Genetic Variation of Physicochemical Properties and Digestibility of Foxtail Millet (Setaria italica) Landraces of Taiwan
Abstract
1. Introduction
2. Results and Discussion
2.1. The Structure and Arrangements of Starch Granules in Foxtail Millet Grains
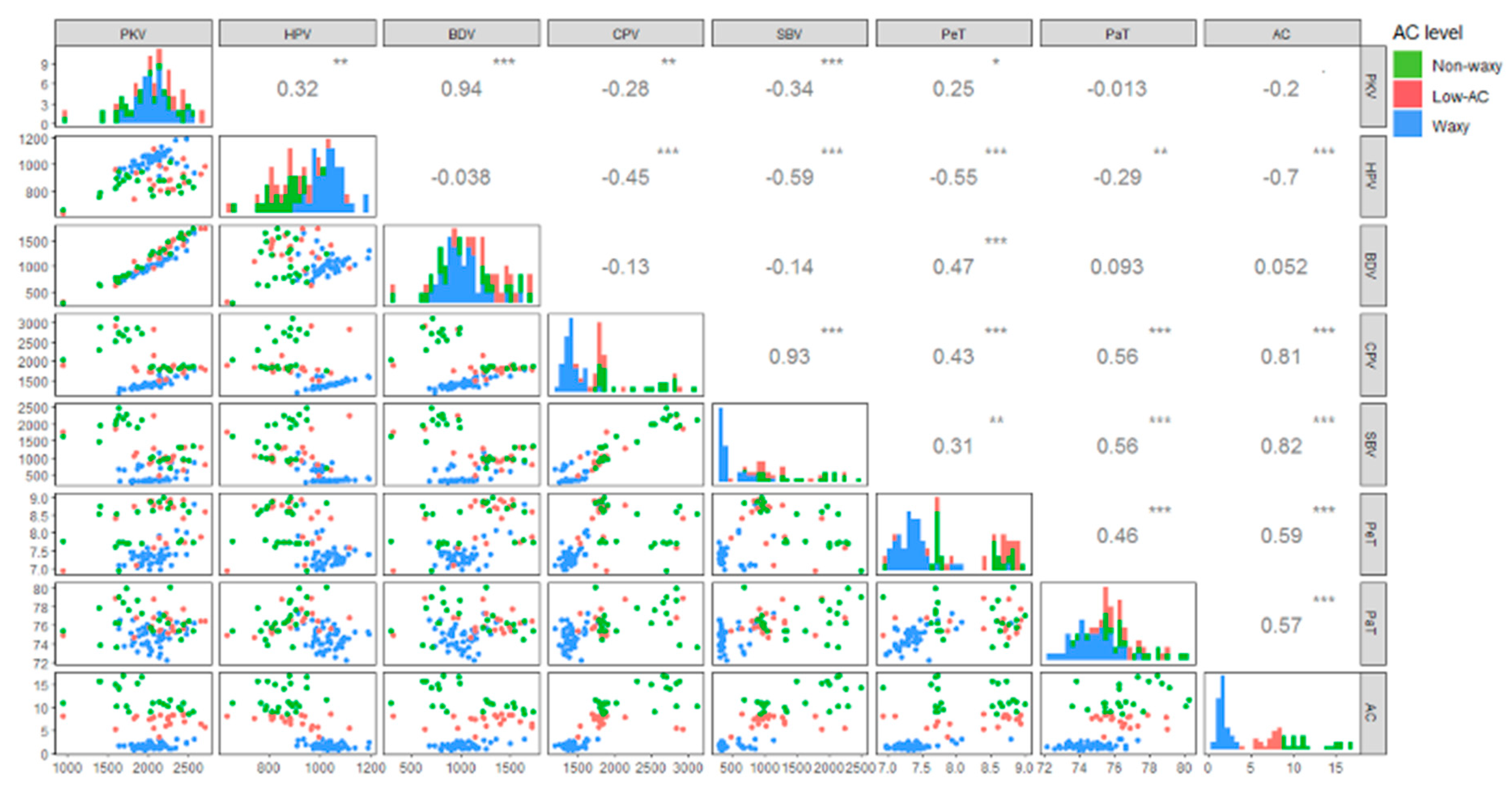
2.2. Physicochemical Properties of Foxtail Millet Grain
2.3. Starch Hydrolysis Analysis of Foxtail Millet
3. Materials and Methods
3.1. Plant Materials and Experimental Design
3.2. Scanning Electron Microscopy (SEM) of Starch Granules
3.3. Amylose Content
3.4. Pasting Properties of Foxtail Millet Grain
3.5. Starch Content
3.6. In Vitro Starch Digestibility
3.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Austin, D.F. Foxtail millets (Setaria: Poaceae)—Abandoned food in two hemispheres. Econ. Bot. 2006, 60, 143–158. [Google Scholar] [CrossRef]
- Baltensperger, D.D. