Green Chemistry Extractions of Carotenoids from Daucus carota L.—Supercritical Carbon Dioxide and Enzyme-Assisted Methods
Abstract
1. Introduction
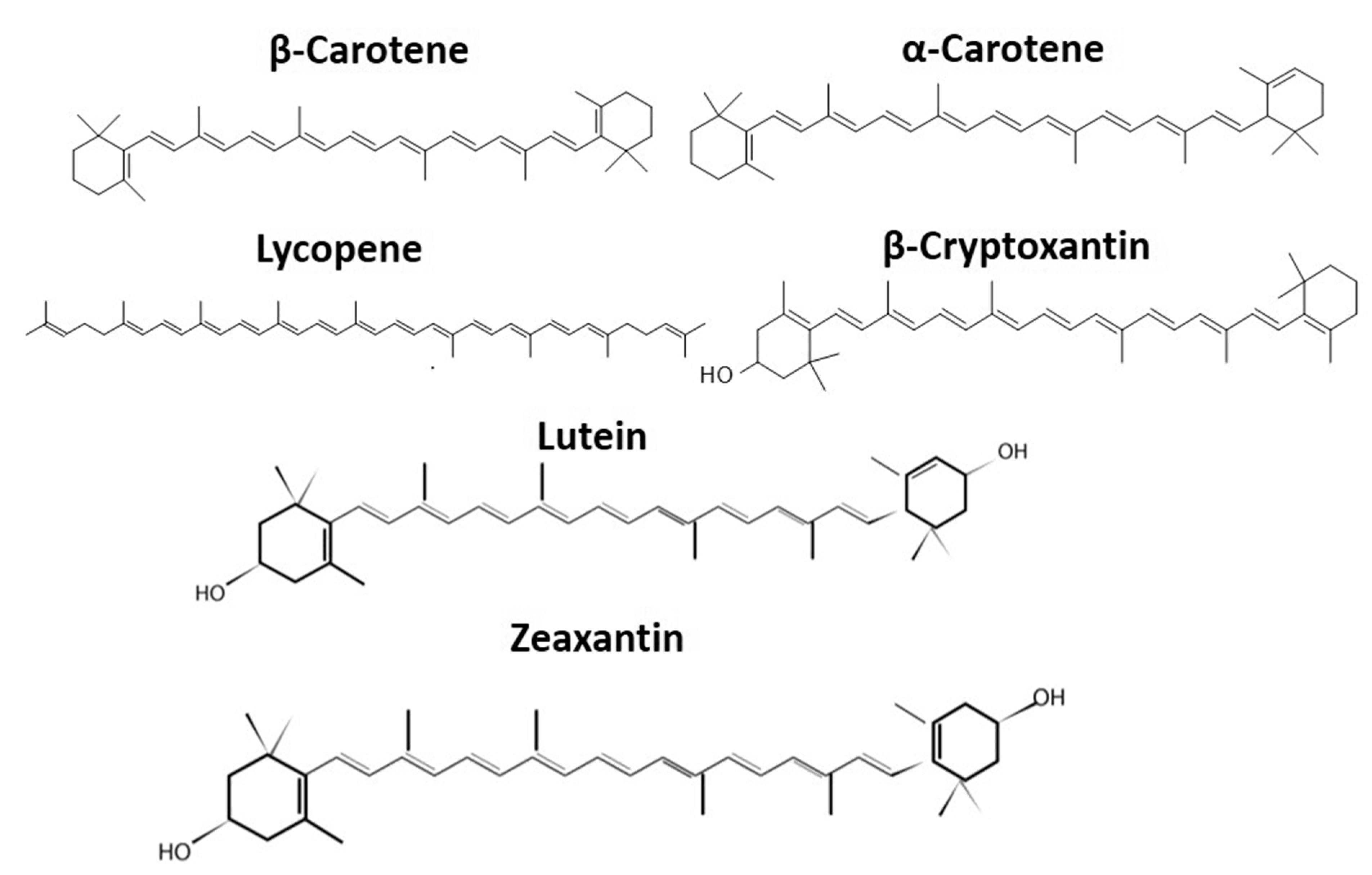
2. Chemical Properties of Carotenoids Important for Their Isolation
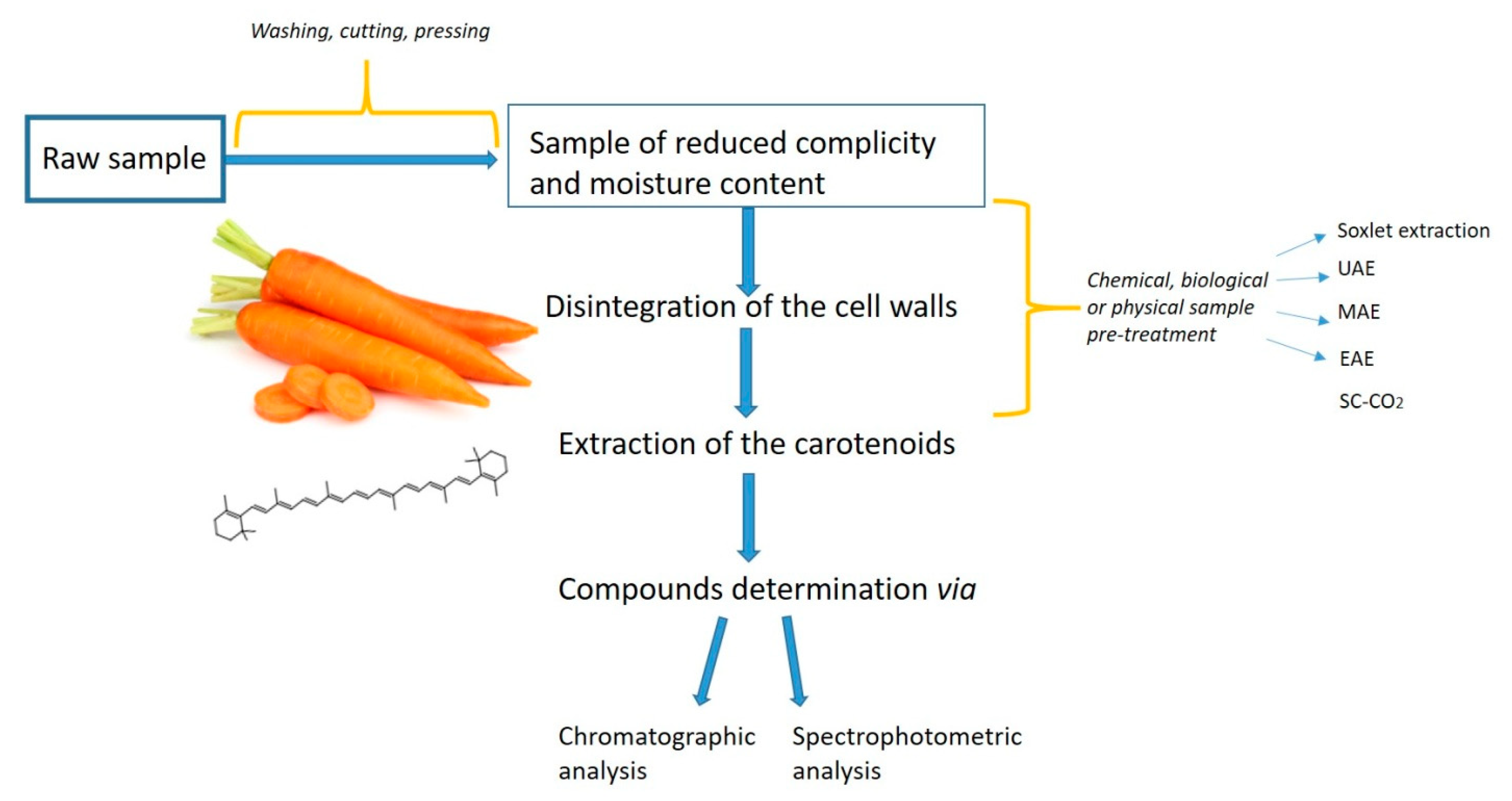
2.1. Aspects to Consider: Carotenoids Extraction
2.2. Pre-Treatments Applied Before Extraction of Carotenoids
2.3. Selection of the Most Appropriate Solvent
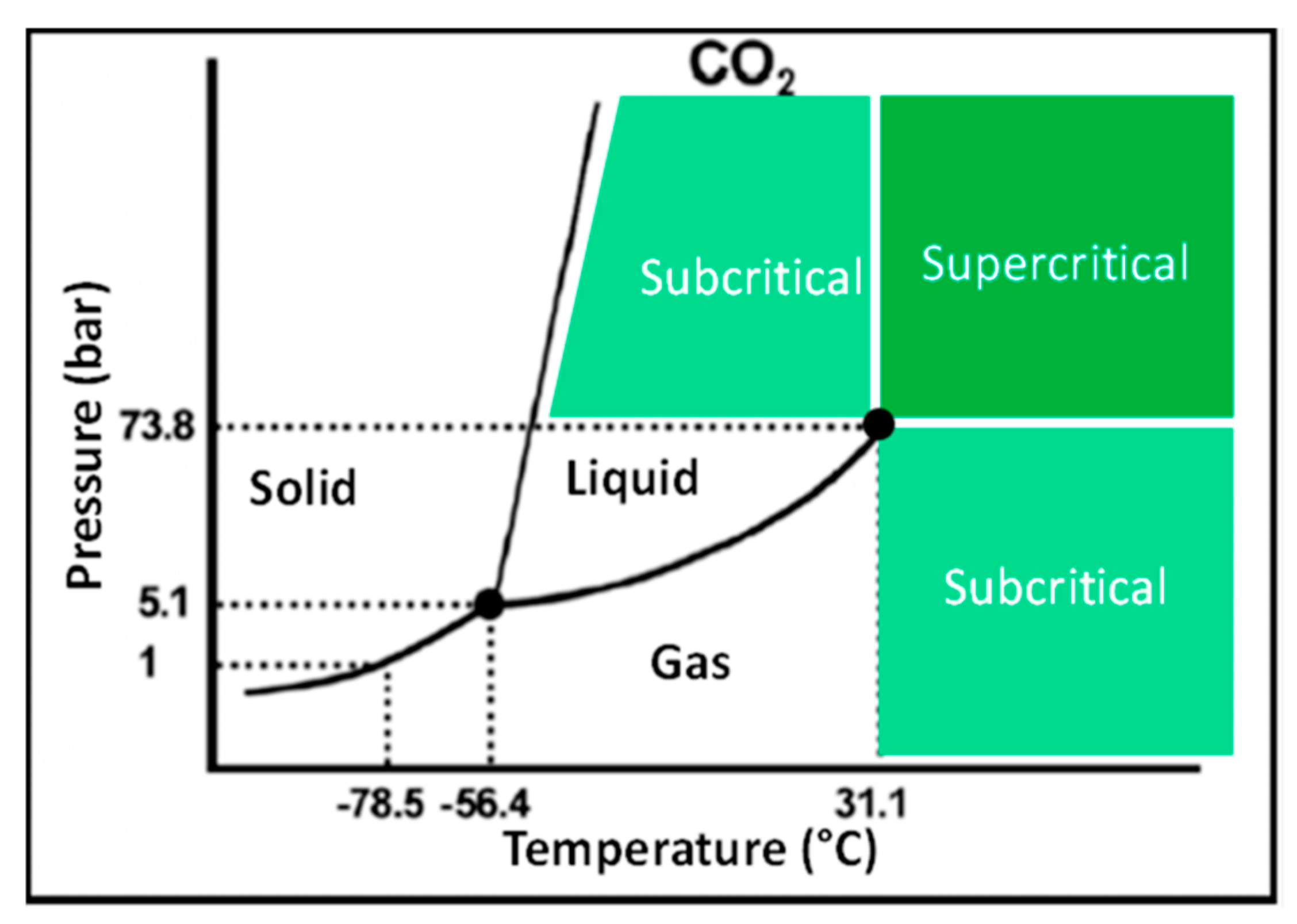
3. Supercritical Carbon Dioxide (SC-CO2) Extraction
SC-CO2 Extraction of Carotenoids from Daucus Carota L.
4. Enzyme-Assisted Extractions (EAE)
4.1. EAE of Carotenoids
4.2. EAE of Carotenoids from Daucus Carota L.
5. Conclusions
Funding
Conflicts of Interest
References
- Moran, N.A.; Jarvik, T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 2010, 328, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Arvayo-Enríquez, H.; Mondaca-Fernández, I.; Gortárez-Moroyoqui, P.; López-Cervantes, J.; Rodríguez-Ramírez, R. Carotenoids extraction and quantification: A review. Anal. Methods 2013, 5, 2916–2924. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plant. Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Nogueira, M.; Berry, H.; Nohl, R.; Klompmaker, M.; Holden, A.; Fraser, P.D. Subchromoplast Fractionation Protocol for Different Solanaceae Fruit Species. Bio-protocol 2016, 6, e1861. [Google Scholar] [CrossRef]
- Kruger, J.; Stuetz, W.; Frank, J. Iron, Catechin, and Ferulic Acid Inhibit Cellular Uptake of β-Carotene by Reducing Micellization. J. Agric. Food Chem. 2019, 67, 5792–5800. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, H.; Shi, J.; Xue, S.J.; Wang, D.; Wang, W.; Cheng, A.; Gong, Z.; Chen, X.; Wang, C. Effect of modifier on the composition and antioxidant activity of carotenoid extracts from pumpkin (Cucurbita maxima) by supercritical CO2. LWT-Food Sci. Technol. 2013, 51, 433–440. [Google Scholar] [CrossRef]
- Sun, M.; Temelli, F. Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J. Supercrit. Fluids 2006, 37, 397–408. [Google Scholar] [CrossRef]
