Potential Risk to Pollinators from Nanotechnology-Based Pesticides
Abstract
:1. Introduction
2. Bees as Environmental Sentinels
3. Properties and Benefits of Nanotechnology-Based Pesticides
4. Size as an Arbitrary Criterion for Regulation of Nano-Agrotechnology
5. Risks Posed by Particulate Contaminants on Pollinators
6. NBP Uncertainties for Pollinators
7. Implications: The Need to Consider NBP Formulations in Risk Assessment
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calderone, N.W. Insect Pollinated Crops, Insect Pollinators and US Agriculture: Trend Analysis of Aggregate Data for the Period 1992–2009. PLoS ONE 2012, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. Long-Term Global Trends in Crop Yield and Production Reveal No Current Pollination Shortage but Increasing Pollinator Dependency. Curr. Biol. 2008, 18, 1572–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 2012, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Vanengelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [Green Version]
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Sheppard, W.S. Honey Bee Exposure to Pesticides: A Four-Year Nationwide Study. Insects 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Mullin, C.A.; Chen, J.; Fine, J.D.; Frazier, M.T.; Frazier, J.L. The formulation makes the honey bee poison. Pestic. Biochem. Phys. 2015, 120, 27–35. [Google Scholar] [CrossRef]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J.B. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kookana, R.S.; Boxall, A.B.A.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudhry, Q.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding Principles for Regulatory Evaluation of Environmental Risks. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorp, R.W. The collection of pollen by bees. Plant Syst. Evol. 2000, 222, 211–223. [Google Scholar] [CrossRef]
- Amador, G.J.; Matherne, M.; Waller, D.; Mathews, M.; Gorb, S.N.; Hu, D.L. Honey bee hairs and pollenkitt are essential for pollen capture and removal. Bioinspir. Biomim. 2017, 12, 026015. [Google Scholar] [CrossRef] [PubMed]
- Doke, M.A.; McGrady, C.M.; Otieno, M.; Grozinger, C.M.; Frazier, M. Colony Size, Rather Than Geographic Origin of Stocks, Predicts Overwintering Success in Honey Bees (Hymenoptera: Apidae) in the Northeastern United States. J. Econ. Entomol. 2019, 112, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Couvillon, M.; Riddell Pearce, F.C.; Accleton, C.; Fensome, K.A.; Quah, S.K.L.; Taylor, E.L.; Ratnieks, F.L.W. Honey bee foraging distance depends on month and forage type. Apidologie 2015, 46, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.W.; Kookana, R.S.; Smith, N.E.; Kah, M.; Doolette, C.L.; Reeves, P.T.; Lovell, W.; Anderson, D.J.; Turney, T.W.; Navarro, D.A. Ecological Risk Assessment of Nano-enabled Pesticides: A Perspective on Problem Formulation. J. Agric. Food Chem. 2018, 66, 6480–6486. [Google Scholar] [CrossRef] [Green Version]
- Sponsler, D.B.; Johnson, R.M. Mechanistic modeling of pesticide exposure: The missing keystone of honey bee toxicology. Environ. Toxicol. Chem. 2017, 36, 871–881. [Google Scholar] [CrossRef]
- Benuszak, J.; Laurent, M.; Chauzat, M.P. The exposure of honey bees (Apis mellifera; Hymenoptera: Apidae) to pesticides: Room for improvement in research. Sci. Total Environ. 2017, 583, 423–438. [Google Scholar] [CrossRef]
- Fisher, R.-L. Bee; Lippert, J., Ed.; Princeton Architectural Press: New York, NY, USA, 2010. [Google Scholar]
- Clarke, D.; Morley, E.; Robert, D. The bee, the flower, and the electric field: Electric ecology and aerial electroreception. J. Comp. Physiol. A 2017, 203, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. The role of electrostatic forces in pollination. Plant Syst. Evol. 2000, 222, 133–142. [Google Scholar] [CrossRef]
- Clarke, D.; Whitney, H.; Sutton, G.; Robert, D. Detection and Learning of Floral Electric Fields by Bumblebees. Science 2013, 340, 66–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greggers, U.; Koch, G.; Schmidt, V.; Durr, A.; Floriou-Servou, A.; Piepenbrock, D.; Gopfert, M.C.; Menzel, R. Reception and learning of electric fields in bees. Proc. R. Soc. Lond. B Biol. Sci. 2013, 280, 1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, G.P.; Clarke, D.; Morley, E.L.; Robert, D. Mechanosensory hairs in bumblebees (Bombus terrestris) detect weak electric fields. Proc. Natl. Acad. Sci. USA 2016, 113, 7261–7265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, L.J.; Muir, R.G. Pollen and Bee Bread. Am. Bee J. 2013, 153, 727–729. [Google Scholar]
- Pochi, D.; Biocca, M.; Fanigliulo, R.; Pulcini, P.; Conte, E. Potential Exposure of Bees, Apis mellifera L., to Particulate Matter and Pesticides Derived from Seed Dressing During Maize Sowing. Bull. Environ. Contam. Toxicol. 2012, 89, 354–361. [Google Scholar] [CrossRef]
- Kevan, P.G. Pollinators as bioindicators of the state of the environment: Species, activity and diversity. Agric. Ecosyst. Environ. 1999, 74, 373–393. [Google Scholar] [CrossRef]
- Skorbilowicz, E.; Skorbilowicz, M.; Ciesluk, I. Bees as Bioindicators of Environmental Pollution with Metals in an Urban Area. J. Ecol. Eng. 2018, 19, 229–234. [Google Scholar] [CrossRef]
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; Larhantec, M.; Delbac, F.; Pouliquen, H. Bees, honey and pollen as sentinels for lead environmental contamination. Environ. Pollut. 2012, 170, 254–259. [Google Scholar] [CrossRef]
- Matin, G.; Kargar, N.; Buyukisik, H.B. Bio-monitoring of cadmium, lead, arsenic and mercury in industrial districts of Izmir, Turkey by using honey bees, propolis and pine tree leaves. Ecol. Eng. 2016, 90, 331–335. [Google Scholar] [CrossRef]
- Panatto, D.; Gasparini, R.; Lai, P.; Rovatti, P.; Gallelli, G. Long-tenn decline of (CS)-C-137 concentration in honey in the second decade after the Chernobyl accident. Sci. Total Environ. 2007, 382, 147–152. [Google Scholar] [CrossRef]
