Abstract
Annona species have been a valuable source of anti-infective and anticancer agents. However, only limited evaluations of their alkaloids have been carried out. This review collates and evaluates the biological data from extracts and purified isolates for their anti-infective and anti-cancer activities. An isoquinoline backbone is a major structural alkaloid moiety of the Annona genus, and more than 83 alkaloids have been isolated from this genus alone. Crude extracts of Annona genus are reported with moderate activities against Plasmodium falciparum showing larvicidal activities. However, no pure compounds from the Annona genus were tested against the parasite. The methanol extract of Annona muricata showed apparent antimicrobial activities. The isolated alkaloids from this genus including liriodenine, anonaine, asimilobine showed sensitivity against Staphylococcus epidermidis. Other alkaloids such as (+)-Xylopine and isocoreximine indicated significant anti-cancer activity against A549 and K-562 cell lines, respectively. This review revealed that the alkaloids from Annona genus are rich in structural diversity and pharmacological activities. Further exploration of this genus and their alkaloids has potential for developing novel anti-infective and anticancer drugs.
1. Introduction
Annona is one of the 129 genera of the Annonaceae family and contains 119 species with eight species grown for commercial uses [1,2]. Most of the species grow in tropical regions; e.g., the soursop fruit tree (Annona muricata) is cultivated commercially and is widespread in the West Indies, North and South Americas, Africa, the Pacific Islands, and Southeast Asia. Annona species have been used as medicines by indigenous people for a wide range of disorders including parasitic infections, inflammation, diabetes, and cancer [3]. The phytochemical investigation of this plant genus has revealed the presence of acetogenins, alkaloids, essential oils, flavonoids, terpenoids, and other chemical classes [4,5]. Acetogenins (ACGs) are the major constituents of the Annona genre and examples were found to possess a variety of pharmacological properties including as antitumor, immunosuppressive, pesticidal, antiprotozoal, antimicrobial, antimalarial, anthelmintic, and antiviral agents, with some being commercially developed for the treatment of oral herpes and treating infestations of head lice, fleas, and ticks [5,6]. However, the available phytochemistry, including information on the composition and bioactivities of constituents from Annona species is limited and scattered [2]. This review evaluates the ethnopharmacological uses, alkaloid constituents, and the anti-infective properties of constituents contained within the genus Annona.
2. Ethnomedicinal Uses of Anonna Genus
The Annona species are moderately erect shrubs or small trees that grow to 5–11 m in height depending upon species and the region they inhabit, and are ferruginous to greyish, and tomentose when young, but later becoming glabrous [7]. Ethnobotanically, the plants from this genus play significant roles as food products and medicinal agents. A recent review on A. muricata showed that it is widely used in traditional decoctions in as many as 35 different countries for treating numerous diseases [8]; e.g., despite reports that the seed is toxic, traditional Mexican pharmacopeia uses powdered toasted seed as a potent emetic and cathartic. The seed was also used as an insecticidal agent and seed powder was used as a lotion when mixed with grease to treat parasitic skin disorders. A decoction of the fruit skin was used to treat pneumonia [9]. To South-East Asian people, decocted leaves of Annona reticulate (“custard apple”) was used internally against worms, and poultice leaves were applied externally to treat abscesses, boils, and ulcers. Unripe fruit was used to treat diarrhea and dysentery, and decocted root was used as febrifuge and to treat toothache [9,10].
In India, Annona squamosa (“sugar apple”) leaves are crushed and applied to wounds, ulcers, and is sniffed to relieve hysteria and fainting spells. Decocted leaves are used systemically to treat dysentery (India), and as a tonic, febrifuge, and cold remedy (tropical America). Crushed ripe fruit was applied to surface tumors (India), whereas the unripe fruit was used to treat dysentery in Elsavador [9]. The stem bark and root were used to treat diarrhea and dysentery [9]. The Annona muricata (“soursop”) has been used in the indigenous medicine of Togo to treat hypertension and diabetes mellitus [11], with the leaves used as an anti-parasitic, anti-rheumatic, astringent, and emetic in Brazil [12]. Decocted leaves were used as an analgesic, antispasmodic agents in Equador, whereas it is used as a remedy for cough, catarrhal inflammation, diarrhea, dysentery, bladder problems, and inflammation in the West Indies. Mashed leaves were also used as a poultice to relief eczema, rheumatism, and skin eruptions [9]. Traditional medicine in Indonesia has used the leaves as a treatment for boils, spasms, and as an aphrodisiac [13]. The fruit juice was used as a diuretic agent and to treat leprosy and liver ailments [9]. Currently, in Indonesia, the fruit is commonly used traditionally to treat breast cancer. A decoction of the seeds was used as a strong emetic agent, and the flower was used to treat catarrhal inflammation. In Materia Medica of British Guiana, a tincture of the powdered seeds and bay rum serves as a strong emetic. Soursop flowers are believed to alleviate catarrhal inflammation. The roots have been used as a vermifuge and an antidote for poisoning [9]. The roots are commonly used in Guinea as anti-parasitic and pesticidal agents. In Indonesia, currently, the stem and root bark are used as an alternative medication to treat malarial fever.
There are less popular Annona species, which were also used in traditional medication. In Guyana, a decoction of the stem bark of A. ambotay Aublet was used to treat ulcers and skin eruptions. Mixed with the bark, the leaf was used as febrifuge and sudorific. A tea made of the stem and leaf of A. glabra L. was consumed to eliminate flatworm and nematodes in Guyana. A decoction of the bark of Annona haematantha Miq. was used as a bath to treat skin ulcers, while its syrup was used to relieve cough. The bark infusion of Annona sericea Dunal was used to treat cramps [14]. In Mexico, the leaf of Annona diversifolia Safford (“Ilama”) was commonly used as an anticonvulsant, anti-inflammatory, and analgesic agent [15]. An infusion of the leaves of A. senegalensis (“wild custard apple”) was used to treat diarrhea and pulmonary complaints. Decocted stem bark was used to treat stomachache, toothache, dysentery, and worm infection. The root was used to treat venereal diseases and intestinal problems, snake bites, and as cancer therapy (Nigeria). Its green fruits was used to treat Guinea worm sores, diarrhea, dysentery [9]. In Brazil, Annona salzmanii A. DC has been used to treat dysentery, ulcers, and inflammation [16].
3. Phytochemical Studies of Secondary Metabolites of Annona Genus
The juicy pulp of the fruit is often a good source of sugar, vitamins, minerals, and phenolic intake. For example, the dried pulp of Annona muricata contains 68% sugars for every 100 g containing 1.0 g protein, 0.97 g fat, 1.28 niacin, and 29 mg ascorbic acid. Moreover, it could supply 3 g of phenolic substances for every 100 g of pulp [9,17]. The 20th century reported preliminary examinations of the Annona plants of the leaves, fruits, and seeds. Since the 1980s, with the advent of pursuing anti-cancer drug leads from medicinal plants, acetogenin was isolated from the Annona genus based on its promising anti-cancer activity. For example, a recent acetogenin, squamocin P, isolated from A. squamosa, possessed significant anticancer activity against SMMC 7721/T, MCF-7/ADR, A549/T with IC50 values of 0.435, 3.34, 6.32 µM, respectively, with the positive control cisplatin having higher IC50 values of 198.85, 178.87, and 219.33 µM against SMMC 7721/T, MCF-7/ADR, and A549/T, respectively. While this encouraged investigations into this species, they were confined to this one polyketide compound, at the expense of other components present. Figure 1a shows the number of compounds isolated from each plant part of Annona muricata. In the previous phytochemical studies of Annona muricata, around 127 compounds were isolated, in which almost 90% were acetogenins (Figure 1b) [18].
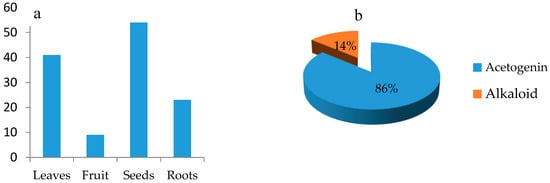
Figure 1.
