Procyanidin-rich Extract from Natural Cocoa Powder on Cellular Viability, Cell Cycle Progression, and Chemoresistance in Human Epithelial Ovarian Carcinoma Cell Lines. Pharmacogn Mag. 2016, 12, 109–115. [Google Scholar]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant of Turkey. Afr. J. tradit Complem. 2014, 11, 481–488. [Google Scholar] [CrossRef]
Sample Availability: Extracts and plant powder are available from the authors. The samples are stored in Department of Pharmaceutical and Pharmacological Sciences, University of Padova. |
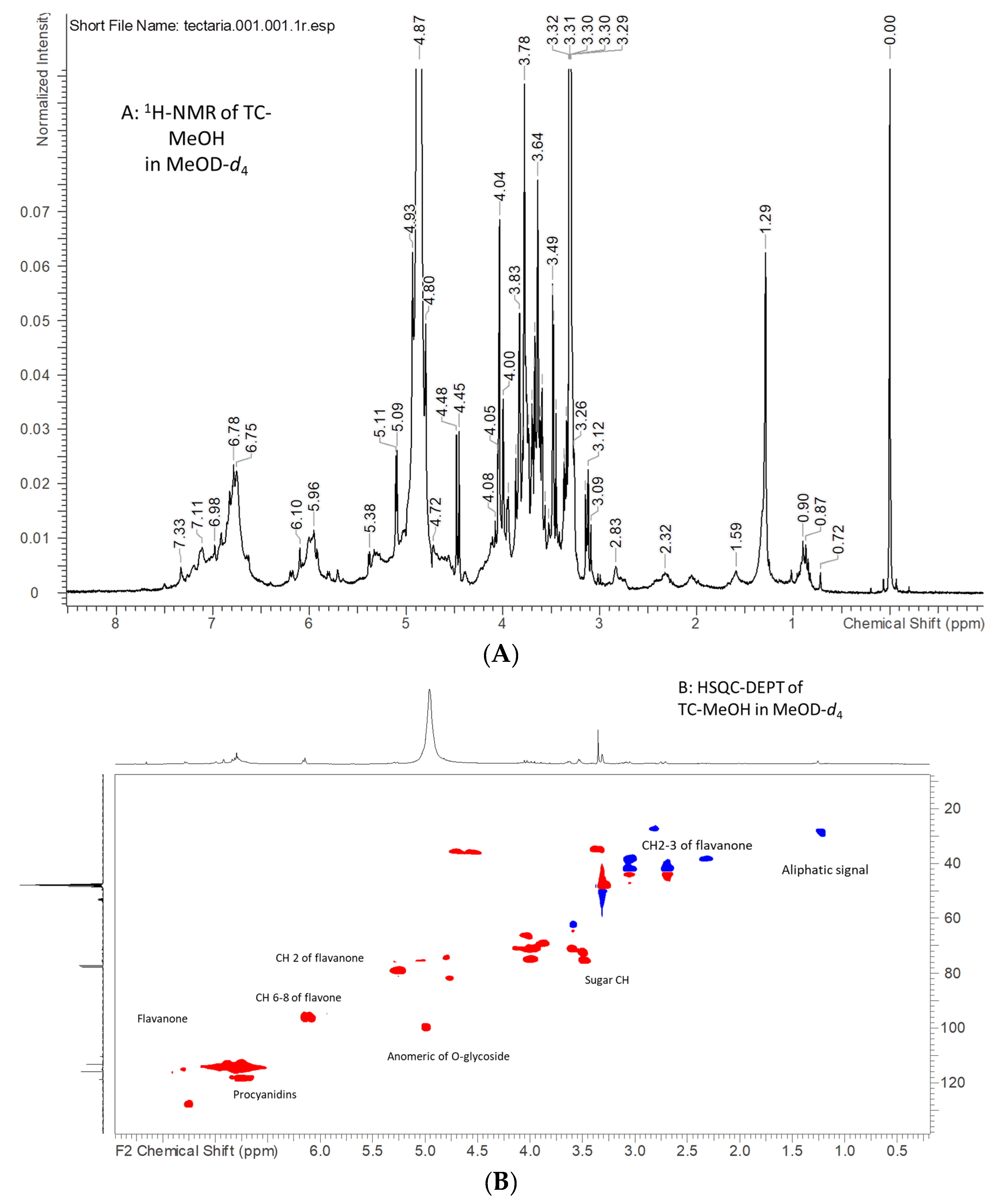

| TC-MeOH | |||
|---|---|---|---|
| δH | δC | Correlations | Assignments |
| 7.45 | 115.3 | 150.5, 125.3 | Aromatic phenol ring of procyanidin or tannin |
| 7.31 | 114.5 | Aromatic phenol ring of procyanidin or tannin | |
| 6.76 | 114.5 | 129.4, 118.7, 116.5 | Aromatic phenol ring of procyanidin or tannin |
| 7.25 | 127.7 | 156.8, 125.7, 79.5 | Flavanol moiety |
| 6.90–6.99 | 114.2–117.2 | 144.7, 118.5, 79.5 | Flavanol moiety |
| 6.16–6.15 | 94.5–95.8 | 196.3, 163.5, 103.5, 94.2 | Flavanol moiety position H-6/8 |
| 5.26 | 78.3 | 196.3, 128.5, 113.5 | Flavanol moiety CH position 2 |
| 2.47 dd | 196.3, 127.8, 79.5 | Flavanol moiety CH2 position 3 | |
| 3.11 dd | 196.3, 127.8, 79.5 | Flavanol moiety CH2 position 3 | |
| 5.01 | 98.9 | 163.4 | Anomeric proton of O-glycoside residue |
| 4.77 | 80.5 | Flavonol or procyanidin CH | |
| 3.50 | 74.1–72.2 | 98.6, 75.6 | Sugar residue CH |
| 3.62 | 71.3 | Sugar residue CH | |
| 3.86 | 68.8 | Sugar residue CH | |
| 4.00 | 70.0 | Sugar residue CH | |
| 4.05 | 74.6 | 70.0 | Sugar residue CH |
| 4.02 | 66.7 | 75.0 | Sugar residue CH |
| 2.32 | 37.3 | 172.6 | Organic acid CH2 |
| 1.25 | 28.6 | Aliphatic |
| TC-EtOAc | |||
|---|---|---|---|
| δH | δC | Correlations | Assignments |
| 7.28 | 126.5 | 144.5, 119.4 | aromatic phenol ring of procyanidin or tannin |
| 7.00 | 115.0 | 144.5, 120.0, 73.6 | catechin moiety H-2′ or H-6′ |
| 6.80 | 118.2 | 144.0, 129.4, 116.5 | catechin H-5′ |
| 5.98–6.01 | 95.0–93.0 | 156.0, 101.0, 93 | H-6/8 of catechin units |
| 5.31 | 79.3 | H-2 of upper unit of catechin/epicatechin moieties | |