- Lenucci, M.S.; De Caroli, M.; Marrese, P.P.; Iurlaro, A.; Rescio, L.; Böhm, V.; Dalessandro, G.; Piro, G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015, 170, 193–202. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Comim, S.R.R.; Madella, K.; Oliveira, J.V.; Ferreira, S.R.S. Supercritical fluid extraction from dried banana peel (Musa spp., genomic group AAB): Extraction yield, mathematical modeling, economical analysis and phase equilibria. J. Supercrit. Fluids 2010, 54, 30–37. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.D.; Basso, R.C.; Meirelles, A.J.A.; Oliveira, J.V.; Batista, E.A.C.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef]
- Mitra, P.; Ramaswamy, H.S.; Chang, K.S. Pumpkin (Cucurbita maxima) seed oil extraction using supercritical carbon dioxide and physicochemical properties of the oil. J. Food Eng. 2009, 95, 208–213. [Google Scholar] [CrossRef]
- Majdoub, S.; El Mokni, R.; Muradalievich, A.A.; Piras, A.; Porcedda, S.; Hammami, S. Effect of pressure variation on the efficiency of supercritical fluid extraction of wild carrot (Daucus carota subsp. maritimus) extracts. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1125, 121713. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Baranda, A.B.; De Marañón, I.M. The effect of High Pressure and High Temperature processing on carotenoids and chlorophylls content in some vegetables. Food Chem. 2014, 163, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Kaur, C.; Rudra, S.G.; Varghese, E. Enzyme-Assisted Extraction of Carotenoid-Rich Extract from Red Capsicum (Capsicum annuum). Agric. Res. 2016, 5, 193–204. [Google Scholar] [CrossRef]
- Tang, G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010, 91, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of carotenoids in carrot: An underground story comes to light. Arch. Biochem. Biophys. 2013, 539, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Zorrilla-López, U.; Farré, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally important carotenoids as consumer products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Saini, R.K.; Assefa, A.D.; Keum, Y.S. Fatty acid and carotenoid composition of bitter melon (Momordica charantia L.) seed arils: a potentially valuable source of lycopene. J. food Meas. Charact. 2017, 11, 1266–1273. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-utilization of fruits and vegetables waste to produce β-carotene in solid-state fermentation: Characterization and antioxidant activity. Process. Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Suite, WA, USA, 2001; Volume 71, ISBN 1578810728. [Google Scholar]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Norshazila, S.; Koy, C.N.; Rashidi, O.; Ho, L.H.; Azrina, I.; Nurul Zaizuliana, R.A.; Zarinah, Z. The effect of time, temperature and solid to solvent ratio on pumpkin carotenoids extracted using food grade solvents. Sains Malays. 2017, 46, 231–237. [Google Scholar] [CrossRef]
- Rodríguez-Villalón, A.; Gas, E.; Rodríguez-Concepción, M. Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedlings. Plant. J. 2009, 60, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Farré, G.; Bai, C.; Twyman, R.M.; Capell, T.; Christou, P.; Zhu, C. Nutritious crops producing multiple carotenoids-a metabolic balancing act. Trends Plant. Sci. 2011, 16, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R. Bioactive Foods and Extracts; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-1619-6. [Google Scholar]
- Honda, M.; Kageyama, H.; Hibino, T.; Zhang, Y.; Diono, W.; Kanda, H.; Yamaguchi, R.; Takemura, R.; Fukaya, T.; Goto, M. Improved carotenoid processing with sustainable solvents utilizing Z-isomerization-induced alteration in physicochemical properties: A review and future directions. Molecules 2019, 24, 2149. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC-Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Soares, B.; Passos, H.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Ionic liquids in chromatographic and electrophoretic techniques: toward additional improvements in the separation of natural compounds. Green Chem. 2016, 18, 4582–4604. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives†. Food Funct. 2017, 8. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Papadaki, S.; Krokida, M. Life cycle analysis of β-carotene extraction techniques. J. Food Eng. 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, L.; Murtaza, A.; Liu, Y.; Liu, S.; Li, J.; Iqbal, A.; Xu, X.; Pan, S.; Hu, W. Ultrasonic Processing Induced Activity and Structural Changes of Polyphenol Oxidase in Orange (Citrus sinensis Osbeck). Molecules 2019, 24, 1922. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Murtaza, A.; Liu, Y.; Hu, W.; Xu, X.; Pan, S. Catalytic and Structural Characterization of a Browning-Related Protein in Oriental Sweet Melon (Cucumis Melo var. Makuwa Makino). Front. Chem. 