- Malhat, F.M.; Haggag, M.N.; Loutfy, N.M.; Osman, M.A.M.; Ahmed, M.T. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 2015, 120, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, R.C.; Queiroz, S.C.D.; da Luz, C.F.P.; Porto, R.S.; Rath, S. Bee pollen as a bioindicator of environmental pesticide contamination. Chemosphere 2016, 163, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Ridding, L.; Freeman, S.N.; Pereira, M.G.; Sleep, D.; Redhead, J.; Aston, D.; Carreck, N.L.; Shore, R.F.; Bullock, J.M.; et al. Neonicotinoid residues in UK honey despite European Union moratorium. PLoS ONE 2018, 13, e0189681. [Google Scholar] [CrossRef]
- Bogdanov, S. Contaminants of bee products. Apidologie 2006, 37, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, D.; Gattavecchia, E.; Ghini, S.; Porrini, C.; Celli, G.; Mercuri, A.M. Honey-Bees and Their Products as Indicators of Environmental Radioactive Pollution. J. Radioan. Nucl. Chem. Ar. 1990, 141, 427–436. [Google Scholar] [CrossRef]
- Conti, M.E.; Botre, F. Honeybees and their products as potential bioindicators of heavy metals contamination. Environ. Monit. Assess. 2001, 69, 267–282. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mozafari, A.A.; Bahmani, R.; Shokri, K. Use of Honeybees as Bio-Indicators of Environmental Pollution in the Kurdistan Province of Iran. J. Apic. Sci. 2012, 56, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Barganska, Z.; Slebioda, M.; Namiesnik, J. Honey bees and their products: Bioindicators of environmental contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-Garcia, J.; Garcia-Martin, S.; Pena-Crecente, R.M. The use of honeybees and honey as environmental bioindicators for metals and radionuclides: A review. Environ. Rev. 2017, 25, 463–480. [Google Scholar] [CrossRef]
- Smith, K.E.; Weis, D.; Amini, M.; Shiel, A.E.; Lai, V.W.M.; Gordon, K. Honey as a biomonitor for a changing world. Nat. Sustain. 2019, 2, 223–232. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.F.; Hasan, M.K.; Ahammed, G.J.; Li, M.Q.; Yin, H.Q.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Yi, Z.; Hussain, H.I.; Feng, C.; Sun, D.; She, F.; Rookes, J.E.; Cahill, D.M.; Kong, L. Functionalized Mesoporous Silica Nanoparticles with Redox-Responsive Short-Chain Gatekeepers for Agrochemical Delivery. ACS Appl. Mater. Interfaces 2015, 7, 9937–9946. [Google Scholar] [CrossRef]
- Grillo, R.; Pereira, A.E.S.; Nishisaka, C.S.; de Lima, R.; Oehlke, K.; Greiner, R.; Fraceto, L.F. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: An environmentally safer alternative for weed control. J. Hazard. Mater. 2014, 278, 163–171. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Qian, W.Y.; Sun, D.M.; Zhu, R.R.; Du, X.L.; Liu, H.; Wang, S.L. pH-sensitive strontium carbonate nanoparticles as new anticancer vehicles for controlled etoposide release. Int. J. Nanomed. 2012, 7, 5781–5792. [Google Scholar]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharmaceut. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef]
- CIPAC. Formulation Codes. Available online: https://www.cipac.org/index.php/methods-publications/further-information/formulation-codes (accessed on 20 October 2019).
- Cox, C.; Surgan, M. Unidentified inert ingredients in pesticides: Implications for human and environmental health. Environ. Health. Perspect. 2006, 114, 1803–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.M.; Beestman, G.B. Recently patented and commercialized formulation and adjuvant technology. Crop. Prot. 2007, 26, 320–327. [Google Scholar] [CrossRef]
- Bleeker, E.A.J.; de Jong, W.H.; Geertsma, R.E.; Groenewold, M.; Heugens, E.H.W.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.P.; et al. Considerations on the EU definition of a nanomaterial: Science to support policy making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Stander, L.; Theodore, L. Environmental implications of nanotechnology—An update. Int. J. Environ. Res. Public Health 2011, 8, 470–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Res 2018, 7, 376. [Google Scholar] [CrossRef] [Green Version]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, 2140. [Google Scholar]
- Borsuk, G.; Paleolog, J.; Olszewski, K.; Strachecka, A. Laboratory assessment of the effect of nanosilver on longevity, sugar syrup ingestion, and infection of honeybees with Nosema spp. Med. Weter. 2013, 69, 730–732. [Google Scholar]
- Culha, M.; Kalay, S.; Sevim, E.; Pinarbas, M.; Bas, Y.; Akpinar, R.; Karaoglu, S.A. Biocidal properties of maltose reduced silver nanoparticles against American foulbrood diseases pathogens. Biometals 2017, 30, 893–902. [Google Scholar] [CrossRef]
- Reitmayer, C.; Newman, T.A.; Girling, R.; Farthing, E. Diesel Exhaust Pollution Affects Learning Abilities and Leads to an Altered Stress Response in the Cns of the Honey Bee (Apis mellifera). Glia 2013, 61, S72. [Google Scholar]
- Girling, R.D.; Lusebrink, I.; Farthing, E.; Newman, T.A.; Poppy, G.M. Diesel exhaust rapidly degrades floral odours used by honeybees. Sci. Rep. 2013, 3, 2779. [Google Scholar] [CrossRef] [PubMed]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef] [PubMed]
- Girolami, V.; Marzaro, M.; Vivan, L.; Mazzon, L.; Greatti, M.; Giorio, C.; Marton, D.; Tapparo, A. Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication. J. Appl. Entomol. 2012, 136, 17–26. [Google Scholar] [CrossRef]
- Cutler, G.C.; Scott-Dupree, C.D.; Drexler, D.M. Honey bees, neonicotinoids and bee incident reports: The Canadian situation. Pest. Manag. Sci. 2014, 70, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Guimarães, F.; Duque, L.; Noronha, F.; Abreu, I. Characterisation of particulate matter on airborne pollen grains. Environ. Pollut. 2015, 206, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.J.; Lehner, Y.; Kunzmann, M.R. Pesticides and Honey Bees—Danger of Microencapsulated Formulations. Z. Naturforsch. C 1979, 34, 153–156. [Google Scholar] [CrossRef]
