Highly Sensitive Detection of PCV2 Based on Tyramide Signals and GNPL Amplification
Abstract
1. Introduction
2. Results and Discussion
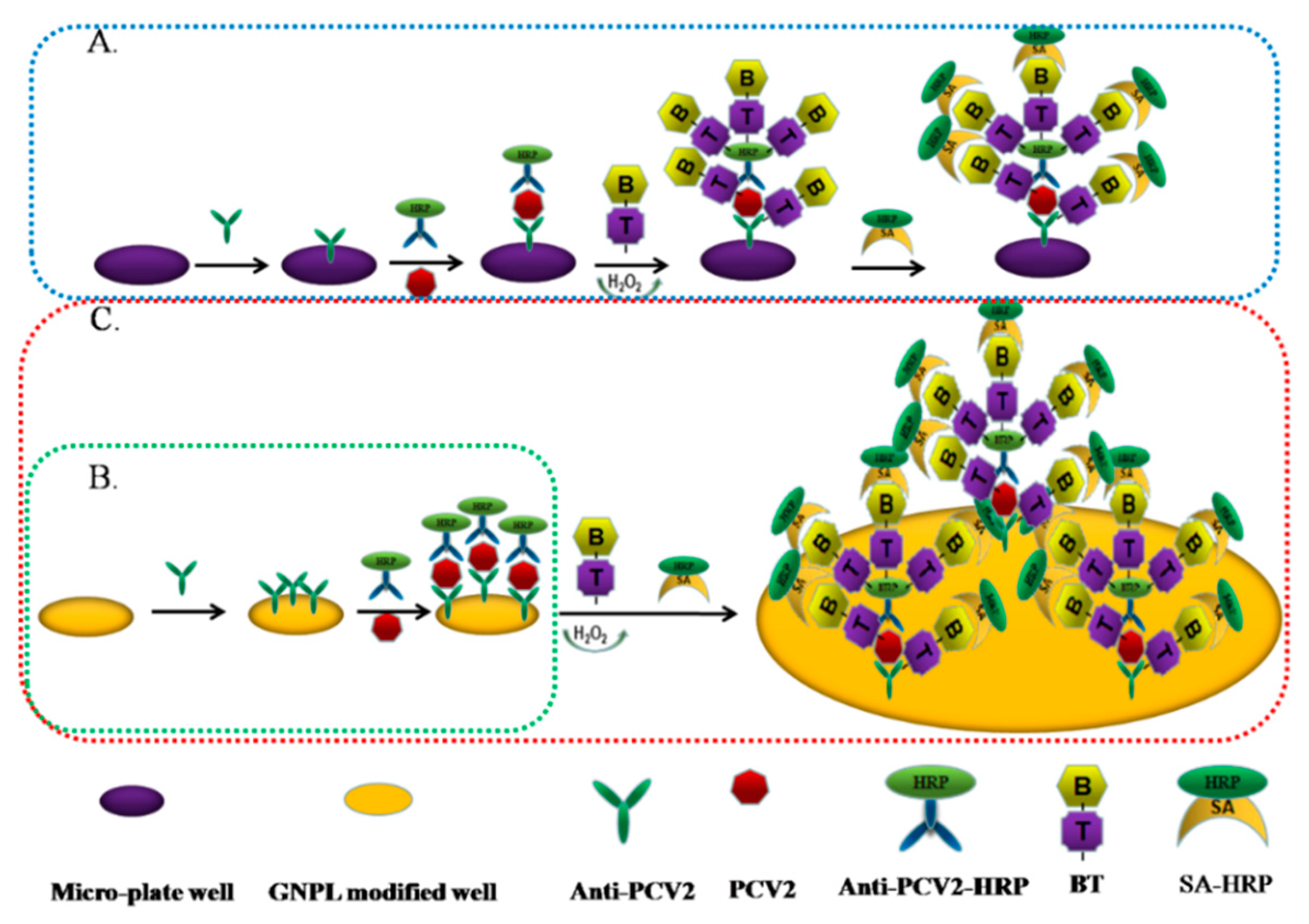
2.1. Basic Principles of the Dual Signal Amplification-Based Detection Method
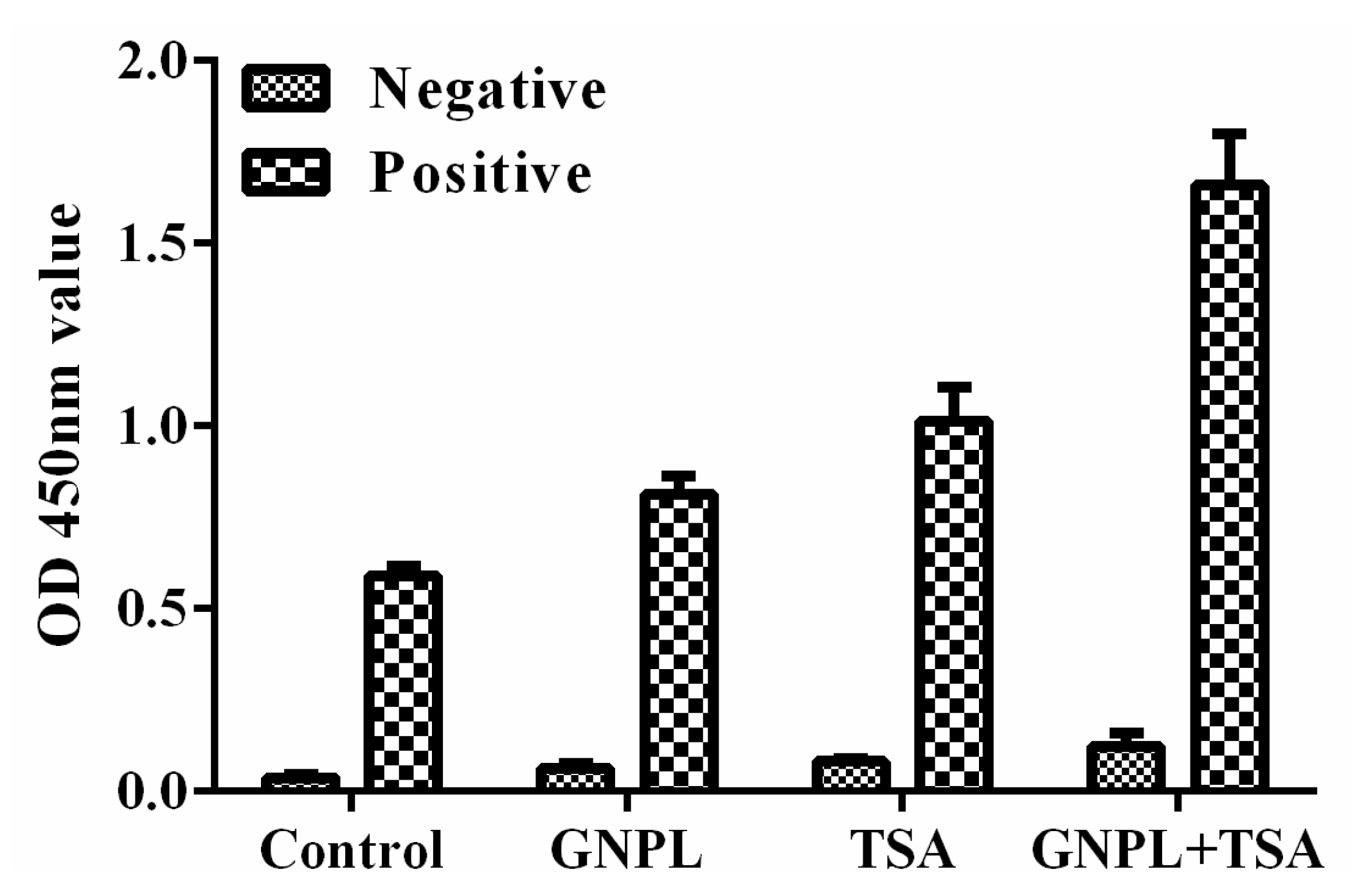
2.2. Verification of the Effect of Dual Signal Amplification
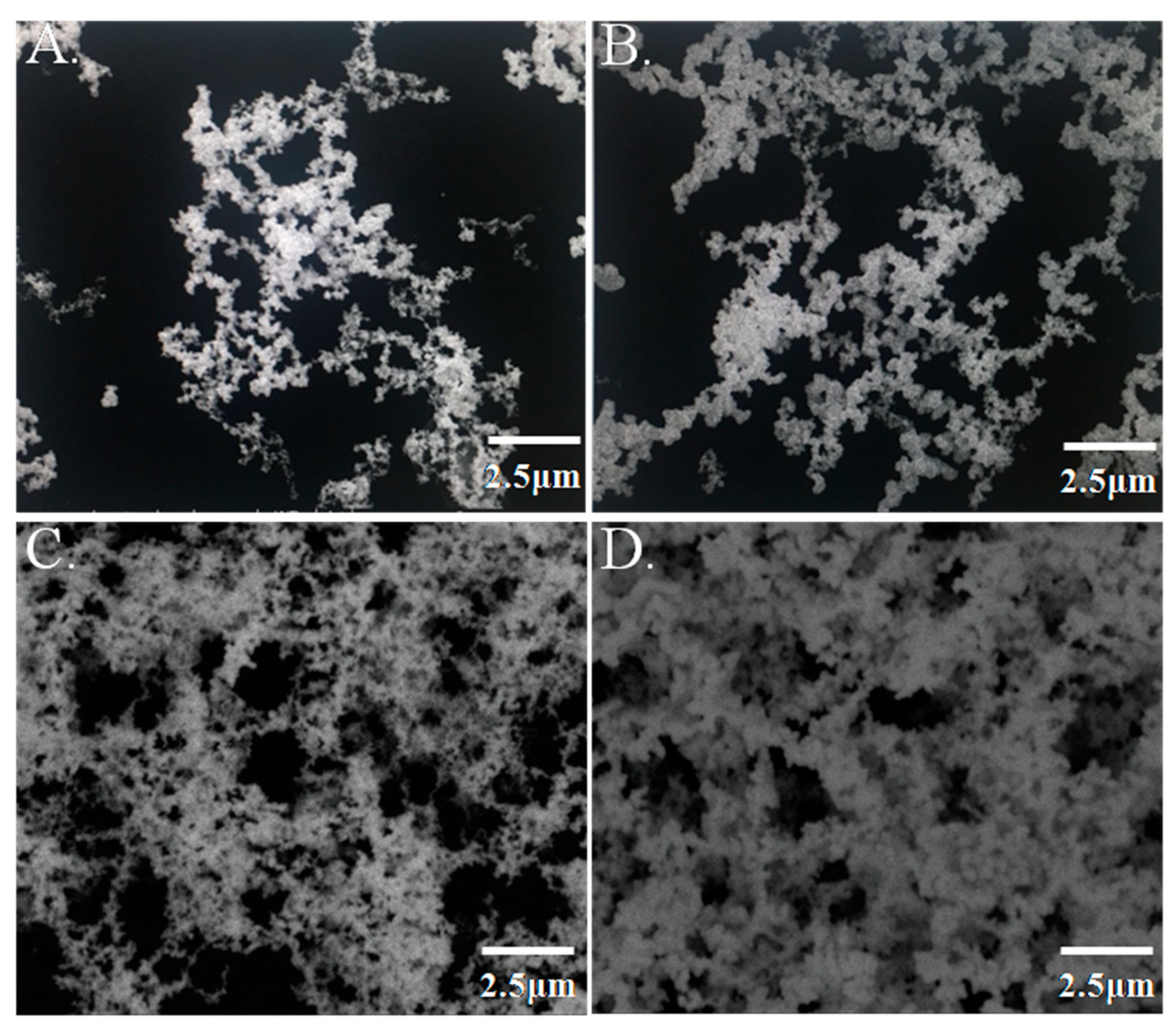
2.3. Characterization of GNPL
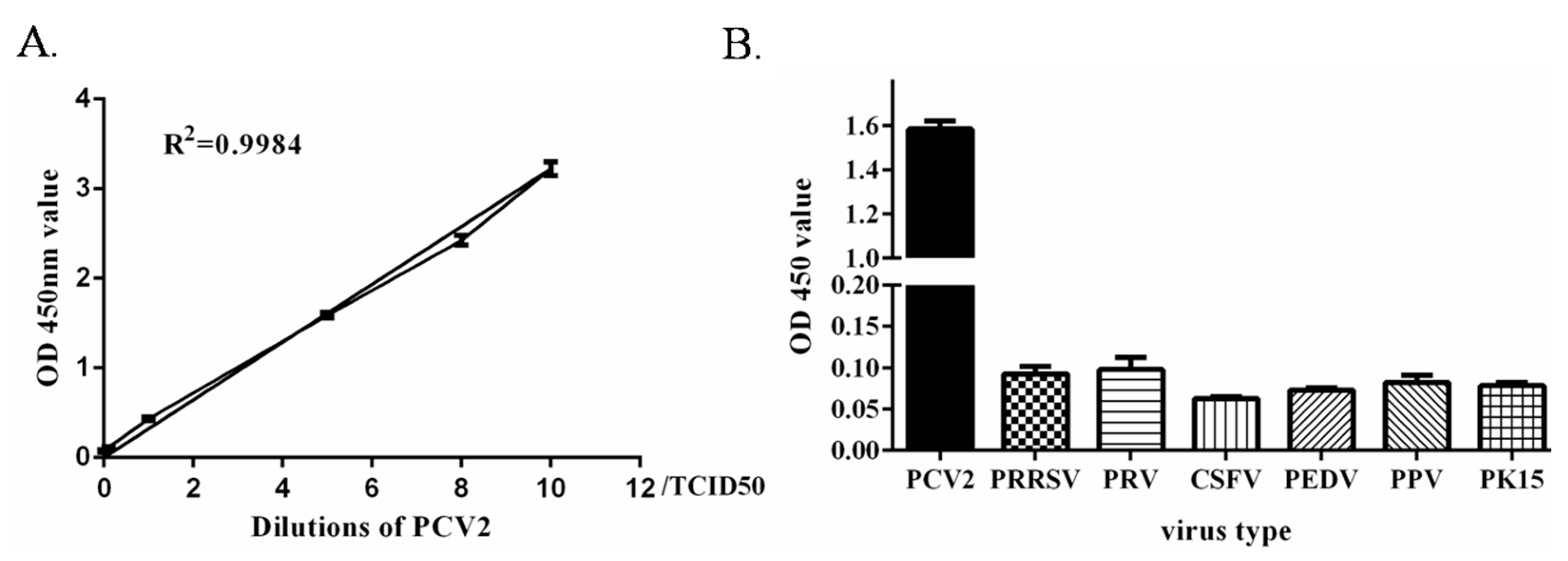
2.4. Establishment and Evaluation of the Dual Signal Amplification-Based Detection Method
2.5. Application of the Dual Signal Amplification-Based Detection Method in Clinical Samples
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of GNPL-Modified Microplate
3.3. Conjuated HRP and PCV2 Antibody
3.4. Procedure of the Amplified ELISA
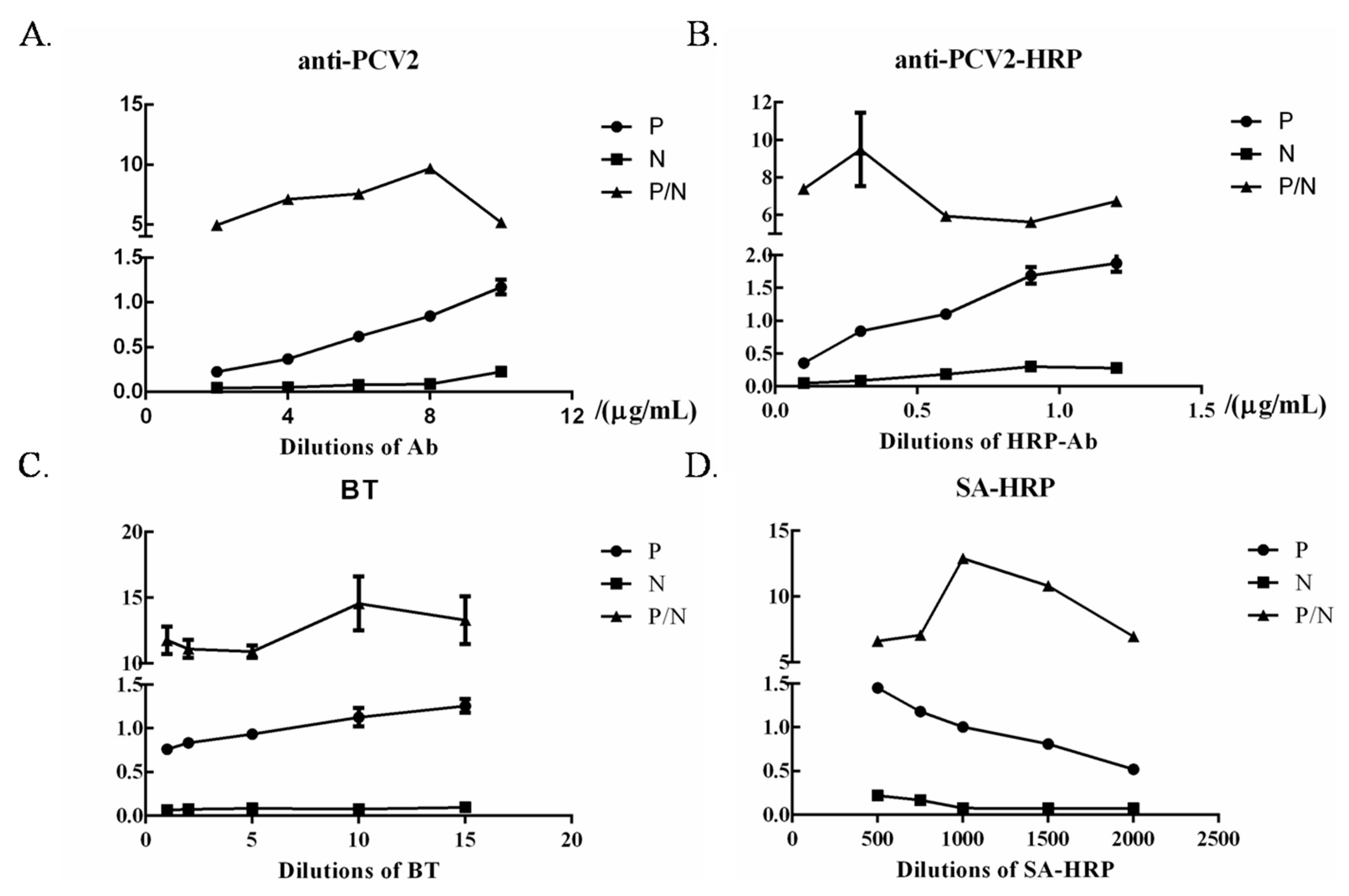
3.5. Optimization of ELISA Working Conditions
3.6. Validation of ELISA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McClenahan, S.D.; Krause, P.R.; Uhlenhaut, C. Molecular and infectivity studies of porcine circovirus in vaccines. Vaccine 2011, 29, 4745–4753. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.R.; Opriessnig, T. Epidemiology and horizontal transmission of porcine circovirus type 2 (PCV2). Anim. Heal. Res. Rev. 2010, 11, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Giannitti, F.; Rossow, S.; Marthaler, D.; Knutson, T.P.; Li, L.; Deng, X.; Resende, T.; Vannucci, F.; Delwart, E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol. J. 2016, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Sun, W.; Li, Z.; Zhuang, X.; Zhao, G.; Xie, C.; Zheng, M.; Jing, J.; Xiao, P.; Wang, M.; et al. The detection of porcine circovirus 3 in Guangxi, China. Transbound. Emerg. Dis. 2018, 65, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, H.; Shi, L.; Li, L. Development of a droplet digital polymerase chain reaction for sensitive and simultaneous identification of porcine circovirus type 2 and 3. J. Virol. Methods 2019, 270, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, X.; Sun, Y.; Cong, G.; Li, Y.; Zhang, Z. Development of isothermal recombinase polymerase amplification assay for rapid detection of porcine circovirus type 2. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, H.-Y.; Fan, L.; Tian, R.-B.; Cui, J.-T.; Li, J.-Y.; Chen, H.-Y.; Yang, M.-F.; Zheng, L.-L.; Li, J.-Y. Development of a TB green II-based duplex real-time fluorescence quantitative PCR assay for the simultaneous detection of porcine circovirus 2 and 3. Mol. Cell. Probes 2019, 45, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Li, R.-C.; Qu, T.; Gong, W.; Yu, X.-L.; Tu, C. Construction of an HRP-streptavidin bound antigen and its application in an ELISA for porcine circovirus 2 antibodies. AMB Express 2017, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Aminabad, N.S.; Farshbaf, M.; Akbarzadeh, A. Recent advances of gold nanoparticles in biomedical applications: State of the art. Cell Biochem. Biophys. 2019, 77, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, K.; Zhu, G.; Sun, Z.; He, S.; Chen, W. Blocking-Free ELISA using a gold nanoparticle layer coated commercial microwell plate. Sensors 2018, 18, 3537. [Google Scholar] [CrossRef] [PubMed]