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. A Comparative Study of the Quality of Strawberry Purée Preserved by Continuous Microwave Heating and Conventional Thermal Pasteurization During Long-Term Cold Storage. Food Bioprocess. Technol. 2016, 9, 1100–1112. [Google Scholar] [CrossRef]
- Wang, B.-J.; Yang, Q.-S.; Chen, T.; Qin, X.-D.; Ma, J.-R.; Zhao, Y. Optimization of enzyme-assisted extraction of carotenoids antioxidants from Cordyceps militaris using response surface methodology. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Kha, T.C.; Phan-tai, H.; Nguyen, M.H. Effects of pre-treatments on the yield and carotenoid content of Gac oil using supercritical carbon dioxide extraction. J. Food Eng. 2014, 120, 44–49. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Barba, F.J.; Skąpska, S.; Lorenzo, J.M.; Zambon, A.; Spilimbergo, S. Enzymatic, physicochemical, nutritional and phytochemical profile changes of apple (Golden Delicious L.) juice under supercritical carbon dioxide and long-term cold storage. Food Chem. 2018, 268, 279–286. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.T.; Benito-Román, O.; Varona, S.; Beltrán, S.; Melgosa, R.; Solaesa, A.G. Effect of thermosonication batch treatment on enzyme inactivation kinetics and other quality parameters of cloudy apple juice. Innov. Food Sci. Emerg. Technol. 2018, 47, 71–80. [Google Scholar] [CrossRef]
- Briongos, H.; Illera, A.E.; Sanz, M.T.; Melgosa, R.; Beltrán, S.; Solaesa, A.G. Effect of high pressure carbon dioxide processing on pectin methylesterase activity and other orange juice properties. LWT-Food Sci. Technol. 2016, 74, 411–419. [Google Scholar] [CrossRef][Green Version]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. TrAC-Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Mustafa, A.; Trevino, L.M.; Turner, C.; Andersson, J.M. Green technology for extraction of high-value compounds from agricultural by-products using supercritical fluid extraction and chromatography Water Extraction and Particle ormation (WEPO) by CO. 2010. Available online: http://www.kilu.lu.se/fileadmin/kilu/CAS/GTG_pdf_files/Mustafa_SFEandSFC_for_carotenoids_Analysdagarna2012.pdf (accessed on 29 October 2019).