- Dahl, G.H.; Lowell, J.R. The Effect of Microencapsulated Pesticides on Non-Target Insects. Abstr. Pap. Am. Chem. Soc. 1983, 186, 53. [Google Scholar]
- Johansen, C.A.; Mayer, D.F. Pollinator Protection, A Bee & Pesticide Handbook; Wicwas Press: Cheshire, CT, USA, 1990; p. 15. 214 p. [Google Scholar]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial Activity of Nanosilver Ions and Particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Erickson, B.E. Court reverses U.S. approval of nanosilver pesticide. C & EN 2017, 95, 15. [Google Scholar]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Marco, G.; Bo, X. Air Quality Legislation and Standards in the European Union: Background, Status and Public Participation. Adv. Clim. Chang. Res. 2013, 4, 50–59. [Google Scholar] [CrossRef]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta 2016, 1860, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- Phogat, N.; Khan, S.A.; Shankar, S.; Ansary, A.A.; Uddin, I. Fate of inorganic nanoparticles in agriculture. Adv. Mater. Lett. 2016, 7, 3–12. [Google Scholar] [CrossRef]
- Firdaus, M.A.M.; Agatz, A.; Hodson, M.E.; Al-Khazrajy, O.S.A.; Boxall, A.B.A. Fate, Uptake, and Distribution of Nanoencapsulated Pesticides in Soil-Earthworm Systems and Implications for Environmental Risk Assessment. Environ. Toxicol. Chem. 2018, 37, 1420–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servin, A.D.; White, J.C. Nanotechnology in agriculture: Next steps for understanding engineered nanoparticle exposure and risk. NanoImpact. 2016, 1, 9–12. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Responses of terrestrial arthropods to air pollution: A meta-analysis. Environ. Sci. Pollut. R. 2010, 17, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Negri, I.; Mavris, C.; Di Prisco, G.; Caprio, E.; Pellecchia, M. Honey Bees (Apis mellifera, L.) as Active Samplers of Airborne Particulate Matter. PLoS ONE 2015, 10, e0132491. [Google Scholar] [CrossRef] [Green Version]
- Pellecchia, M.; Negri, I. Particulate matter collection by honey bees (Apis mellifera, L.) near to a cement factory in Italy. PeerJ 2018, 6, e5322. [Google Scholar] [CrossRef] [Green Version]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Dubey, R.; Shami, T.C.; Rao, K.U.B. Microencapsulation Technology and Applications. Def. Sci. J. 2009, 59, 82–95. [Google Scholar]
- Slattery, M.; Harper, B.; Harper, S. Pesticide Encapsulation at the Nanoscale Drives Changes to the Hydrophobic Partitioning and Toxicity of an Active Ingredient. Nanomater-Basel 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Singh, S.K.; Bajpai, J.; Bajpai, A.K. Controlled pesticide release from biodegradable polymers. Cent. Eur. J. Chem. 2014, 12, 453–469. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Hwang, I.-C.; Park, J.-W.; Park, H.-J. Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest. Manag. Sci. 2012, 68, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Peteu, S.F.; Oancea, F.; Sicuia, O.A.; Constantinescu, F.; Dinu, S. Responsive Polymers for Crop Protection. Polymers-Basel 2010, 2, 229–251. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Chin, C.-P.; Wu, H.-S.; Wang, S.S. New Approach to Pesticide Delivery Using Nanosuspensions: Research and Applications. Ind. Eng. Chem. Res. 2011, 50, 7637–7643. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Sun, C.; Zhao, X.; Cui, B. Construction and evaluation of controlled-release delivery system of Abamectin using porous silica nanoparticles as carriers. Nanoscale Res. Lett. 2014, 9, 655. [Google Scholar] [CrossRef] [Green Version]
- Pérez-de-Luque, A.; Hermosín, M.C. Nanotechnology and its Use in Agriculture. In Bio-Nanotechnology; Bagchi, D., Ed.; Blackwell Publishing Ltd.: Chichester, UK, 2013; pp. 383–398. [Google Scholar]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Lagaly, G. Pesticide–clay interactions and formulations. Appl. Clay Sci. 2001, 18, 205–209. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T.; Williams, M.A.; Horner, E.; Nel, A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. Pract. 2016, 138, 386–396. [Google Scholar]
- Wang, L.; Li, X.; Zhang, G.; Dong, J.; Eastoe, J. Oil-in-water nanoemulsions for pesticide formulations. J. Colloid Interface Sci. 2007, 314, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of Knowledge, Environmental Fate, and Exposure Modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Burgett, M.; Fisher, G.C. Recovery of Penncap-M from Foraging Honey Bees and Pollen Storage-Cells. Environ. Entomol. 1980, 9, 430–431. [Google Scholar] [CrossRef]
- Hooven, L.; Sagili, R.; Johansen, E. How to Reduce Bee Poisoning from Pesticides. Extension; Oregon State University Extension Service: Corvallis, OR, USA, 2013. [Google Scholar]
- Krupke, C.H.; Holland, J.D.; Long, E.Y.; Eitzer, B.D. Planting of neonicotinoid-treated maize poses risks for honey bees and other non-target organisms over a wide area without consistent crop yield benefit. J. Appl. Ecol. 2017, 54, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Limay-Rios, V.; Forero, L.G.; Xue, Y.; Smith, J.; Baute, T.; Schaafsma, A. Neonicotinoid insecticide residues in soil dust and associated parent soil in fields with a history of seed treatment use on crops in southwestern Ontario. Environ. Toxicol. Chem. 2016, 35, 303–310. [Google Scholar] [CrossRef]
- Nuyttens, D.; Devarrewaere, W.; Verboven, P.; Foqué, D. Pesticide-laden dust emission and drift from treated seeds during seed drilling: A Review. Pest. Manag. Sci. 2013, 69, 564–575. [Google Scholar] [CrossRef]
- Clausnitzer, H.; Singer, M.J. Respirable-Dust Production from Agricultural Operations in the Sacramento Valley, California. J. Environ. Qual. 1996, 25, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Rogge, W.F.; Medeiros, P.M.; Simoneit, B.R.T. Organic marker compounds in surface soils of crop fields from the San Joaquin Valley fugitive dust characterization study. Atmos. Environ. 2007, 41, 8183–8204. [Google Scholar] [CrossRef]
- Aneja, V.P.; Schlesinger, W.H.; Nyogi, D.; Jennings, G.; Gilliam, W.; Knighton, R.E.; Duke, C.S.; Blunden, J.; Krishnan, S. Emerging national research needs for agricultural air quality. Eos Trans. Am. Geophys. Union 2006, 87, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, J.D.; Chamecki, M.; Roulston, T.A.; Chen, B.; Pratt, K.R. Air pollutants degrade floral scents and increase insect foraging times. Atmos. Environ. 2016, 141, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.S.; Masciocchi, M.; Villacide, J.M.; Huerta, G.; Daneri, L.; Bruchhausen, A.; Rozas, G.; Corley, J.C. Ashes in the air: The effects of volcanic ash emissions on plant-pollinator relationships and possible consequences for apiculture. Apidologie 2013, 44, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Lighthart, B.; Prier, K.; Loper, G.M.; Bromenshenk, J. Bees scavenge airborne bacteria. Microb. Ecol. 2000, 39, 314–321. [Google Scholar] [PubMed]
- Lighthart, B.; Prier, K.R.S.; Bromenshenk, J.J. Flying honey bees adsorb airborne viruses. Aerobiologia 2005, 21, 147–149. [Google Scholar] [CrossRef]
- Shaw, D.E. The Incidental Collection of Fungal Spores by Bees and the Collection of Spores in Lieu of Pollen. Bee World 1990, 71, 158–176. [Google Scholar] [CrossRef]
- Pattemore, D.E.; Goodwin, R.M.; McBrydie, H.M.; Hoyte, S.M.; Vanneste, J.L. Evidence of the role of honey bees (Apis mellifera) as vectors of the bacterial plant pathogen Pseudomonas syringae. Australas. Plant Pathol. 2014, 43, 571–575. [Google Scholar] [CrossRef]
- Mommaerts, V.; Smagghe, G. Entomovectoring in plant protection. Arthropod-Plant Interact. 2011, 5, 81–95. [Google Scholar] [CrossRef]
- Meredith, A.N.; Harper, B.; Harper, S.L. The influence of size on the toxicity of an encapsulated pesticide: A comparison of micron- and nano-sized capsules. Environ. Int. 2016, 86, 68–74. [Google Scholar] [CrossRef]
- Farooq, M.; Walker, T.W.; Heintschel, B.P.; Hoffmann, W.C.; Fritz, B.K.; Smith, V.L.; Robinson, C.A.; English, T. Impact of electrostatic and conventional sprayers characteristics on dispersion of barrier spray. J. Am. Mosq. Control. Assoc. 2010, 26, 422–429. [Google Scholar] [CrossRef]
- Andriessen, R.; Snetselaar, J.; Suer, R.A.; Osinga, A.J.; Deschietere, J.; Lyimo, I.N.; Mnyone, L.L.; Brooke, B.D.; Ranson, H.; Knols, B.G.J.; et al. Electrostatic coating enhances bioavailability of insecticides and breaks pyrethroid resistance in mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 12081–12086. [Google Scholar] [CrossRef] [Green Version]
- Latheef, M.A.; Carlton, J.B.; Kirk, I.W.; Hoffmann, W.C. Aerial electrostatic-charged sprays for deposition and efficacy against sweet potato whitefly (Bemisia tabaci) on cotton. Pest. Manag. Sci. 2009, 65, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Stelinski, L.L.; Gut, L.J. Mating Behaviors of Cydia pomonella (Lepidoptera: Tortricidae) as Influenced by Sex Pheromone in Electrostatic Powder. J. Econ. Entomol. 2010, 103, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Nansen, C.; MacDonald, K.M.; Rogers, C.D.; Thomas, M.; Poppy, G.M.; Baxter, I.H. Effects of sex pheromone in electrostatic powder on mating behaviour by Lobesia botrana males. J. Appl. Entomol. 2007, 131, 303–310. [Google Scholar] [CrossRef]
- Davis, B.N.K.; Williams, C.T. Buffer zone widths for honeybees from ground and aerial spraying of insecticides. Environ. Pollut. 1990, 63, 247–259. [Google Scholar] [CrossRef]
- Long, E.Y.; Krupke, C.H. Non-cultivated plants present a season-long route of pesticide exposure for honey bees. Nat. Commun. 2016, 7, 11629. [Google Scholar] [CrossRef]
- Damalas, C.A. Pesticide Drift: Seeking Reliable Environmental Indicators of Exposure Assessment. In Environmental Indicators; Armon, H.R., Hänninen, O., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2015; pp. 251–261. [Google Scholar]
- Teske, M.E.; Thistle, H.W.; Schou, W.C.; Miller, P.C.H.; Strager, J.M.; Richardson, B.; Ellis, M.C.B.; Barry, J.W.; Twardus, D.B.; Thompson, D.G. A Review of Computer Models for Pesticide Deposition Prediction. Trans. ASABE 2011, 54, 789–801. [Google Scholar] [CrossRef]
- Hilz, E.; Vermeer, A.W.P. Spray drift review: The extent to which a formulation can contribute to spray drift reduction. Crop. Prot. 2013, 44, 75–83. [Google Scholar] [CrossRef]
- Degrendele, C.; Okonski, K.; Melymuk, L.; Landlová, L.; Kukučka, P.; Audy, O.; Kohoutek, J.; Čupr, P.; Klánová, J. Pesticides in the atmosphere: A comparison of gas-particle partitioning and particle size distribution of legacy and current-use pesticides. Atmos. Chem. Phys. 2016, 16, 1531–1544. [Google Scholar] [CrossRef] [Green Version]
- Coscollà, C.; Muñoz, A.; Borrás, E.; Vera, T.; Ródenas, M.; Yusà, V. Particle size distributions of currently used pesticides in ambient air of an agricultural Mediterranean area. Atmos. Environ. 2014, 95, 29–35. [Google Scholar] [CrossRef]
- Coscollà, C.; Yahyaoui, A.; Colin, P.; Robin, C.; Martinon, L.; Val, S.; Baeza-Squiban, A.; Mellouki, A.; Yusà, V. Particle size distributions of currently used pesticides in a rural atmosphere of France. Atmos. Environ. 2013, 81, 32–38. [Google Scholar] [CrossRef]
- Mast, M.A.; Foreman, W.T.; Skaates, S.V. Current-Use Pesticides and Organochlorine Compounds in Precipitation and Lake Sediment from Two High-Elevation National Parks in the Western United States. Arch. Environ. Contam. Toxicol. 2007, 52, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Corbin, M.; Eckel, W.; Ruhman, M.; Spatz, D.; Thurman, N.; Gangaraju, R.; Kuchnicki, T.; Mathew, R.; Nicholson, I. NAFTA Guidance Document for Conducting Terrestrial Field Dissipation Studies; United States Environmental Protection Agency: Washington, DC, USA, 2006.
- Banerjee, S.; Law, S.E. Electrostatic Induction Charging of Pollen Suspensions. In Proceedings of the 1996 IEEE Industry Applications Conference Thirty-First IAS Annual Meeting, San Diego, CA, USA, 6–10 October 1996; pp. 1775–1781. [Google Scholar]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Stone, D.; Harper, B.J.; Lynch, I.; Dawson, K.; Harper, S.L. Exposure Assessment: Recommendations for Nanotechnology-Based Pesticides. Int. J. Occup. Environ. Health 2010, 16, 467–474. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Guidance for Assessing Pesticide—Risks to Bees; United States Environmental Protection Agency: Washington, DC, USA, 2014.
- Berenbaum, M.R. Does the Honey Bee “Risk Cup” Runneth Over? Estimating Aggregate Exposures for Assessing Pesticide Risks to Honey Bees in Agroecosystems. J. Agric. Food Chem. 2016, 64, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Burlew, D.A. The Effects of Pesticide-Contaminated Pollen on Larval Development of the Honey Bee, Apis mellifera. Ph.D. Thesis, Evergreen State College, Olympia, WA, USA, 2010. [Google Scholar]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Efficiency of QuEChERS approach for determining 52 pesticide residues in honey and honey bees. MethodsX 2016, 3, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orrick, G.; Rothman, G.; Bohaty, R.; White, K.; Koper, C.; Miller, R.; Liu, L. Guidance for Reviewing Environmental Fate Studies; Environmental Fate and Effects Division, USEPA.: Washington, DC, USA, 2012. [Google Scholar]
- Smodis Skerl, M.I.; Kmecl, V.; Gregorc, A. Exposure to pesticides at sublethal level and their distribution within a honey bee (Apis mellifera) colony. Bull. Environ. Contam. Toxicol. 2010, 85, 125–128. [Google Scholar] [CrossRef]
- Bonzini, S.; Tremolada, P.; Bernardinelli, I.; Colombo, M.; Vighi, M. Predicting pesticide fate in the hive (part 1): Experimentally determined tau-fluvalinate residues in bees, honey and wax. Apidologie 2011, 42, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Smart, M.D.; Anelli, C.M.; Sheppard, W.S. Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J. Invertebr. Pathol. 2012, 109, 326–329. [Google Scholar] [CrossRef]
- Fischer, D.; Moriarty, T. Pesticide Risk Assessment for Pollinators: Summary of a SETAC Pellston Workshop. Pensacola FL (USA): Society of Environmental Toxicology and Chemistry (SETAC). 2011; p. 29. 45p, Available online: https://c.ymcdn.com/sites/www.setac.org/resource/resmgr/publications_and_resources/executivesummarypollinators_.pdf?hhSearchTerms=S (accessed on 3 December 2019).
- Chen, W.-Y. Toxicokinetic modeling challenges for aquatic nanotoxicology. Front. Mar. Sci. 2016, 2, 114. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, M. Microbiology of Pollen and Bee Bread—Genus Bacillus. Apidologie 1979, 10, 269–274. [Google Scholar] [CrossRef]
- Gilliam, M. Microbiology of Pollen and Bee Bread—The Yeasts. Apidologie 1979, 10, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, M.; Lorenz, B.J.; Richardson, G.V. Digestive Enzymes and Microorganisms in Honey Bees, Apis-Mellifera - Influence of Streptomycin, Age, Season and Pollen. Microbios 1988, 55, 95–114. [Google Scholar]
- Gilliam, M.; Prest, D.B.; Lorenz, B.J. Microbiology of Pollen and Bee Bread - Taxonomy and Enzymology of Molds. Apidologie 1989, 20, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, M.; Roubik, D.W.; Lorenz, B.J. Microorganisms Associated with Pollen, Honey, and Brood Provisions in the Nest of a Stingless Bee, Melipona-Fasciata. Apidologie 1990, 21, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Villa, S.; Vighi, M.; Finizio, A.; Serini, G.B. Risk assessment for honeybees from pesticide-exposed pollen. Ecotoxicology 2000, 9, 287–297. [Google Scholar] [CrossRef]
- Henein, M. How California Almonds Are Killing Bees. Available online: http://www.honeycolony.com/article/california-almonds-killing-bees/ (accessed on 10 July 2019).