Phytochemical study on Annona muricata. (a) Number of isolated compounds in different regions of the plants; (b) comparison between total isolated acetogenins and alkaloids.
Acetogenins from the Annona genus were reviewed together with other genus in the same family Annonaceae [8,19,20,21,22,23], which covered the isolation, molecular properties, and biosynthesis of their pharmacological activities. Here, we collected records on alkaloids which were isolated in the Annona plant genus from 1960–2019 (Table 1). The alkaloids present have been of interest since the first, annonaine (8, Figure 2), was isolated in 1931 from the stem bark of Annona muricata L. collected in the Philippines [24]. Table 1 shows the alkaloids isolated from the specific plants of each species and their structures are presented in Figure 2.

Table 1.
Alkaloid Constituents of Annona.

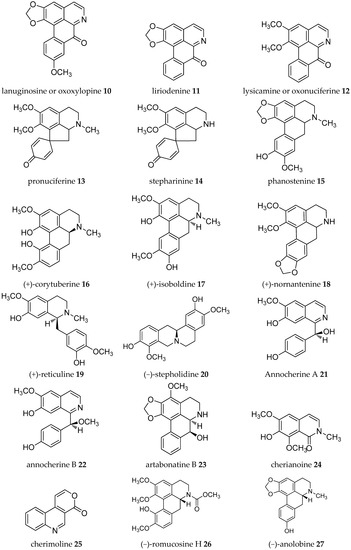

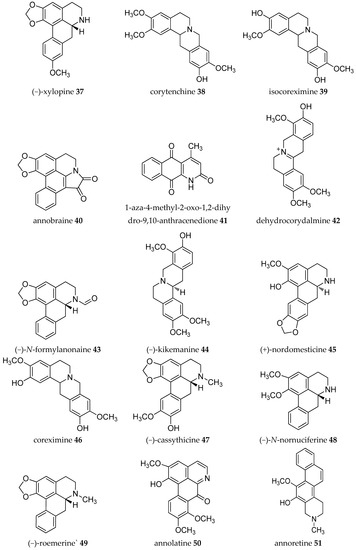
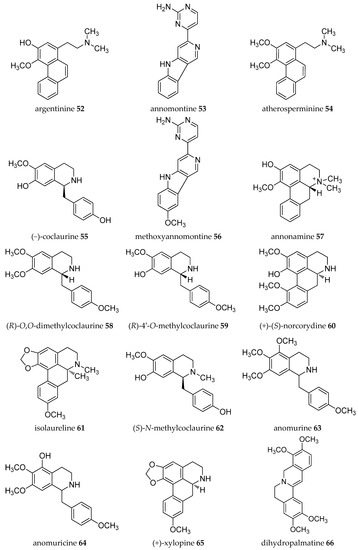
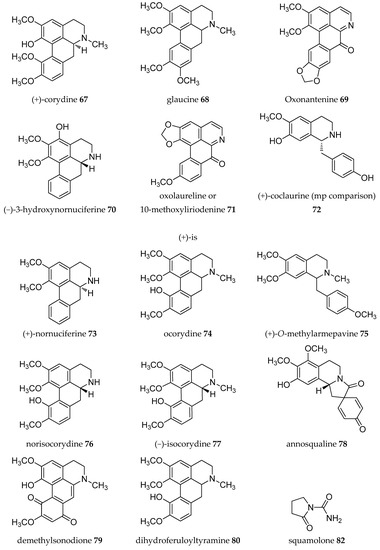
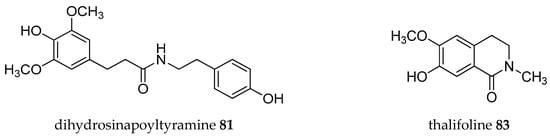
Figure 2.
Structures of Annona alkaloids (1–83).
4. Anti-Infective Alkaloids from the Genus Annona
Plants from the genus Annona plants have been used in traditional medication for the treatment of both infectious and non-infectious diseases. This led to the pharmacological and chemical screening of numerous species to confirm these pharmacological claims and to isolate the compounds which might be responsible for these activities. The Annona genus has been studied for activity against parasites, cancer, and as anti-oxidant agents.
4.1. Antiprotozoal Activities
Ethnopharmacological studies have revealed the Annona species Annona crassiflora, A. muricata, A. senegalensis, and A. squamosa were prescribed in malarial fever therapy. Further studies revealed leaf extract from A. crassiflora was rich in flavonoids and alkaloids, and was able to reduce the Plamsodium berghei NK65 infection level in mice by 57–75% with a daily dosage of 12.5 µg/kg/day [62]. Another study of the crude methanol extract of A. squamosa indicated moderate activity against Plasmodium falciparum 3D7 with an IC50 value of 30 µg/mL compared to the chloroquine control, which gave an IC50 value of 0.021 µg/mL [63]. Moderate anti-plasmodium activity was also shown using crude extracts of A. muricata (Table 2).

Table 2.
Anti-protozoal activity of several extract of Annona muricata and Annona reticulata [68,69].
In an animal model test, an aqueous leaf extract of A. muricata showed a dose dependent antimalarial effect with the highest inhibition of 85.61% observed from a 1000 µg/kg dose. However, the treatment was unable to completely cure the mice, but prolonged the survival time [64]. An essential oil extract of A. squamosa demonstrated inhibition against the erythrocitic stages of P. falciparum, against epimastigotes forms of T. cruzi and against trypomastigotes forms of T. cruzi with IC50 values of 14.7, 16.2, and 12.7 µg/mL, respectively [65].
Although there was a limited record regarding traditional uses of Annona plants to treat other parasitic protozoal infections, e.g., leishmaniasis and trypanosomiasis, several crude extracts of Annona plant (A. muricata) were also tested against L. amazonensis, L. braziliensis, L. Donovani, and T. cruzi (Table 2). The crude ethyl acetate extract from the leaves of A. muricata indicated potent activity against L. amazonensis, L. braziliensis, L. Donovani, and T. cruzi with IC50 values of 10–25 µg/mL. A different strategy to control malarial infection involves controlling its vector. Past larvicidal studies have indicated that the crude methanol extract from the bark of A. squamosa resulted in 100% mortality of Anopheles subpictus (which carry human malaria parasites) at 500 µg/mL [66]. The extract from the stem and root bark were even more toxic toward malarial larvae (Anopheles gambiaes.s. Giles) with 50% mortality at 24 and 21 µg/mL, respectively [67].
The same protocol was applied to other disease vectors, including Aedes (dengue virus vector) and Culex (encephalitis virus). For example, the seeds of Annonas pecies were generally reported to be toxic with LC50 values <1 µg/mL against both Aedes and Culex larvae (Table 3). These results demonstrated that the Annona plants can be used for controlling the vector especially in rural areas where modern, and likely more expensive, vector controls were limited.

Table 3.
Larvicidal of several extract of Annona genus [44,66,73,74,75,76].
Despite numerous alkaloids being isolated from Annona species, reports detailing pharmacological studies on single compounds remains limited. There are reports on the same alkaloids being isolated from different plant genus. For example, (+)-reticuline 19 was isolated from Croton linearis and was previously shown to possess a weak antriprotozoal activity against Lesihmania infatum with IC50 values of 148.0 ± 1.2 µM [70]. Asimilobine 9 and isoboldine 17 isolated from the bark of Beilschmiedia alloiophylla (Costa Rica) possessed anti-leishmanial activity with IC50 values of 29.8 ± 1.5 µM and 50.0 ± 4.0 µM, respectively [71]. A previous study on the leaves and fruits of Annona mucosa (Brazil) produced liriodenine 11, which was highly active against Leishmania amazonensis with an IC50 value of 1.43 ± 0.58 μg/mL and was moderately active against Leishmania braziliensis with an IC50 value of 55.92 ± 3.55 μg/mL [72].