| 4.81 | 74.2 | 67.5, 113.3, 119.8, 129.5 | H-2 of lower units of catechin/epicatechin moieties |
| 4.07 | 74.7 | H-2 of lower units of catechin/epicatechin moieties | |
| 3.84 | 68.9 | 101.5, 37.4, 38.5 | H-3 of upper units of catechin/epicatechin moieties |
| 3.31 | 47.6 | C-4 of upper units of catechin/epicatechin moieties | |
| 3.14–2.72 | 42.6 | C-4 of terminal units | |
| 3.05–2.39 | 37.4 | C-4 terminal units |
| Tr | [M − H]− | Identification | Fragmentation | UV s (nm) | mg/g in TC-MeOH | mg/g in TC-EtOAc | mg/g in TC-H2O |
|---|---|---|---|---|---|---|---|
| 14.6 | 447 | Naringenin-7-O-glucuronide | MS 2 [447]: 271(100) MS 3 [271]: 151(100)-175(25) MS 4 [151]: 107(100) | 200, 280 | * | 0.24 ± 0.06 | 0.006 ± 0.0003 |
| 16.0 | 463 | Eriodictyol-7-O-glucuronide | MS 2 [463]: 287(100) MS 3 [287]: 151(100) MS 4 [151]: 107(100) | 230, 280 | 0.57 ± 0.09 | 7.64 ± 0.8 | 0.48 ± 0.06 |
| 17.9 | 847 | A-type proanthocyanidin trimer with one unit of (epi)afzelechin Isomer 1 | MS2 [847]: 711(98)-559(100)-327(7) | 280 | 0.95 ± 0.06 | 7.40 ± 0.4 | 0.06 ± 0.003 |
| 19.1 | 847 | A-type proanthocyanidin trimer with one unit of (epi)afzelechin Isomer 2 | MS 2 [847]: 711(92)-559(100) MS 3 [711]: 585(100)-559(75)-423(60) MS 4 [585]: 423(100) MS 3 [559]: 389(100) MS 4 [389]: 362(50)-345(100)-273(3) | 280 | 8.96 ± 0,45 | 11.7 ± 2.1 | 0.05 ± 0.007 |
| 19.5 | 577 | B-type procyanidin dimer | MS 2 [877]: 425(100)-407(60)-289(30) MS 3 [425]: 407(100)-273(10)-281(8) MS 4 [407]: 389(20)-339(30)-285(100)-281(98)-256(40)-269(20)-243(22)-213(10) | 280 | 0.39 ± 0.07 | 11.13 ± 0.3 | 0.48 ± 0.02 |
| 20.3 | 461 | Luteolin-7-O-glucuronide | MS 2 [461]: 285(100) MS 3 [285]: 257(45)-243(25)-241(90)-213(50)-199(100)-175(90)-151(35) | 225, 280 | 2.13 ± 0.2 | 16.4 ± 1.2 | 1.25 ± 0.07 |
| 20.8 | 863 | A-type procyanidin trimer | MS 2 [863]: 711(100)-573(50)-451(70)-411(70) MS 3 [711]: 559(100)-407(27) MS 4 [559]: 415(90)-327(60)-255(100) | 280 | 9.73 ± 0, 91 | 38.69 ± 2.6 | 2.58 ± 0.21 |
| 22.4 | 1135 | A-type proanthocyanidin tetramer with one unit of (epi)afzelechin | MS 2 [1135]: 999(70)-847(100)-707(70)-634(58) | 280 | 25.2 ± 0.17 | 5.70 ± 1.8 | 0.09 ± 0.004 |
| 25.1 | 1151 | A-type procyanidin tetramer | MS 2 [1151]: 1025(60)-863(100)-709(60)-573(25) | 280 | 6.5 ± 0.76 | 0.44 ± 0.8 | * |