- Vanengelsdorp, D.; Evans, J.D.; Donovall, L.; Mullin, C.; Frazier, M.; Frazier, J.; Tarpy, D.R.; Hayes, J.; Pettis, J.S. “Entombed Pollen”: A new condition in honey bee colonies associated with increased risk of colony mortality. J. Invertebr. Pathol. 2009, 101, 147–149. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.P.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar]
- David, A.; Botías, C.; Abdul-Sada, A.; Nicholls, E.; Rotheray, E.L.; Hill, E.M.; Goulson, D. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 2016, 88, 169–178. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides?—A brief review. Environ. Int. 2016, 89, 7–11. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Katoch, R.; Sethi, A.; Thakur, N.; Murdock, L.L. RNAi for Insect Control: Current Perspective and Future Challenges. Appl. Biochem. Biotechnol. 2013, 171, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.L.; Gurman, P.; Stelinski, L.L.; Elman, N.M. Functional nano-dispensers (FNDs) for delivery of insecticides against phytopathogen vectors. Green Chem. 2015, 17, 4173–4177. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [Green Version]
- Harper, S.; Usenko, C.; Hutchison, J.E.; Maddux, B.L.S.; Tanguay, R.L. In vivo biodistribution and toxicity depends on nanomaterial composition, size, surface functionalisation and route of exposure. J. Exp. Nanosci. 2008, 3, 195–206. [Google Scholar] [CrossRef]
- Bourguet, D.; Guillemaud, T. The Hidden and External Costs of Pesticide Use. In Sustainable Agriculture Reviews: Volume 19; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 35–120. [Google Scholar]
- Milner, A.M.; Boyd, I.L. Toward pesticidovigilance. Science 2017, 357, 1232–1234. [Google Scholar] [CrossRef] [Green Version]
- Guidance, D. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Biotechnol. Law Rep. 2011, 30, 613–616. [Google Scholar]