4.2. Antimicrobial Activities
Traditionally, Annona plants have been prepared for use against infection related diseases, such as ulcer, dysentery, and boils, and therefore became a driving force for conducting anti-microbial studies against common bacteria; preliminary results on the crude extracts are shown in Table 4. In general, the crude extract possessed moderate to inactive anti-microbial values ranging from 6.25–4096 µg/mL. Most of the studies were based on the anti-microbial activity of crude extracts with no separate non-polar to polar fractions tested or individual constituents isolated. Therefore, further investigations are required to substantiate the traditional claims for these Annona plants by the isolation and identification of individual constituents. As a result, discussion here is confined to the anti-microbial activities from isolated alkaloid constituents (Table 5). A. muricata, A. squamosa, A. cherimola, and A. ambotay showed reasonable antimicrobial activities, whereas A. reticulata did not present antimicrobial activity, with reported MIC values of more than 1000 µg/mL against Bacillus cereus, Staphylococcus aureus [77]. Antimicrobial testing of the methanol extract of A. squamosa fruit against multidrug resistant MRSA reported MIC values of 5000 µg/mL, but no information was given against ESBLEC (extended-spectrum beta-lactamase producing E. coli), CRPA (carbapenem-resistant P. aeruginosa) and MDRAB (multidrug-resistant A. baumannii) [78]. The benzoquinoline alkaloid, anonaine 8, indicated comparable anti-microbial activities with positive control, with the exception against Staphylococcus aureus. Another study reported annoquinone A, isolated from A. Montana, possessed anti-microbial activity against Bacillus subtilis and Micrococcus luteus with IC50 value of 10, 10 µg/mL, respectively [79].

Table 4.
Anti-microbial activities of crude extracts or fractions of Annona genus.

Table 5.
Anti-microbial activities of alkaloids isolated from Annona genus.
Previous studies using alkaloid samples from sources other than Annona revealed, (−)-asimilobine 9 isolated from the bark of Beilschmiedia alloiophylla (Costa Rica) and B. kunstleri (Malaysia) indicated anti-fungal activity with an IC50 value of 16.0 µg/mL [71]. (−)-Stepholidine 20 isolated from rattan stem of Fibraurea recisa had antifungal activity against drug resistant Candida albicans SM372, Candida krusei KM066, Candida parapsilosis SM304160, Cryptococcus neofarms SM9406204 with similar MIC value of 320 µg/mL [91]. Alkaloid (−)-roemerine 49 from the same stem indicated significant inhibition of C. albican transition from yeast to hyphae in a dose dependent manner [92]. Glaucine 68 isolated from the aerial component of Glaucium oxylobum showed moderate skin anti-fungal activities against Microsporum canis, Microsporum gypseum, and Trichophyton mentagrophytes [93]. Antifungal activities of the non-Annona isolated alkaloids were evaluated against non-pathogenic fungi including liriodenine 11 from the wood of Michelia formosa which indicated a low activity against several wood decaying fungi both white and brown rot-fungi, Lenzites betulina, Trametes versicolor, Laetiporus sulphureus, Gloeophyllum trabeum, and Fomitopsis pinicola [94]. Similar alkaloids were also previously evaluated against pathogenic bacteria, including liriodenine from the roots of Zanthoxylum nitidum which showed a good antimicrobial activity against MRSA with MIC value of 93.8 µg/mL [95]. Liriodenine 11 from the stem of Mitrephira glabra Scheff was active against non-pathogenic bacteria, Micrococcus luteus, Mycobacterium sinegmatis, Saccharomyces cerevisae, and Aspergilus niger with an MIC value of 6.3, 12, 12, and 25 µg/mL, respectively [96].
5. Anticancer Alkaloids Present in the Genus Annona
In addition to the above antiprotozoal and antimicrobial activities, both the crude extracts from annona plants and the individual alkaloids have shown potent anticancer/antitumour activities.Many crude extracts of Annona species showed significant anti-cancer activities, but most of the bioactive constituents present in those crude extracts were acetogenins, fatty acids, and peptides [7]. However, wherever studied, it was known that some aporphine alkaloids, especially (−)-roemerine 49, which was isolated from the leaves of the wild custard apple, improved the response produced by vinblastine against multidrug-resistant KB-V1 or KB-3 cells (ED50 > 20 µg/mL). This alkaloid appears to function by interacting with P-glycoprotein in the multidrug-resistant KB-V1 cell membrane vesicles [59]. The leaves of Annona muricata also showed potency to reduce gastric lesion, to expel parasitic worms and, moreover, the crude extract from the bark possessed anti-viral activity against herpes simplex virus type 1. The extracts and compounds also showed anticancer activities against breast cancer. Alkaloids, (−)-coclaurine 55, (+)-reticuline 19, argentinine 52, atherosperminine 54, and (+)-xylopine 65 were isolated from the root of Indonesian Annona muricata in which (−)-coclaurine 55, (+)-reticuline 19 were non-toxic against a human suspension cancer cell line (HL-60 leukemia cells) and two fibroblastic cell lines (A549 lung cancer cells and HepG2 liver cancer cells). (+)-Xylopine 65 exhibited the lowest IC50 value ranging from approximately 20–80 µM [18].The alkaloid isocoreximine 39 isolated from Annona cherimola, at concentration of 50 µg/mL indicated cytotoxicity against K-562, U-251, PC-3, HCT-15, and MCF-7 with % inhibition of cell viability 94.15%, 65.23%, 78.71%, 63.05%, and 85.76%, respectively. Isocoreximine 39 showed in vitro cytotoxic activity against K-562, U-251, PC-3, HCT-15, and MCF-7 with % of inhibition of cell viability 94.15%, 65.23%, 78.71%, 63.05%, and 85.76%, respectively [34].
Although most of the alkaloids isolated from Annona species were reported with no anticancer activity data, there were cytotoxicity activity data on similar molecules obtained from non-Annona genus (Table 6). Interestingly, annomontine 54, a carbolated pyrimidine alkaloid was previously reported from the marine sponge Acanthostrongylophora ingens collected from Indonesian water. The alkaloid possessed pronounced anticancer activity against mouse lymphoma L5178Y compared to a standard control kahallide F [97]. The oxoaporphine alkaloid, liriodenine 11, was found in at least in twenty different species, ranging across flowering plants but mostly in annonaceae family. The alkaloid isolated from Brazilian Guatteria blepharophylla stem bark possessed anticancer activity against MCF-7 cell line with a more potent result compared to a standard drug doxorubicin with TGI value of 36.67 compared to 46.04 µM [98].

Table 6.
Anticancer/cytotoxicityactivities ofalkaloids that were obtained from non-Annona genera.