- Pacheco, S.; Peixoto, F.M.; Borguini, R.G.; Nascimento, L.d.S.d.M.d.; Bobeda, C.R.R.; Santiago, M.C.P.d.; Godoy, R.L.D. Microscale extraction method for HPLC carotenoid analysis in vegetable matrices. Sci. Agric. 2014, 71, 416–419. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Carotenes and xanthophylls as antioxidants. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 17–50. ISBN 978-1-78242-089-7. [Google Scholar]
- Ombódi, A.; Daood, H.G.; Helyes, L. Carotenoid and tocopherol composition of an orange-colored carrot as affected by water supply. HortScience 2014, 49, 729–733. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Shetty, N.P.; Giridhar, P. Carotenoid content in vegetative and reproductive parts of commercially grown Moringa oleifera Lam. cultivars from India by LC-APCI-MS. Eur. Food Res. Technol. 2014, 238, 971–978. [Google Scholar] [CrossRef]
- Murtaza, A.; Muhammad, Z.; Iqbal, A.; Ramzan, R.; Liu, Y.; Pan, S.; Hu, W. Aggregation and Conformational Changes in Native and Thermally Treated Polyphenol Oxidase From Apple Juice (Malus domestica). Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cernelic, K.; Prosek, M.; Golc-Wondra, A.; Rodic, Z.; Simonovska, B.; Puklavec, M. Influence of Synthetic Antioxidants on Extraction of All-trans-Lutein from Spinach under Air and Nitrogen Atmosphere. Food Nutr. Sci. 2013, 4, 195–200. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process. Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Mezzomo, N.; Martínez, J.; Maraschin, M.; Ferreira, S.R.S. Pink shrimp (P. brasiliensis and P. paulensis) residue: Supercritical fluid extraction of carotenoid fraction. J. Supercrit. Fluids 2013, 74, 22–33. [Google Scholar] [CrossRef]
- Castro-Ibáñez, I.; Gil, M.I.; Allende, A. Ready-to-eat vegetables: Current problems and potential solutions to reduce microbial risk in the production chain. LWT-Food Sci. Technol. 2017, 85, 284–292. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Urreta, I.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Suárez-Alvarez, S. Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4607–4616. [Google Scholar] [CrossRef]
- Irakli, M.N.; Samanidou, V.F.; Papadoyannis, I.N. Development and validation of an HPLC method for the simultaneous determination of tocopherols, tocotrienols and carotenoids in cereals after solid-phase extraction. J. Sep. Sci. 2011, 34, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Rigi, A.; Abbasi, S. Microemulsion-based lycopene extraction: Effect of surfactants, co-surfactants and pretreatments. Food Chem. 2016, 197, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ho, S.-H.; Chen, C.-N.N.; Chen, C.-Y.; Jing, K.; Ng, I.-S.; Chen, J.; Chang, J.-S.; Lu, Y. Disruption of thermo-tolerant Desmodesmus sp. F51 in high pressure homogenization as a prelude to carotenoids extraction. Biochem. Eng. J. 2016, 109, 243–251. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2019, 125549. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Vijayan, D.; Praveenkumar, R.; Han, J.-I.; Lee, K.; Park, J.-Y.; Chang, W.-S.; Lee, J.-S.; Oh, Y.-K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Hu, W.; Ahmad, I.; Ahmed, A.; Xu, X. Food and Bioproducts Processing Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod. Process. 2019, 117, 170–182. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Muhammad, Z.; Elkhedir, A.E.; Tao, M. Inactivation, Aggregation and Conformational Changes of Polyphenol Oxidase from Quince (Cydonia oblonga Miller) Juice Subjected to Thermal and High-Pressure Carbon Dioxide Treatment. Molecules 2018, 23, 1743. [Google Scholar] [CrossRef]
- Murtaza, A.; Iqbal, A.; Linhu, Z.; Liu, Y.; Xu, X.; Pan, S.; Hu, W. Effect of high-pressure carbon dioxide on the aggregation and conformational changes of polyphenol oxidase from apple (Malus domestica) juice. Innov. Food Sci. Emerg. Technol. 2019. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, L.; Xu, Z.; Zhang, Y.; Liao, X. Enzyme Inactivation in Food Processing using High Pressure Carbon Dioxide Technology. Crit. Rev. Food Sci. Nutr. 2013, 53, 145–161. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Hu, W.; Liao, X. Changes in the activity, dissociation, aggregation, and the secondary and tertiary structures of a thaumatin-like protein with a high polyphenol oxidase activity induced by high pressure CO2. Innov. Food Sci. Emerg. Technol. 2014, 23, 68–78. [Google Scholar] [CrossRef]