| 25.3 | 1424 [M − 2H]2− | B-type proanthocyanidin decamer with two units of (epi)afzelechin | MS 2 [1424]: 1271(100) | 280 | 0.69 ± 0.09 | 4.74 ± 0.1 | 0.02 ± 0.005 |
| 26.1 | 1151 [M − 2H]2− | A-type procyanidin octamer | MS 2 [1151]: 863(42)-777(55) | 280 | 8.97 ± 0.06 | 0.62 ± 0.05 | 0.09 ± 0.03 |
| 26.9 | 1438 [M−2H]2− | A-type procyanidin decamer with two A bonds | MS 2 [1438]: 1191(100) | 280 | 11.4 ± 0.1 | 0.61 ± 0.04 | 0.013 ± 0.001 |
| 28.5 | 720 [M − 2H]2− | B-type procyanidin pentamer | MS 2 [720]: 643(100) MS 3 [643]: 559(65)-407(25) | 280 | 5.67 ± 0.1 | 5.97 ± 0.08 | 0.09 ± 0.006 |
| 28.6 | 719 [M − 2H]2− | A-type procyanidin pentamer | MS 2 [719]: 567(50)-451(20) | 280 | 10.8 ± 1.1 | 0.99 ± 0.09 | 0.07 ± 0.007 |
| 29.8 | 1440 [M − 2H]2− | B-type procyanidin decamer | MS 2 [1440]: 1313(100)-961(55)-817(70) | 280 | 0.51 ± 0.2 | 2.79 ± 0.06 | 0.14 ± 0.004 |
| 33.0 | 864 [M − 2H]2− | B-type procyanidin esamer | MS 2 [864]: 779(90)-575(70)-532(75)-411(100)-289(20) | 280 | 5.85 ± 0.4 | 5.44 ± 0.1 | 0.15 ± 0.003 |
| 34.3 | 1008 [M − 2H]2− | B-type procyanidin heptamer | MS 2 [1008]: 777(55) | 280 | 9.87 ± 1.3 | 1.78 ± 0.03 | 0.08 ± 0.005 |
| Sample | Total Flavonoid (mg/g) | Total PAC (mg/g) | PAC Dimers (mg/g) | PAC Trimers (mg/g) | PAC Tetramers and Polymers (mg/g) |
|---|---|---|---|---|---|
| TC-MeOH | 2.70 ± 0.05 | 105.49 ± 0.15 | 0.39 ± 0.01 | 19.64 ± 0.13 | 85.46 ± 0.16 |
| TC-EtOAc | 24.28 ± 0.15 | 98.00 ± 0.12 | 11.13 ± 0.15 | 57.79 ± 0.15 | 29.08 ± 0.15 |
| TC-H2O | 1.74 ± 0.05 | 3.91 ± 0.05 | 0.48 ± 0.01 | 2.69 ± 0.05 | 0.74 ± 0.02 |
| Samples | Total Phenolic Content (mg GAE/g) | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Metal chelating (mg EDTAE/g) | Phosphomolybdenum (mmol TE/g) |
|---|---|---|---|---|---|---|---|
| TC-EtOAc | 276.70 ± 2.58 a | 948.59 ± 30.92 a | 1661.21 ± 9.01 a | 1510.63 ± 31.55 a | 931.18 ± 17.74 a | na | 6.32 ± 0.41 a |
| TC-H2O | 235.85 ± 1.82 b | 933.97 ± 12.12 a | 1269.30 ± 21.75 b | 1108.66 ± 4.44 b | 713.07 ± 11.98 b | 6.26 ± 0.73 a | 6.25 ± 0.18 a |
| TC-MeOH | 234.30 ± 0.99 b | 762.62 ± 34.65 b | 1097.10 ± 14.02 c | 1089.99 ± 6.42 b | 645.59 ± 4.83 c | 2.61 ± 0.34 b | 5.70 ± 0.59 a |