6. Conclusions
This review presents the ethnomedicinal, alkaloidal and biological, properties of Annona species with respect to reported anti-infective and anti-cancer activities. The Anonna species: A. muricata (soursop), A. squamosa (custard apple), A. senegalensis (wild custard apple), and A. cherimola (cherimola) are renowned traditionally for their anti-tumor properties. Among these, A. muricata is widely studied and has shown broad range of biological activities including anti-protozoal, anti-cancer, anti-tumour, antimicrobial, and antiparasitic properties. This species has also produced several patents and commercial products. Investigations into extracts from the leaves, bark, fruit, and seeds of this plant genus have found terpenoids, steroids, flavonoids, cardiac glycosides, tannins, phenols, sugars, fatty acids, acetogenins, and alkaloids. As many as 200 phytochemicals belonging to these chemotypes have been identified and isolated from this Annona muricata species alone, with the most important being acetogenins, phenols and anonaine alkaloids. Anonaine and its structurally related alkaloids were the most abundant and commonly available alkaloids in Annonaceae family. The oxoaporphine alkaloid, liriodenine, was found in at least in twenty different species, ranging across flowering plants but mostly in annonaceae family. The alkaloids from Annona species have rarely been explored for their medicinal applications. However, wherever studied, Annona alkaloids have been reported to possess anti-inflammatory, anti-cancer, antitumor, anti-HIV, antiprotozoal, antiparasitic, antidiabetic, analgesics, gastroprotective, antihypertensive, hepatoprotective, nephroprotective, and neuroprotective properties. Amongst these broad-ranging properties, anti-cancer and anti-tumour activities of both the crude extracts and alkaloids is commendable. Most interesting and noteworthy of this Annona genera is that the pharmacological properties accentuate the ethnomedicinal utilization of this plant, as well as its usefulness in the agrifood sector. Liriodenine, annonaine, glaucine and cleistopholine showed potent anti-cancer, anti-tumour, and cytotoxicity activities against many human cancer cell lines, and it is worthwhile to pursue detailed clinical investigations of these alkaloids. To the best of our knowledge, there is no clinical study that was successfully completed on the extracts rich in acetogenins or alkaloids. In this respect, it is also necessary to conduct scientific studies to establish optimal and safe doses of consumption of both the plant extracts and their phytochemicals especially alkaloids. This is because the use of the Annona plants is popular not only in Indonesia, but wide across the tropical countries.
Author Contributions
Conceptualization, A.S.N., P.W., P.A.K.; data curation and analysis 1960–2015, A.S.N.; data curation and analysis 2015–2019, A.S.N., Y.D.D.; making and editing of the figures, A.S.N., Y.D.D.; writing—original draft preparation, A.S.N., Y.D.D., P.W., P.A.K.; writing—review and editing, A.S.N., Y.D.D., P.W., P.A.K.
Funding
This research received no external funding.
Acknowledgments
A.S.N. thanks to University of Jember and University of Wollongong for research support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The Plant List Version 1. Available online: http://www.theplantlist.org/ (accessed on 5 December 2018).
- Badrie, N.; Schauss, A.G. Soursop (Annona muricata L.): Composition, nutritional value, medicinal uses, and toxicology. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Elsevier Inc.: London, UK, 2010. [Google Scholar]
- Mishra, S.; Ahmad, S.; Kumar, N.; Sharma, B.K. Annona muricata (the cancer killer): A Review. Glob. J. Pharm. Res. 2013, 2, 1613–1618. [Google Scholar]
- Oliveira, B.H.; Sant’Ana, A.E.G.; Bastos, D.Z.L. Determination of the diterpenoid, kaurenoic acid, in Annona glabra by HPLC. Phytochem. Anal. 2002, 13, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.; de Goulart, R.; Vasques Farinazzi-Machado, F.; da Soares de Souza, M.; Santos Bueno, P.; Guiguer, E.; Araujo, A.; Groppo, M. Annona sp: Plants with Multiple Applications as Alternative Medicine - A Review. Curr. Bioact. Compd. 2012, 8, 277–286. [Google Scholar] [CrossRef]
- Asare, G.A.; Afriyie, D.; Ngala, R.A.; Abutiate, H.; Doku, D.; Mahmood, S.A.; Rahman, H. Antiproliferative activity of aqueous leaf extract of Annona muricata L. on the prostate, BPH-1 cells, and some target genes. Integr. Cancer Ther. 2015, 14, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef]
- Morton, J.F.; Dowling, C.F. Fruits of Warm Climates; Wipf and Stock Publishers: Miami, FL, USA, 1987. [Google Scholar]
- Jansen, P.C.M.; Jukema, J.; Oyen, L.P.A.; van Lingen, T.G. Annona reticulata L. In Plant Resources of South-East Asia No. 2: Edible fruits and nuts; Verheij, E.W.M., Coronel, R.E., Eds.; Pudoc: Wageningen, The Netherlands, 1991; p. 316. [Google Scholar]
- Karou, S.D.; Tchacondo, T.; Djikpo Tchibozo, M.A.; Abdoul-Rahaman, S.; Anani, K.; Koudouvo, K.; Batawila, K.; Agbonon, A.; Simpore, J.; de Souza, C. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus and hypertension in the Central Region of Togo. Pharm. Biol. 2011, 49, 1286–1297. [Google Scholar] [CrossRef]
- Dos, S.A.F.; Sant’Ana, A.E. The molluscicidal activity of plants used in Brazilian folk medicine. Phytomedicine 2000, 6, 431–438. [Google Scholar]
- Syamsuhidayat, S.; Hutapea, J.R. Inventaris Tanaman Obat Indonesia; Departemen Kesehatan RI, Badan Penelitian dan Pengembangan Kesehatan: Jakarta, Indonesia, 1991.
- DeFilipps, R.A.; Maina, S.L.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2004. [Google Scholar]
- González-Trujano, M.E.; Navarrete, A.; Reyes, B.; Hong, E. Some pharmacological effects of the ethanol extract of leaves of Annona diversifolia on the central nervous system in mice. Phyther. Res. 1998, 12, 600–602. [Google Scholar] [CrossRef]
- Oliveira da Cruz, P.E.; Costa, E.V.; de S. Moraes, V.R.; de L. Nogueira, P.C.; Vendramin, M.E.; Barison, A.; Ferreira, A.G.; do N. Prata, A.P. Chemical constituents from the bark of Annona salzmannii (Annonaceae). Biochem. Syst. Ecol. 2011, 39, 872–875. [Google Scholar] [CrossRef]
- Ribeiro da Silva, L.M.; Teixeira de Figueiredo, E.A.; Ricardo, N.M.P.S.; Vieira, I.G.P.; Wilane de Figueiredo, R.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.S.; Haritakun, R.; Lambert, J.M.; Dillon, C.T.; Keller, P.A. Alkaloids from the root of Indonesian Annona muricata L. Nat. Prod. Res. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, J.K.; Hui, Y.-H.; McLaughlin, J.L. Annonaceous Acetogenins: A Review. J. Nat. Prod. 1990, 53, 237–278. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-P.; Rieser, M.J.; Gu, Z.-M.; Zhao, G.-X.; McLaughlin, J.L. Annonaceous acetogenins: An updated review. Phytochem. Anal. 1993, 4, 27–48. [Google Scholar] [CrossRef]