- Laboureur, L.; Ollero, M.; Touboul, D. Lipidomics by supercritical fluid chromatography. Int. J. Mol. Sci. 2015, 16, 13868–13884. [Google Scholar] [CrossRef] [PubMed]
- Uquiche, E.; Antilaf, I.; Millao, S. Enhancement of pigment extraction from B. braunii pretreated using CO2 rapid depressurization. Braz. J. Microbiol. 2016, 47, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.F. Extracting Carotenoids from Corn Industry Coproducts. Master’s Thesis, North Dakota State Universityof Agriculture and Applied Science, Fargo, ND, USA, 2016. [Google Scholar]
- Hu, W.; Zhang, Y.; Wang, Y.; Zhou, L.; Leng, X.; Liao, X.; Hu, X. Aggregation and homogenization, surface charge and structural change, and inactivation of mushroom tyrosinase in an aqueous system by subcritical/supercritical carbon dioxide. Langmuir 2011, 27, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmad, S.; Ahmad, A. Green extraction methods and environmental applications of carotenoids-a review. RSC Adv. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar]
- Kolak, J.J. A Procedure for the Supercritical Fluid Extraction of Coal Samples, with Subsequent Analysis of Extracted Hydrocarbons; U.S. Department of the Interior: Reston, VA, USA, 2006.
- Wimmer, Z.; Zarevúcka, M. A review on the effects of supercritical carbon dioxide on enzyme activity. Int. J. Mol. Sci. 2010, 11, 233–253. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Pontvianne, S.; Framboisier, X.; Achard, M.; Kudaibergenova, R.; Ayadi-Trabelsi, M.; Kalthoum-cherif, J.; Vanderesse, R.; Frochot, C.; Guiavarc’h, Y. Accelerated solvent extraction of carotenoids from: Tunisian Kaki (Diospyros kaki L.), peach (Prunus persica L.) and apricot (Prunus armeniaca L.). Food Chem. 2015, 184, 131–139. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Framboisier, X.; Frochot, C.; Vanderesse, R.; Barth, D.; Kalthoum-Cherif, J.; Blanchard, F.; Guiavarc’h, Y. Response surface methodology applied to Supercritical Fluid Extraction (SFE) of carotenoids from Persimmon (Diospyros kaki L.). Food Chem. 2016, 208, 209–219. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E.; Lubián, L.M.; Montero, O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Fernandez-Sevilla, J.M.; Fernández, F.G.A.; García, M.C.C.; Grima, E.M. Supercritical fluid extraction of carotenoids from Scenedesmus almeriensis. Food Chem. 2010, 123, 928–935. [Google Scholar] [CrossRef]
- Pour Hosseini, S.R.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Prado, J.; Veggi, P.; Meireles, M. Extraction Methods for Obtaining Carotenoids from Vegetables-Review. Curr. Anal. Chem. 2013, 10, 29–66. [Google Scholar] [CrossRef]
- Khajeh, M. Optimization of process variables for essential oil components from Satureja hortensis by supercritical fluid extraction using Box-Behnken experimental design. J. Supercrit. Fluids 2011, 55, 944–948. [Google Scholar] [CrossRef]
- Stinco, C.M.; Szczepańska, J.; Marszałek, K.; Pinto, C.A.; Inácio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Meléndez-Martínez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef]
- Liu, G.; Xu, X.; Hao, Q.; Gao, Y. Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT-Food Sci. Technol. 2009, 42, 1491–1495. [Google Scholar] [CrossRef]
- Leitão, N.C.M.C.S.; Prado, G.H.C.; Veggi, P.C.; Meireles, M.A.A.; Pereira, C.G. Anacardium occidentale L. leaves extraction via SFE: Global yields, extraction kinetics, mathematical modeling and economic evaluation. J. Supercrit. Fluids 2013, 78, 114–123. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot-A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.P.; Paula, J.T.; Paviani, L.C.; Cabral, F.A.; Martinez-Correa, H.A. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT-Food Sci. Technol. 2015, 62, 131–137. [Google Scholar] [CrossRef]
- de França, L.F.; Reber, G.; Meireles, M.A.A.; Machado, N.T.; Brunner, G. Supercritical extraction of carotenoids and lipids from buriti (Mauritia flexuosa), a fruit from the Amazon region. J. Supercrit. Fluids 1999, 14, 247–256. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Mendes, R.L.; Fernandes, H.L.; Coelho, J.; Reis, E.C.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem. 1995, 53, 99–103. [Google Scholar] [CrossRef]