- Alavanja, M.C. Introduction: Pesticides use and exposure extensive worldwide. Rev. Environ. Health 2009, 24, 303–309. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yanez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. UK 2015, 5, 14621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straub, L.; Villamar-Bouza, L.; Bruckner, S.; Chantawannakul, P.; Gauthier, L.; Khongphinitbunjong, K.; Retschnig, G.; Troxler, A.; Vidondo, B.; Neumann, P.; et al. Neonicotinoid insecticides can serve as inadvertent insect contraceptives. Proc. R. Soc. B Biol. Sci. 2016, 283, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu-Smart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. UK 2016, 6, 32108. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.R. Modern Insect Extinctions, the Neglected Majority. Extinciones Modernas de Insectos, la Mayoría Desatendida. Conserv. Biol. 2005, 19, 1030–1036. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Aizen, M.A.; Alcantara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; Garcia, M.B.; Garcia, D.; Gomez, J.M.; Jordano, P.; et al. Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 2015, 29, 299–307. [Google Scholar] [CrossRef]
- Hennig, E.I.; Ghazoul, J. Pollinating animals in the urban environment. Urban Ecosyst. 2012, 15, 149–166. [Google Scholar] [CrossRef] [Green Version]
- Badger, M.; Ortega-Jimenez, V.M.; von Rabenau, L.; Smiley, A.; Dudley, R. Electrostatic Charge on Flying Hummingbirds and Its Potential Role in Pollination. PLoS ONE 2015, 10, e0138003. [Google Scholar] [CrossRef]


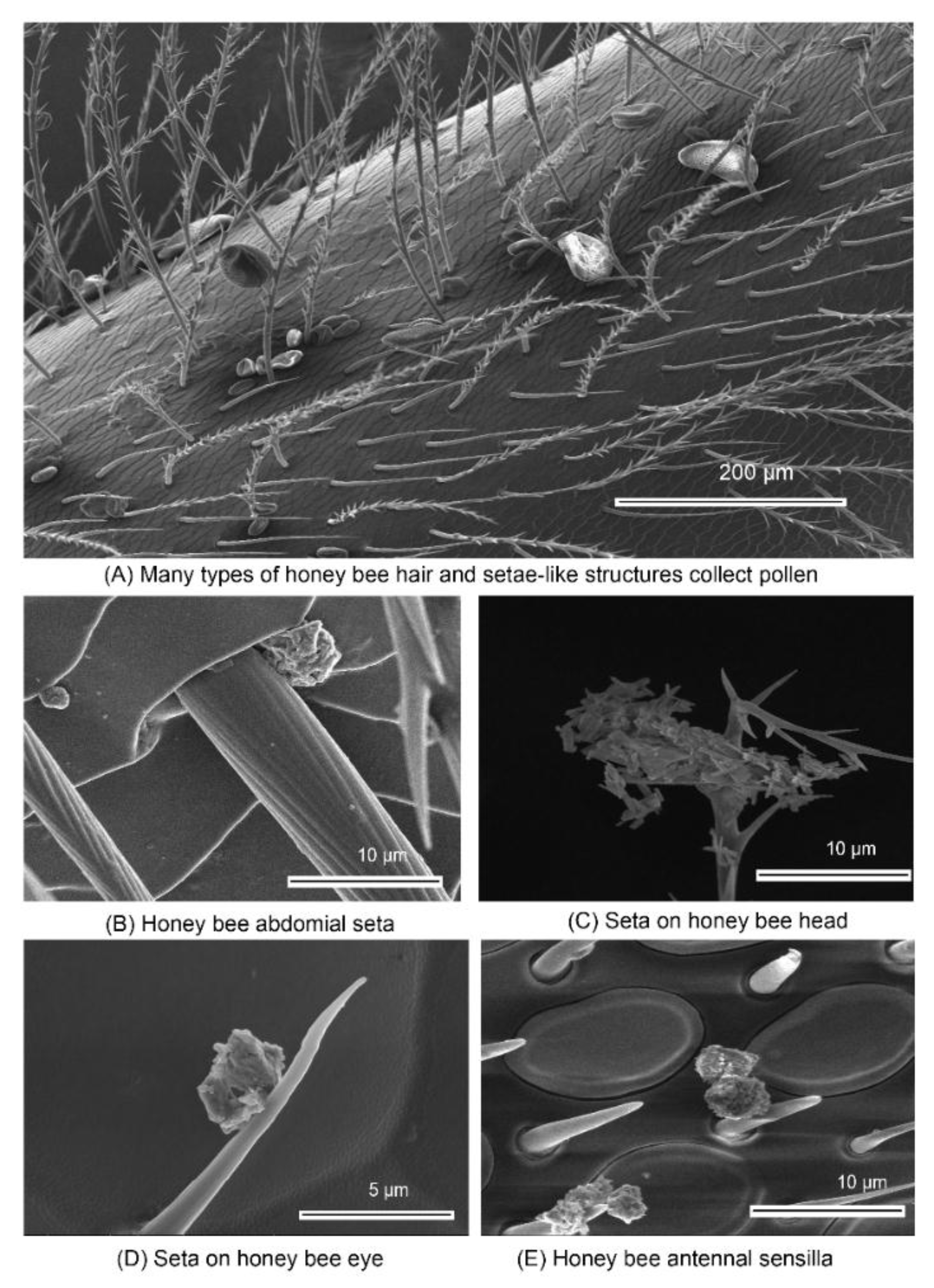
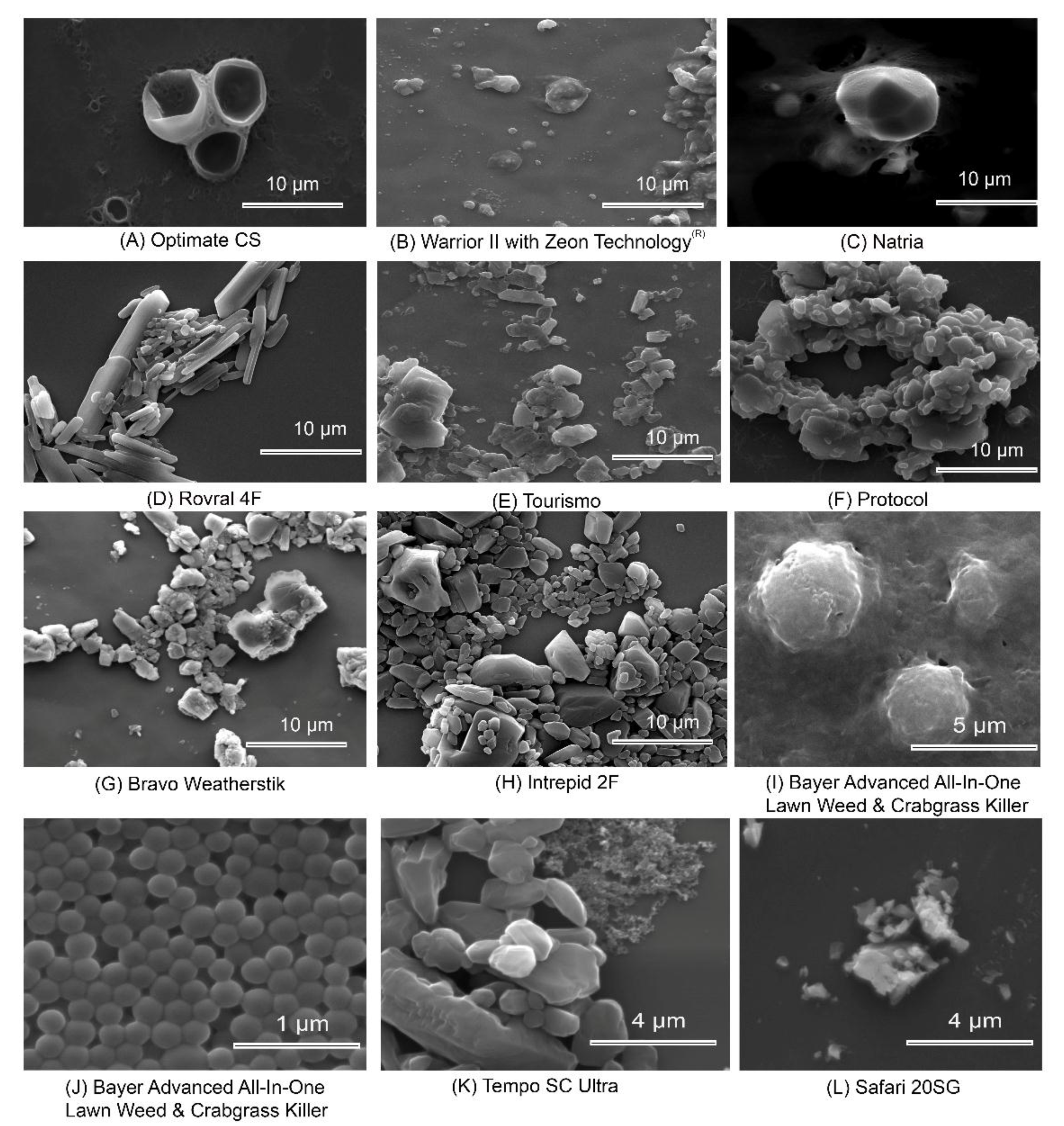
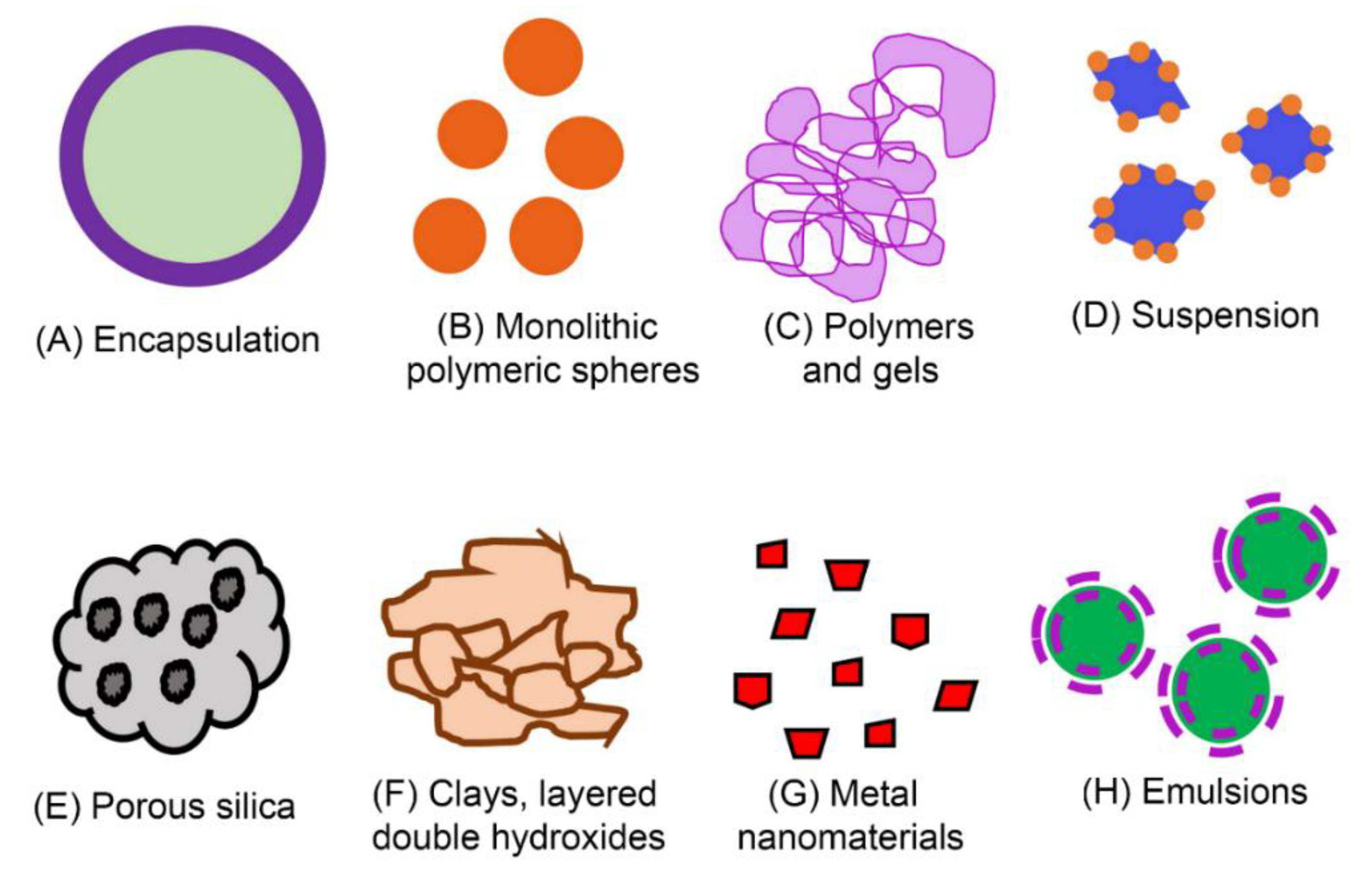
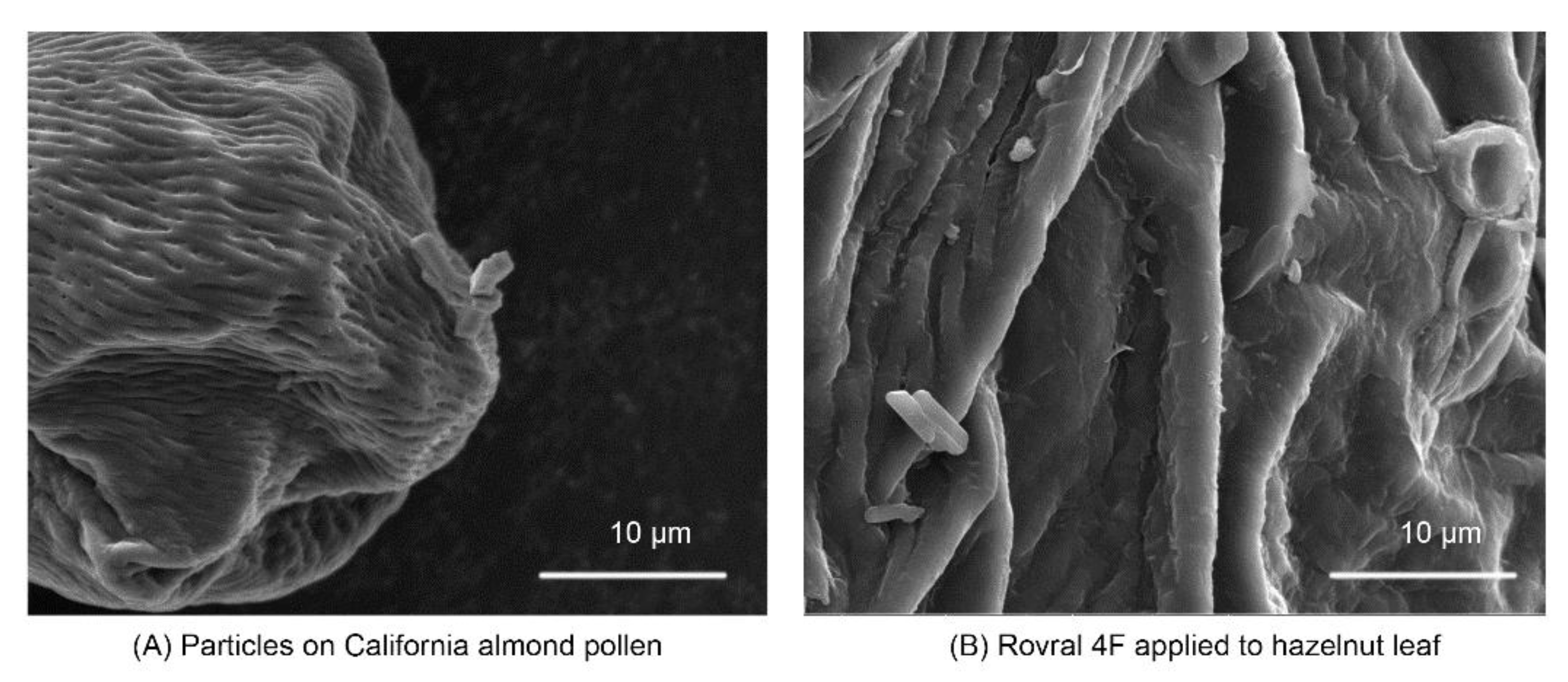

| Particle Classification | Particle Examples | Range | Effects on Bees |
|---|---|---|---|
| Nano (definition varies) PM0.1 <100 nm ultrafine particles | Nanosilver | 1.5–5 nm | Decreased Nosema spores, variable effects on longevity [60], biocides against American foulbrood pathogens [61]. |