- Feras, Q.A.; Liu, A.; McLaughlin, J.L. Annonaceous Acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar]
- Zeng, L.; Ye, Q.; Oberlies, H.; Shi, G.; Gu, Z.-M.; He, K.; McLaughlin, J.L. Recent advances in Annonaceous acetogenins. Nat. Prod. Rep. 1996, 13, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Abdul Kadir, H. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef]
- Reyes, F.R.; Santos, A.C. Isolation of anonaine from Anona squamosa Linn. Philipp. J. Sci. 1931, 44, 409–410. [Google Scholar]
- De Oliveira, A.B.; De Oliveira, G.G.; Carazza, F.; Maia, J.G.S. Geovanine, a new azaanthracene alkaloid from Annona ambotay Aubl. Phytochemistry 1987, 26, 2650–2651. [Google Scholar] [CrossRef]
- Rabelo, S.V.; Costa, E.V.; Barison, A.; Dutra, L.M.; Nunes, X.P.; Tomaz, J.C.; Oliveira, G.G.; Lopes, N.P.; de F.C. Santos, M.; da Silva Almeida, J.R.G. Alkaloids isolated from the leaves of atemoya (Annona cherimola × Annona squamosa). Rev. Bras. Farmacogn. 2015, 25, 419–421. [Google Scholar] [CrossRef]
- Raju, D.U.; Babu, K.S.; Ravada, S.C.R.; Golakoti, T. Isoquinoline alkaloid, flavonoids and a triol from leaves of Annona cherimola. J. Appl. Chem. (Lumami, India) 2015, 4, 120–126. [Google Scholar]
- Villar, A.; Mares, M.; Rios, J.L.; Cortes, D. Alkaloids from Annona cherimolia leaves. J. Nat. Prod. 1985, 48, 151–152. [Google Scholar] [CrossRef]
- Rios, J.L.; Cortes, D.; Valverde, S. Acetogenins, aporphinoids, and azaanthraquinone from Annona cherimolia seeds. Planta Med. 1989, 55, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Villar del Fresno, A.; Rios Canavate, J.L. Alkaloids from Annona cherimolia seed. J. Nat. Prod. 1983, 46, 438. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, F.-R.; Wu, Y.-C. Cherimoline, a novel alkaloid from the stems of Annona cherimola. Tetrahedron Lett. 1997, 38, 6247–6248. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Pan, W.B.; Wu, Y.C. Four alkaloids from Annona cherimola. Phytochemistry 2001, 56, 753–757. [Google Scholar] [CrossRef]
- Simeon, S.; Rios, J.L.; Villar, A. Alkaloids from Annona cherimolia (Mill.) stem bark. Plant. Med. Phytother. 1989, 23, 159–161. [Google Scholar]
- Martinez-Vazquez, M.; De la Cueva Lozano, D.G.; Estrada-Reyes, R.; Gonzalez-Lugo, N.M.; Ramirez Apan, T.; Heinze, G. Bio-guided isolation of the cytotoxic corytenchine and isocoreximine from roots of Annona cherimolia. Fitoterapia 2005, 76, 733–736. [Google Scholar] [CrossRef]
- de la Cruz Chacon, I.; Gonzalez-Esquinca, A.R. Liriodenine alkaloid in Annona diversifolia during early development. Nat. Prod. Res. 2012, 26, 42–49. [Google Scholar] [CrossRef]
- Chang, F.-R.; Chen, C.-Y.; Hsieh, T.-J.; Cho, C.-P.; Wu, Y.-C. Chemical constituents from Annona glabra III. J. Chin. Chem. Soc. 2000, 47, 913–920. [Google Scholar] [CrossRef]
- Riley-Saldana, C.A.; del R. Cruz-Ortega, M.; Martinez Vazquez, M.; De-la-Cruz-Chacon, I.; Castro-Moreno, M.; Gonzalez-Esquinca, A.R. Acetogenins and alkaloids during the initial development of Annona muricata L. (Annonaceae). Zeitschrift fuer Naturforschung C 2017, 72, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Chen, C.-M.; Kuan, S.-S. Alkaloids of Annona glabra. I. Isolation of (−)-N-methylactinodaphnine. J. Chin. Chem. Soc. 1971, 18, 133–136. [Google Scholar] [CrossRef]
- Yang, T.-H.; Chen, C.-M. Studies on the alkaloids of Anona glabra. II. T’ai-wan Yao Hsueh Tsa Chih 1973, 25, 1–7. [Google Scholar]
- Yang, T.-H.; Chen, C.-M. Studies on the alkaloids of Anona glabra L. II. Proc. Natl. Sci. Counc., Part. 2 1974, 7, 177–184. [Google Scholar]
- Wu, Y.C.; Chang, G.Y.; Duh, C.Y.; Wang, S.K. Cytotoxic alkaloids of Annona montana. Phytochemistry 1993, 33, 497–500. [Google Scholar] [CrossRef]
- Leboeuf, M.; Cave, A.; Forgacs, P.; Tiberghien, R.; Provost, J.; Touche, A.; Jacquemin, H. Alkaloids of the genus Annona. XL. Chemical and pharmacological study of alkaloids from Annona montana Macf. Plant. Med. Phytother. 1982, 16, 169–184. [Google Scholar]
- Yokomori, Y.; Sekido, K.; Wu, T.S.; Tien, H.J.; Hirokawa, S. The crystal and molecular structure of 1-(2-amino-4-pyrimidinyl)-β-carboline. Bull. Chem. Soc. Jpn. 1982, 55, 2236–2238. [Google Scholar] [CrossRef]
- Magadula, J.J.; Innocent, E.; Otieno, J.N. Mosquito larvicidal and cytotoxic activities of 3 Annona species and isolation of active principles. J. Med. Plants Res. 2009, 3, 674–680. [Google Scholar]
- Matsushige, A.; Kotake, Y.; Matsunami, K.; Otsuka, H.; Ohta, S.; Takeda, Y. Annonamine, a new aporphine alkaloid from the leaves of Annona muricata. Chem. Pharm. Bull. 2012, 60, 257–259. [Google Scholar] [CrossRef]
- Fofana, S.; Keita, A.; Balde, S.; Ziyaev, R.; Aripova, S.F. Alkaloids from leaves of Annona muricata. Chem. Nat. Compd. 2012, 48, 714. [Google Scholar] [CrossRef]
- Fofana, S.; Ziyaev, R.; Abdusamatov, A.; Zakirov, S.K. Alkaloids from Annona muricata leaves. Chem. Nat. Compd. 2011, 47, 321. [Google Scholar] [CrossRef]
- Leboeuf, M.; Legueut, C.; Cave, A.; Desconclois, J.F.; Forgacs, P.; Jacquemin, H. [Alkaloids of Annonaceae. XXIX. Alkaloids of Annona muricata]. Planta Med. 1981, 42, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Laprevote, O.; Leboeuf, M.; Cave, A.; Provost, J.; Forgacs, P.; Jacquemin, H. Alkaloids of the Annonaceae. 88. Alkaloids of Annona paludosa Aublet. Plant. Med. Phytother. 1988, 22, 159–164. [Google Scholar]
- Chang, F.-R.; Chen, K.-S.; Ko, F.-N.; Teng, C.-M.; Wu, Y.-C. Bioactive alkaloids from Annona reticulata. Chin. Pharm. J. 1995, 47, 483–491. [Google Scholar]
- Yang, T.H.; Cheng, M.Y. The alkaloids of Annona reticulata L. II. T’ai-wan Yao Hsueh Tsa Chih 1987, 39, 195–201. [Google Scholar]
- Xu, L.; Li, K.; Sun, N.; Kong, J. Alkaloids of Annona reticulata. Zhongguo Zhongyao Zazhi 1992, 17, 295–296. [Google Scholar]
- Campos, F.R.; Batista, R.L.; Batista, C.L.; Costa, E.V.; Barison, A.; dos Santos, A.G.; Pinheiro, M.L.B. Isoquinoline alkaloids from leaves of Annona sericea (Annonaceae). Biochem. Syst. Ecol. 2008, 36, 804–806. [Google Scholar] [CrossRef]
- Pinto, N.C.C.; Silva, J.B.; Menegati, L.M.; Guedes, M.C.M.R.; Scio, E.; Fabri, R.L.; Marques, L.B.; Souza-Fagundes, E.M.D.E.; Silva, T.P.D.A.; Melo, R.C.N.D.E.; et al. Cytotoxicity and bacterial membrane destabilization induced by Annona squamosa L. extracts. An. Acad. Bras. Cienc. 2017, 89, 2053–2073. [Google Scholar] [CrossRef]