| Samples | AChE Inhibition (mg GALAE/g) | BChE Inhibition (mg GALAE/g) | Tyrosinase Inhibition (mg KAE/g) | Amylase Inhibition (mmol ACAE/g) | Glucosidase Inhibition (mmol ACAE/g) |
|---|---|---|---|---|---|
| TC-EtOAc | 6.22 ± 0.06 a | 9.82 ± 0.68 a | 153.89 ± 1.61 a | 1.50 ± 0.02 a | 5.46 ± 0.05 a |
| TC-H2O | 1.35 ± 0.03 c | 1.70 ± 0.67 c | 66.85 ± 1.22 c | 0.42 ± 0.04 c | 5.48 ± 0.01 a |
| TC-MeOH | 5.58 ± 0.10 b | 6.31 ± 0.71 b | 149.41 ± 0.96 b | 1.04 ± 0.05 b | 5.48 ± 0.01 a |
| Samples | 2008 | BxPC3 |
|---|---|---|
| TC-EtOAc | 28, 7 | 12, 5 |
| TC-H2O | >50 | >50 |
| TC-MeOH | >50 | >50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, S.S.; Sut, S.; Barbon Di Marco, S.; Zengin, G.; Gandin, V.; De Franco, M.; Pant, D.R.; Mahomoodally, M.F.; Dall’Acqua, S.; Rajbhandary, S. Phytochemical Fingerprinting and In Vitro Bioassays of the Ethnomedicinal Fern Tectaria coadunata (J. Smith) C. Christensen from Central Nepal. Molecules 2019, 24, 4457. https://doi.org/10.3390/molecules24244457
Shrestha SS, Sut S, Barbon Di Marco S, Zengin G, Gandin V, De Franco M, Pant DR, Mahomoodally MF, Dall’Acqua S, Rajbhandary S. Phytochemical Fingerprinting and In Vitro Bioassays of the Ethnomedicinal Fern Tectaria coadunata (J. Smith) C. Christensen from Central Nepal. Molecules. 2019; 24(24):4457. https://doi.org/10.3390/molecules24244457
Chicago/Turabian StyleShrestha, Shyam Sharan, Stefania Sut, Serena Barbon Di Marco, Gokhan Zengin, Valentina Gandin, Michele De Franco, Deepak Raj Pant, Mohamad Fawzi Mahomoodally, Stefano Dall’Acqua, and Sangeeta Rajbhandary. 2019. "Phytochemical Fingerprinting and In Vitro Bioassays of the Ethnomedicinal Fern Tectaria coadunata (J. Smith) C. Christensen from Central Nepal" Molecules 24, no. 24: 4457. https://doi.org/10.3390/molecules24244457
APA StyleShrestha, S. S., Sut, S., Barbon Di Marco, S., Zengin, G., Gandin, V., De Franco, M., Pant, D. R., Mahomoodally, M. F., Dall’Acqua, S., & Rajbhandary, S. (2019). Phytochemical Fingerprinting and In Vitro Bioassays of the Ethnomedicinal Fern Tectaria coadunata (J. Smith) C. Christensen from Central Nepal. Molecules, 24(24), 4457. https://doi.org/10.3390/molecules24244457