- Kohout, C.; Santi, C.; Polito, L. Anisotropic gold nanoparticles in biomedical applications. Int. J. Mol. Sci. 2018, 19, 3385. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Cradduck, M.; Cheung, L.; Olive, D.M. Development of a near-infrared fluorescence ELISA method using tyramide signal amplification. Anal. Biochem. 2012, 426, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Ian, D.A.; Tim, H.D. Tyramide signal amplification for DNA and mRNA in situ hybridization. In In Situ Hybridization Protocols; Humana Press: Totowa, NJ, USA, 2006; pp. 33–60. [Google Scholar]
- Zhang, Z.; Kitching, P. A sensitive method for the detection of foot and mouth disease virus by in situ hybridisation using biotin-labelled oligodeoxynucleotides and tyramide signal amplification. J. Virol. Methods 2000, 88, 187–192. [Google Scholar] [CrossRef]
- Trang, N.T.; Hirai, T.; Ngan, P.H.; Lan, N.T.; Fuke, N.; Toyama, K.; Yamamoto, T.; Yamaguchi, R. Enhanced detection of Porcine reproductive and respiratory syndrome virus in fixed tissues by in situ hybridization following tyramide signal amplification. J. Vet. Diagn. Investig. 2015, 27, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, M.; Yuan, L.; Cheng, Z.; Wu, Z.; Chen, H. Sensitive sandwich ELISA based on a gold nanoparticle layer for cancer detection. Analyst 2012, 137, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Stack, E.C.; Wang, C.; Roman, K.A.; Hoyt, C.C. Multiplexed immunohistochemistry, imaging, and quantitation: A review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 2014, 70, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Camargo, I.F.; Gaspar, A.M.C.; Yoshida, C.F.T. Comparative ELISA reagents for detection of hepatitis B surface antigen (HBsAg). Memórias do Inst. Oswaldo Cruz 1987, 82, 181–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
Sample Availability: Samples of the compounds are available from the authors. |
| Sample | A | B | C |
|---|---|---|---|
| Coefficient variation of interassay | 5.24% | 4.76% | 4.25% |
| Coefficient variation of intra-assay | 6.13% | 4.86% | 5.13% |
| Sample Source | Number of Positive | Number of Negative |
|---|---|---|
| Lung | 3 | 9 |