- Philipov, S.; Kande, K.M.; Machev, K. Alkaloids of Annona senegalensis. Fitoterapia 1995, 66, 275–276. [Google Scholar]
- Fofana, S.; Ziyaev, R.; Diallo, S.K.; Camara, M.; Aripova, S.F. Alkaloids of Annona senegalensis. Chem. Nat. Compd. 2013, 49, 587–588. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Tewari, S.; Dhar, M.M. Aporphine alkaloids of Annona squamosa. Phytochemistry 1972, 11, 1819–1822. [Google Scholar] [CrossRef]
- Bhaumik, P.K.; Mukherjee, B.; Juneau, J.P.; Bhacca, N.S.; Mukerjee, R. Alkaloids from leaves of Annona squamosa. Phytochemistry 1979, 18, 1584–1586. [Google Scholar] [CrossRef]
- You, M.; Mahinda Wickramaratne, D.B.; Silva, G.L.; Chai, H.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Kinghorn, A.D.; Pezzuto, J.M. (-)-Roemerine, an aporphine alkaloid from Annona senegalensis that reverses the multidrug-resistance phenotype with cultured cells. J. Nat. Prod. 1995, 58, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Chang, F.-R.; Wu, Y.-C. Annosqualine: A novel alkaloid from the stems of Annona squamosa. Helv. Chim. Acta 2004, 87, 1392–1399. [Google Scholar] [CrossRef]
- Yang, T.-H.; Chen, C.-M. Constituents of Annona squamosa. J. Chin. Chem. Soc. 1970, 17, 243–250. [Google Scholar] [CrossRef]
- Pimenta, L.P.S.; Garcia, G.M.; do V. Goncalves, S.G.; Dionisio, B.L.; Braga, E.M.; Mosqueira, V.C.F. In vivo antimalarial efficacy of acetogenins, alkaloids and flavonoids enriched fractions from Annona crassiflora Mart. Nat. Prod. Res. 2014, 28, 1254–1259. [Google Scholar] [CrossRef]
- Kamaraj, C.; Kaushik, N.K.; Mohanakrishnan, D.; Elango, G.; Bagavan, A.; Zahir, A.A.; Rahuman, A.A.; Sahal, D. Antiplasmodial potential of medicinal plant extracts from Malaiyur and Javadhu hills of South India. Parasitol Res. 2012, 111, 703–715. [Google Scholar] [CrossRef]
- Somsak, V.; Polwiang, N.; Chachiyo, S. In vivo antimalarial activity of Annona muricata leaf extract in mice infected with Plasmodium berghei. J. Pathog. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Meira, C.S.; Guimaraes, E.T.; Macedo, T.S.; da Silva, T.B.; Menezes, L.R.A.; Costa, E.V.; Soares, M.B.P. Chemical composition of essential oils from Annona vepretorum Mart. and Annona squamosa L. (Annonaceae) leaves and their antimalarial and trypanocidal activities. J. Essent. Oil Res. 2015, 27, 160–168. [Google Scholar] [CrossRef]
- Kamaraj, C.; Bagavan, A.; Elango, G.; Zahir, A.A.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T.; Abdul Rahuman, A. Larvicidal activity of medicinal plant extracts against Anopheles subpictus & Culex tritaeniorhynchus. Indian J. Med. Res. 2011, 134, 101–106. [Google Scholar]
- Kihampa, C.; Joseph, C.C.; Nkunya, M.H.H.; Magesa, S.M.; Hassanali, A.; Heydenreich, M.; Kleinpeter, E. Larvicidal and IGR activity of extract of Tanzanian plants against malaria vector mosquitoes. J. Vector Borne Dis. 2009, 46, 145–152. [Google Scholar]
- Osorio, E.; Arango, G.J.; Jimenez, N.; Alzate, F.; Ruiz, G.; Gutierrez, D.; Paco, M.A.; Gimenez, A.; Robledo, S. Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J. Ethnopharmacol 2007, 111, 630–635. [Google Scholar] [CrossRef]
- Yamthe, L.R.T.; Fokou, P.V.T.; Mbouna, C.D.J.; Keumoe, R.; Ndjakou, B.L.; Djouonzo, P.T.; Mfopa, A.N.; Legac, J.; Tsabang, N.; Gut, J.; et al. Extracts from Annona muricata L. and Annona reticulata L. (Annonaceae) potently and selectively inhibit Plasmodium falciparum. Medicines 2015, 2, 55–66. [Google Scholar] [CrossRef]
- Garcia Diaz, J.; Tuenter, E.; Escalona Arranz, J.C.; Llaurado Maury, G.; Cos, P.; Pieters, L. Antimicrobial activity of leaf extracts and isolated constituents of Croton linearis. J. Ethnopharmacol. 2019, 236, 250–257. [Google Scholar] [CrossRef]
- Mollataghi, A.; Coudiere, E.; Hadi, A.H.A.; Mukhtar, M.R.; Awang, K.; Litaudon, M.; Ata, A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia 2012, 83, 298–302. [Google Scholar] [CrossRef]
- de Lima, J.P.S.; Pinheiro, M.L.B.; Santos, A.M.G.; Pereira, J.L.S.; Santos, D.M.F.; Barison, A.; Silva-Jardim, I.; Costa, E. V In vitro antileishmanial and cytotoxic activities of Annona mucosa (Annonaceae). Rev. Virtual Quim. 2012, 4, 692–702. [Google Scholar]
- de Omena, M.C.; Navarro, D.M.A.F.; de Paula, J.E.; Luna, J.S.; Ferreira de Lima, M.R.; Sant’Ana, A.E.G. Larvicidal activities against Aedes aegypti of some Brazilian medicinal plants. Bioresour. Technol. 2007, 98, 2549–2556. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; De Paula, J.E.; Degallier, N.; Molez, J.E.; Espindola, L.S. Larvicidal activity of some Cerrado plant extracts against Aedes aegypti. J. Am. Mosq Control. Assoc. 2006, 22, 314–317. [Google Scholar] [CrossRef]
- Hoe, P.K.; Yiu, P.H.; Eea, G.C.L.; Wong, S.C.; Rajan, A.; Bong, C.F.J. Biological Activity of Annona muricata Seed Extracts. Malaysian J. Sci. 2010, 29, 153–159. [Google Scholar] [CrossRef]
- Das, N.G.; Goswami, D.; Rabha, B. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J. Vector Borne Dis 2007, 44, 145–148. [Google Scholar]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.-H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lu, W.; Zhou, X. Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: Persimmon, guava, and sweetsop. Biomed. Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-S.; Jong, T.-T.; Tien, H.-J.; Kuoh, C.-S.; Furukawa, H.; Lee, K.-H. Annoquinone-A, an antimicrobial and cytotoxic principle from Annona montana. Phytochemistry 1987, 26, 1623–1625. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Pereira, C.R.; Pimenta, L.P.S.; Boaventura, M.A.D.; Silva, L.G.F.E. Antibacterial activity of eight Brazilian annonaceae plants. Nat. Prod. Res. 2006, 20, 21–26. [Google Scholar] [CrossRef]
- Bories, C.; Loiseau, P.; Cortes, D.; Myint, S.H.; Hocquemiller, R.; Gayral, P.; Cave, A.; Laurens, A. Antiparasitic activity of Annona muricata and Annona cherimolia seeds. Planta Med. 1991, 57, 434–436. [Google Scholar] [CrossRef]
- Bento, E.B.; Matias, E.F.F.; Brito, F.E.; Oliveira, D.R.; Coutinho, H.D.M.; Costa, J.G.M.; Kerntopf, M.R.; Menezes, I.R.A. Association between food and drugs: Antimicrobial and synergistic activity of Annona muricata L. Int. J. Food Prop. 2012, 16, 738–744. [Google Scholar] [CrossRef]
- Tsobou, R.; Mapongmetsem, P.-M.; Voukeng, K.I.; Van Damme, P. Phytochemical screening and antibacterial activity of medicinal plants used to treat typhoid fever in Bamboutos division, West Cameroon. J. Appl. Pharm. Sci. 2015, 5, 34–49. [Google Scholar]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial activities of the methanol extracts of Canarium schweinfurthii and four other Cameroonian dietary plants against multi-drug resistant Gram-negative bacteria. Saudi J. Biol. Sci. 2016, 23, 565–570. [Google Scholar] [CrossRef]
- Essama, S.H.R.; Nyegue, M.A.; Foe, C.N.; Silihe, K.K.; Tamo, S.P.B.; Etoa, F.X. Antibacterial and antioxidant activities of hydro-ehanol extracts of barks, leaves and stems of Annona muricata. Am. J. Pharmacol. Sci. 2015, 3, 126–131. [Google Scholar]
- Darji, B.; Ratani, J.; Doshi, M.; Kothari, V. In vitro antimicrobial activity in certain plant products/seed extracts against selected phytopathogens. Res. Pharm. 2012, 2, 1–10. [Google Scholar]
- Mohamad, N.; Majid, E.-M.; Falah, A.; Layla, C.; Akram, H.; Ali, C.; Hassan, R. Antibacterial, antioxidant and antiproliferative activities of the hydroalcoholic extract of the Lebanese Annona squamosa L. seeds. Int. Res. J. Pharm. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- More, G.; Tshikalange, T.E.; Lall, N.; Botha, F.; Meyer, J.J.M. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol 2008, 119, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; da Cruz, P.E.O.; de Lourenço, C.C.; de Souza Moraes, V.R.; de Lima Nogueira, P.C.; Salvador, M.J. Antioxidant and antimicrobial activities of aporphinoids and other alkaloids from the bark of Annona salzmannii A. DC. (Annonaceae). Nat. Prod. Res. 2012, 27, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Bettarini, F.; Borgonovi, G.E.; Fiorani, T.; Gagliardi, I.; Caprioli, V.; Massardo, P.; Ogoche, J.I.J.; Hassanali, A.; Nyandat, E.; Chapya, A. Antiparasitic compounds from East African plants: Isolation and biological activity of anonaine, matricarianol, canthin-6-one and caryophyllene oxide. Insect Sci. Its Appl. 1993, 14, 93–99. [Google Scholar] [CrossRef]
- Rao, G.-X.; Zhang, S.; Wang, H.-M.; Li, Z.-M.; Gao, S.; Xu, G.-L. Antifungal alkaloids from the fresh rattan stem of Fibraurea recisa Pierre. J. Ethnopharmacol. 2009, 123, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Du, F.; Yan, L.; He, G.; He, J.; Wang, C.; Rao, G.; Jiang, Y.; Xu, G. Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules 2015, 20, 17913–17928. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Amin, G.; Shidfar, M.R.; Hadizadeh, H.; Shafiee, A. Antifungal activity of the methanolic extract and alkaloids of Glaucium oxylobum. Fitoterapia 2003, 74, 493–496. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wu, C.-L.; Huang, S.-L.; Chang, H.-T. Antifungal activity of Liriodenine from Michelia formosana heartwood against wood-rotting fungi. Wood Sci. Technol. 2012, 46, 737–747. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, J.; Liu, X.; Qiu, J.; Min, H.; Zheng, R.; Xu, H.; Li, H.; Zhan, R.; Chen, W. Antibacterial constituents from roots of Zanthoxylum nitidum. Zhongcaoyao 2013, 44, 1546–1551. [Google Scholar]
- Li, C.; Lee, D.; Graf, T.N.; Phifer, S.S.; Nakanishi, Y.; Riswan, S.; Setyowati, F.M.; Saribi, A.M.; Soejarto, D.D.; Farnsworth, N.R.; et al. Bioactive constituents of the stem bark of Mitrephora glabra. J. Nat. Prod. 2009, 72, 1949–1953. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Zayed, M.F.; Sayed, H.M. Ingenines A and B, Two new alkaloids from the Indonesian sponge Acanthostrongylophora ingens. Drug Res. 2015, 65, 361–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, E.V.; Marques, F. de A.; Pinheiro, M.L.B.; Braga, R.M.; Delarmelina, C.; Duarte, M.C.T.; Ruiz, A.L.T.G.; Ernesto de Carvalho, J.; Maia, B.H.L.N.S. Chemical constituents isolated from the bark of Guatteria blepharophylla (Annonaceae) and their antiproliferative and antimicrobial activities. J. Braz. Chem. Soc. 2011, 22, 1111–1117. [Google Scholar]
- Liu, C.-M.; Kao, C.-L.; Wu, H.-M.; Li, W.-J.; Huang, C.-T.; Li, H.-T.; Chen, C.-Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Liu, T.-Z.; Tseng, W.-C.; Lu, F.-J.; Hung, R.-P.; Chen, C.-H.; Chen, C.-H. (-)-Anonaine induces apoptosis through Bax- and caspase-dependent pathways in human cervical cancer (HeLa) cells. Food Chem. Toxicol. 2008, 46, 2694–2702. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Ebel, R.; Ebel, R.; Proksch, P. Acanthomine A, a new pyrimidine-β-carboline alkaloid from the sponge Acanthostrongylophora ingens. Nat. Prod. Commun. 2008, 3, 175–178. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Chang, F.-R.; Chia, Y.-C.; Chen, C.-Y.; Lin, H.-C.; Chiu, H.-F.; Wu, Y.-C. The alkaloids of Artabotrys uncinatus. J. Nat. Prod. 2001, 64, 1157–1161. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Chang, F.-R.; Chia, Y.-C.; Chen, C.-Y.; Chiu, H.-F.; Wu, Y.-C. Cytotoxic Constituents of the Fruits of Cananga odorata. J. Nat. Prod. 2001, 64, 616–619. [Google Scholar] [CrossRef]
- Nordin, N.; Majid, N.A.; Mohan, S.; Dehghan, F.; Karimian, H.; Rahman, M.A.; Ali, H.M.; Hashim, N.M. Cleistopholine isolated from Enicosanthellum pulchrum exhibits apoptogenic properties in human ovarian cancer cells. Phytomedicine 2016, 23, 406–416. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.-Y.; Han, C.-R.; Yuan, Y.; Yang, B.; Zhang, Y.; Wang, J.; Zhong, X.-Q.; Huang, X. Two novel alkaloids from the stem of Saprosma hainanense and their cytotoxic activities in vitro. Chem. Pharm. Bull. 2011, 59, 338–340. [Google Scholar] [CrossRef]
- Sun, R.; He, Q.; Deng, Z.; Geng, Z.; Jiang, H.; Zhang, W.; Yang, K.; Du, S.; Wang, C.; Fan, L.; et al. Cytotoxicity of Aporphine, Protoberberine, and Protopine Alkaloids from Dicranostigma leptopodum (Maxim.) Fedde. Evid. Based. Complement. Alternat. Med. 2014, 2014, 1–6. [Google Scholar]
- Del Rayo Camacho, M.; Kirby, G.C.; Warhurst, D.C.; Croft, S.L.; Phillipson, J.D. Oxoaporphine alkaloids and quinones from Stephania dinklagei and evaluation of their antiprotozoal activities. Planta Med. 2000, 66, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Goeren, A.C.; Zhou, B.; Kingston, D.G.I. Cytotoxic and DNA damaging activity of some aporphine alkaloids from Stephania dinklagei. Planta Med. 2003, 69, 867–868. [Google Scholar]
- Rong, L.; Hu, D.; Wang, W.; Zhao, R.; Xu, X.; Jing, W. Alkaloids from root tubers of Stephania kwangsiensis H.S.Lo and their effects on proliferation and apoptosis of lung NCI-H446 cells. Biomed. Res. 2016, 27, 893–896. [Google Scholar]
- Chen, J.J.; Ishikawa, T.; Duh, C.Y.; Tsai, I.L.; Chen, I.S. New dimeric aporphine alkaloids and cytotoxic constituents of Hernandia nymphaefolia. Planta Med. 1996, 62, 528–533. [Google Scholar] [CrossRef]
- Soonthornchareonnon, N.; Suwanborirux, K.; Bavovada, R.; Patarapanich, C.; Cassady, J.M. New cytotoxic 1-azaanthraquinones and 3-aminonaphthoquinone from the stem bark of Goniothalamus marcanii. J. Nat. Prod. 1999, 62, 1390–1394. [Google Scholar] [CrossRef]
- Hoet, S.; Stevigny, C.; Block, S.; Opperdoes, F.; Colson, P.; Baldeyrou, B.; Lansiaux, A.; Bailly, C.; Quetin-Leclercq, J. Alkaloids from Cassytha filiformis and related aporphines: Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 2004, 70, 407–413. [Google Scholar]
- Hassan, E.M.; Hassan, R.A.; Salib, J.Y.; Mohamed, S.M.; El-Toumy, S.A. Chemical constituents and cytotoxic activity of Codiaeum variegatum CV. petra. J. Appl. Sci. Res. 2013, 9, 4884–4888. [Google Scholar]
- Kim, K.H.; Piao, C.J.; Choi, S.U.; Son, M.W.; Lee, K.R. New cytotoxic tetrahydroprotoberberine-aporphine dimeric and aporphine alkaloids from Corydalis turtschaninovii. Planta Med. 2010, 76, 1732–1738. [Google Scholar] [CrossRef]
- Demirgan, R.; Karagoz, A.; Pekmez, M.; Onay-Ucar, E.; Artun, F.T.; Gurer, C.; Mat, A. In vitro anticancer activity and cytotoxicity of some papaver alkaloids on cancer and normal cell lines. African J. Tradit. Complement. Altern. Med. 2016, 13, 22–26. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Hassan, E.M.; Ibrahim, N.A. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat. Prod. Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef]
- Ubonopas, L.; Wongsinkongman, P.; Chuakul, W.; Suwanborirux, K.; Lee, K.H.; Soonthornchareonnon, N. Bioactive flavonoids and alkaloids from Anomianthus dulcis (Dunal) J. Sinclair stem bark. Mahidol Univ. J. Pharm. Sci. 2014, 41, 13–22. [Google Scholar]
- Pang, S.-Q.; Wang, G.-Q.; Lin, J.; Diao, Y.; Xu, R. Cytotoxic activity of the alkaloids from Broussonetia papyrifera fruits. Pharm. Biol. 2014, 52, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lopa, S.S.; Sadik, G.; Harun-or-Rashid; Islam, R.; Khondkar, P.; Alam, A.H.M.K.; Rashid, M.A. Antibacterial and cytotoxic compounds from the bark of Cananga odorata. Fitoterapia 2005, 76, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Khamis, S.; Bibby, M.C.; Brown, J.E.; Cooper, P.A.; Scowen, I.; Wright, C.W. Phytochemistry and preliminary biological evaluation of Cyathostemma argenteum, a Malaysian plant used traditionally for the treatment of breast cancer. Phyther. Res. 2004, 18, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jian, L.; Chen, G.; Song, X.; Han, C.; Wang, J. Chemical constituents and in vitro anticancer cytotoxic activities of Polyalthia plagioneura. Chem. Nat. Compd. 2014, 49, 1172–1174. [Google Scholar] [CrossRef]
- Nordin, N.; Abdul Majid, N.; Hashim, N.M.; Abd Rahman, M.; Hassan, Z.; Ali, H.M. Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression. Drug Des. Devel. Ther. 2015, 9, 1437–1448. [Google Scholar]
- Macabeo, A.P.G.; Lopez, A.D.A.; Schmidt, S.; Heilmann, J.; Dahse, H.-M.; Alejandro, G.J.D.; Franzblau, S.G. Antitubercular and cytotoxic constituents from Goniothalamus gitingensis. Rec. Nat. Prod. 2014, 8, 41–45. [Google Scholar]
- Costa, E.V.; Pinheiro, M.L.B.; Maia, B.H.L.N.S.; Marques, F.A.; Ruiz, A.L.T.G.; Marchetti, G.M.; de Carvalho, J.E.; Soares, M.B.P.; Costa, C.O.S.; Galvao, A.F.C.; et al. 7,7-Dimethylaporphine and Other Alkaloids from the Bark of Guatteria friesiana. J. Nat. Prod. 2016, 79, 1524–1531. [Google Scholar] [CrossRef]
- Dong, X.; Mondranondra, I.O.; Che, C.T.; Fong, H.H.S.; Farnsworth, N.R. Kmeriol and other aromatic constituents of Kmeria duperreana. Pharm. Res. 1989, 6, 637–640. [Google Scholar] [CrossRef]
- Mondranondra, I.O.; Che, C.T.; Rimando, A.M.; Vajrodaya, S.; Fong, H.H.S.; Farnsworth, N.R. Sesquiterpene lactones and other constituents from a cytotoxic extract of Michelia floribunda. Pharm. Res. 1990, 7, 1269–1272. [Google Scholar] [CrossRef]
- Chan, Y.-Y.; Juang, S.-H.; Huang, G.-J.; Liao, Y.-R.; Chen, Y.-F.; Wu, C.-C.; Chang, H.-T.; Wu, T.-S. The constituents of Michelia compressa var. formosana and their bioactivities. Int. J. Mol. Sci. 2014, 15, 10926–10935. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-W.; Liu, C.-M.; Chung, M.-I.; Chen, C.-Y. Biofunctional constituents from Michelia compressa var. lanyuensis with anti-melanogenic properties. Molecules 2015, 20, 12166–12174. [Google Scholar] [CrossRef]
- Still, P.C.; Yi, B.; Gonzalez-Cestari, T.F.; Pan, L.; Pavlovicz, R.E.; Chai, H.-B.; Ninh, T.N.; Li, C.; Soejarto, D.D.; McKay, D.B.; et al. Alkaloids from Microcos paniculata with Cytotoxic and Nicotinic Receptor Antagonistic Activities. J. Nat. Prod. 2013, 76, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Quan, T.D.; Nguyen, T.H.A.; Sung, T.V. A new hydrochalcone from Miliusa sinensis. Nat. Prod. Res. 2011, 25, 1361–1365. [Google Scholar] [CrossRef]
- Chang, F.-R.; Hwang, T.-L.; Yang, Y.-L.; Li, C.-E.; Wu, C.-C.; Issa, H.H.; Hsieh, W.-B.; Wu, Y.-C. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med. 2006, 72, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Wirasathien, L.; Boonarkart, C.; Pengsuparp, T.; Suttisri, R. Biological activities of alkaloids from Pseuduvaria setosa. Pharm. Biol. 2006, 44, 274–278. [Google Scholar] [CrossRef]
- Waechter, A.-I.; Cave, A.; Hocquemiller, R.; Bories, C.; Munoz, V.; Fournet, A. Antiprotozoal activity of aporphine alkaloids isolated from Unonopsis buchtienii (Annonaceae). Phyther. Res. 1999, 13, 175–177. [Google Scholar] [CrossRef]
- Zhao, L.-N.; Wang, J.; Wang, Z.; Tan, N.-H. Chemical and cytotoxic constituents of Zanthoxylum nitidum. Zhongguo Zhong Yao Za Zhi 2018, 43, 4659–4664. [Google Scholar]
- Yang, C.-H.; Cheng, M.-J.; Lee, S.-J.; Yang, C.-W.; Chang, H.-S.; Chen, I.-S. Secondary metabolites and cytotoxic activities from the stem bark of Zanthoxylum nitidum. Chem. Biodivers. 2009, 6, 846–857. [Google Scholar] [CrossRef]
- Amna, U.; Hasnan, M.H.H.; Ahmad, K.; Abdul Manaf, A.; Awang, K.; Nafiah, M.A. In vitro cytotoxic of aporphine and proaporphine alkaloids from phoebe grandis (Ness) merr. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 15–20. [Google Scholar]
- Chang, Y.-C.; Chang, F.-R.; Khalil, A.T.; Hsieh, P.-W.; Wu, Y.-C. Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z. Naturforsch. C. 2003, 58, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Zahari, A.; Cheah, F.K.; Mohamad, J.; Sulaiman, S.N.; Litaudon, M.; Leong, K.H.; Awang, K. Antiplasmodial and antioxidant isoquinoline alkaloids from Dehaasia longipedicellata. Planta Med. 2014, 80, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chen, J.J.; Duh, C.Y.; Tsai, J.L.; Chang, C.T. New aporphine alkaloids and cytotoxic constituents of Hernandia nymphaefolia. Planta Med. 1997, 63, 154–157. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).