- Macıas-Sánchez, M.D.; Mantell, C.; Rodrıguez, M.; de La Ossa, E.M.; Lubián, L.M.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. J. Food Eng. 2005, 66, 245–251. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E.; Lubián, L.M.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Synechococcus sp. J. Supercrit. Fluids 2007, 39, 323–329. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Process optimization and extraction rate analysis of carotenoids extraction from rosehip fruit using supercritical CO2. J. Supercrit. Fluids 2008, 44, 308–314. [Google Scholar] [CrossRef]
- Genival Filho, L.; De Rosso, V.V.; Meireles, M.A.A.; Rosa, P.T.V.; Oliveira, A.L.; Mercadante, A.Z.; Cabral, F.A. Supercritical CO2 extraction of carotenoids from pitanga fruits (Eugenia uniflora L.). J. Supercrit. Fluids 2008, 46, 33–39. [Google Scholar] [CrossRef]
- Juan, C.; Oyarzún, B.; Quezada, N.; del Valle, J.M. Solubility of carotenoid pigments (lycopene and astaxanthin) in supercritical carbon dioxide. Fluid Phase Equilib. 2006, 247, 90–95. [Google Scholar]
- Espinosa-Pardo, F.A.; Martinez, J.; Martinez-Correa, H.A. Extraction of bioactive compounds from peach palm pulp (Bactris gasipaes) using supercritical CO2. J. Supercrit. Fluids 2014, 93, 2–6. [Google Scholar] [CrossRef]
- Katherine, L.S.V.; Edgar, C.C.; Jerry, W.K.; Luke, R.H.; Julie, C.D. Extraction conditions affecting supercritical fluid extraction (SFE) of lycopene from watermelon. Bioresour. Technol. 2008, 99, 7835–7841. [Google Scholar] [CrossRef]
- Di Giacomo, G.; Scimia, F.; Taglieri, L. Application of Supercritical Carbon Dioxide for the Preservation of Fresh-Like Carrot Juice. Int. J. New Technol. Res. 2016, 2, 71–77. [Google Scholar]
- Santeramo, F.G.; Carlucci, D.; De Devitiis, B.; Seccia, A.; Stasi, A.; Viscecchia, R.; Nardone, G. Emerging trends in European food, diets and food industry. Food Res. Int. 2018, 104, 39–47. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Barzana, E.; Rubio, D.; Santamaria, R.I.; Garcia-Correa, O.; Garcia, F.; Sanz, V.E.R.; López-Munguía, A. Enzyme-mediated solvent extraction of carotenoids from Marigold flower (Tagetes erecta). J. Agric. Food Chem. 2002, 50, 4491–4496. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Villalobos-Gutierrez, M.G.; Esquivel, P.; Carle, R. Development and optimization of low temperature enzyme-assisted liquefaction for the production of colouring foodstuff from purple pitaya (Hylocereus sp. [Weber] Britton & Rose). Eur. Food Res. Technol. 2009, 230, 269–280. [Google Scholar]
- Ghandahari Yazdi, A.P.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H. Optimization of the enzyme-assisted aqueous extraction of phenolic compounds from pistachio green hull. Food Sci. Nutr. 2019, 7, 356–366. [Google Scholar] [CrossRef]
- Boulila, A.; Hassen, I.; Haouari, L.; Mejri, F.; Amor, I.B.; Casabianca, H.; Hosni, K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Ind. Crops Prod. 2015, 74, 485–493. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.T.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT-Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Fernández, K.; Vega, M.; Aspé, E. An enzymatic extraction of proanthocyanidins from País grape seeds and skins. Food Chem. 2015, 168, 7–13. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Purnima, K.T.; Florence, S.P.; Appu Rao, A.G.; Srinivas, P. Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem. 2009, 113, 1234–1238. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Mai, H.C.; Truong, V.; Debaste, F. Optimization of enzyme-aided extraction of oil rich in carotenoids from gac fruit (Momordica cochinchinensis Spreng.). Food Technol. Biotechnol. 2013, 51, 488–499. [Google Scholar]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef]
- Encalada, A.M.I.; Pérez, C.D.; Flores, S.K.; Rossetti, L.; Fissore, E.N.; Rojas, A.M. Antioxidant pectin enriched fractions obtained from discarded carrots (Daucus carota L.) by ultrasound-enzyme assisted extraction. Food Chem. 2019, 289, 453–460. [Google Scholar] [CrossRef]
- Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant. Cell Environ. 2006, 29, 435–445. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant. Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Holanda, H.D.D. Hidrólise enzimática do resíduo do camarão sete-barbas (Xiphopenaeus kroyeri) e caracterização dos subprodutos. 2004. Available online: http://repositorio.unicamp.br/jspui/handle/REPOSIP/256397 (accessed on 19 October 2019). (In Portuguese).