| Diesel exhaust | 7.5–1000 nm | Affects learning and stress response [62], degrades floral odors [63]. | |
| Nanopesticide particles approximate lower range | ≈50 nm | Whether NBP size or properties affect exposure or toxicity to bees remains to be investigated | |
| Fugitive dust from seed planting | 230 nm–32 μm | Associated with bee mortality [64,65,66]. Particle fraction under 1 μm contains more active ingredient | |
| PM2.5 <2500 nm fine particles | Nanopesticide particles approximate upper range | ≈1–10 μm | Whether NBP size or properties affect exposure or toxicity to bees remains to be investigated |
| PM10 <10,000 nm coarse particles | Pollen | 6–100 μm | Source of protein [25]. Can be a vector of contaminants and smaller particles into the hive [67] |
| Microencapsulated methyl parathion (PENNCAP-M) | 30–50 μm | Colony mortality, storage in pollen [68,69,70] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooven, L.A.; Chakrabarti, P.; Harper, B.J.; Sagili, R.R.; Harper, S.L. Potential Risk to Pollinators from Nanotechnology-Based Pesticides. Molecules 2019, 24, 4458. https://doi.org/10.3390/molecules24244458
Hooven LA, Chakrabarti P, Harper BJ, Sagili RR, Harper SL. Potential Risk to Pollinators from Nanotechnology-Based Pesticides. Molecules. 2019; 24(24):4458. https://doi.org/10.3390/molecules24244458
Chicago/Turabian StyleHooven, Louisa A., Priyadarshini Chakrabarti, Bryan J. Harper, Ramesh R. Sagili, and Stacey L. Harper. 2019. "Potential Risk to Pollinators from Nanotechnology-Based Pesticides" Molecules 24, no. 24: 4458. https://doi.org/10.3390/molecules24244458
APA StyleHooven, L. A., Chakrabarti, P., Harper, B. J., Sagili, R. R., & Harper, S. L. (2019). Potential Risk to Pollinators from Nanotechnology-Based Pesticides. Molecules, 24(24), 4458. https://doi.org/10.3390/molecules24244458