| Spleen | 9 | 3 |
| Lymph node | 10 | 2 |
| Serum | 1 | 11 |
| Oral | 4 | 8 |
| Total | 27 | 33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Hu, B.; Xia, X.; Xu, Y.; Hang, B.; Jiang, J.; Hu, J. Highly Sensitive Detection of PCV2 Based on Tyramide Signals and GNPL Amplification. Molecules 2019, 24, 4364. https://doi.org/10.3390/molecules24234364
Zhang S, Hu B, Xia X, Xu Y, Hang B, Jiang J, Hu J. Highly Sensitive Detection of PCV2 Based on Tyramide Signals and GNPL Amplification. Molecules. 2019; 24(23):4364. https://doi.org/10.3390/molecules24234364
Chicago/Turabian StyleZhang, Shouping, Bin Hu, Xiaojing Xia, Yanzhao Xu, Bolin Hang, Jinqing Jiang, and Jianhe Hu. 2019. "Highly Sensitive Detection of PCV2 Based on Tyramide Signals and GNPL Amplification" Molecules 24, no. 23: 4364. https://doi.org/10.3390/molecules24234364
APA StyleZhang, S., Hu, B., Xia, X., Xu, Y., Hang, B., Jiang, J., & Hu, J. (2019). Highly Sensitive Detection of PCV2 Based on Tyramide Signals and GNPL Amplification. Molecules, 24(23), 4364. https://doi.org/10.3390/molecules24234364