- Krichnavaruk, S.; Shotipruk, A.; Goto, M.; Pavasant, P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2008, 99, 5556–5560. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Eren, A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: use of agricultural wastes as a carbon source. Process. Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]
- Hrncic, M.K.; Cör, D.; Verboten, M.T.; Knez, Z. Application of supercritical and subcritical fluids in food processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
| Methods’ Parameters | Extraction with Organic Solvent | CO2 Extraction | EAE |
|---|---|---|---|
| Extraction yield | High | High | Low |
| Cost of the analysis | Medium | High | Low |
| Cost of the solvents | High | Low | Low |
| Ecological safety | Low | High | High |
| Time | Rapid | Rapid | Medium |
| Trace solvent residues in final product | Yes | No | No |
| Handling | Difficult | Difficult | Simple |
| Carotenoid | Concentrations [mg/g] | Extraction Methods | References | ||
|---|---|---|---|---|---|
| Total carotenoids | 0.16–0.38 | 10 g extracted with n-hexane/ethanol 96% (1:1, v/v) until colorless; kept at 20 °C and analyzed within 72 h | [51,52] | ||
| Carotenes (Hydrophobic Carotenoids) | Color | Application | Activities | References | |
| β-carotene | 0.046–0.10 | Orange | Nutraceutical; cosmetic; animal feed industries | Antioxidant, anticancer, precursor of vitamin A | [52] |
| α-carotene | 0.046 | Red | Nutraceutical and functional nutrients | Antioxidant, counteract heart disease and cancer | [53] |
| Xanthophylls (Hydrophilic Carotenoids) | Color | Application | Activities | References | |
| Lutein | 0.0011–0.0056 | Golden Yellow | Poultry feed; functional nutrient | Antioxidant | [54] |
| Zeaxanthin | 0.031 | Yellow | Poultry and fish | Eye disease, Age-related macular degeneration | [55] |
| Sample | Analyte | Conditions | References |
|---|---|---|---|
| Buriti (Mauritia flexuosa) pulp | β-carotene | 20 MPa (pressure), 40 °C (temperature) | [98] |
| Microalgae Chlorella vulgaris (Chlorophyta) | carotenoids: canthaxanthin and astaxanthin | 10 to 35 MPa (pressure) 40 and 60 °C (temperature) | [99,100] |
| Marine microalgae Microchloropsis gaditana (formerly Nannochloropsis gaditana, Ochrophyta, Eustigmatophyceae) | carotenoids and chlorophyll | 10 to 50 MPa (pressure 40 and 60° C (temperature) | [101] |
| Cyanobacterium Synechococcus sp., | carotenoids and chlorophyll | 10 to 50 MPa (pressure) from 40 to 60 °C (temperature) | [102] |
| Rose fruit (Rosa canina) | carotenoids: lycopene, the β-carotene and lutein | 15 to 45 MPa (pressure) from 40 to 80 °C (temperature) CO2 flow rate from 2 to 4 mL/min | [103] |
| Pitanga (Eugenia uniflora L.). | carotenoids: lycopene and rubixanthin | 10, 15, 20, 25, 30, 35, and 40 MPa (seven levels of pressure) 40 and 60 °C (temperature) | [104] |
| Tomato Paste | lycopene and astaxanthin | pressure (from 10 to 42 MPa) temperature (40 to 60 °C) | [105] |
| Pink shrimp (P. paulensis and P. brasiliensis) | carotenoid components | from 10 to 30 MPa (pressure) 40 and 60 °C temperature | [62] |
| peach palm fruit (Bactris gasipaes) | total phenolic content, total flavonoids, total carotenoids | 300 bar (pressure) 40 °C (temperature) | [106] |
| Gac fruit (Momordica cochinchinensis Spreng.) aril | lycopene, β-carotene | 20 MPa (pressure) 50 °C (temperature) | [47] |
| Carrot | Total carotenoid content, α- and β-carotene, and lutein | 27.6–55.1 MPa (pressure) from 40 to 70 °C (temperature) | [15] |
| Frozen watermelon | Lycopene | 20.7–41.4 MPa (pressure) 70–90 °C (temperature) | [107] |
| Pumpkin (Cucurbita maxima) | Total carotenoid content, carotenoid profile | 25 MPa (pressure) 50 and 80 °C (temperature) | [14] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miękus, N.; Iqbal, A.; Marszałek, K.; Puchalski, C.; Świergiel, A. Green Chemistry Extractions of Carotenoids from Daucus carota L.—Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules 2019, 24, 4339. https://doi.org/10.3390/molecules24234339
Miękus N, Iqbal A, Marszałek K, Puchalski C, Świergiel A. Green Chemistry Extractions of Carotenoids from Daucus carota L.—Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules. 2019; 24(23):4339. https://doi.org/10.3390/molecules24234339
Chicago/Turabian StyleMiękus, Natalia, Aamir Iqbal, Krystian Marszałek, Czesław Puchalski, and Artur Świergiel. 2019. "Green Chemistry Extractions of Carotenoids from Daucus carota L.—Supercritical Carbon Dioxide and Enzyme-Assisted Methods" Molecules 24, no. 23: 4339. https://doi.org/10.3390/molecules24234339
APA StyleMiękus, N., Iqbal, A., Marszałek, K., Puchalski, C., & Świergiel, A. (2019). Green Chemistry Extractions of Carotenoids from Daucus carota L.—Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules, 24(23), 4339. https://doi.org/10.3390/molecules24234339







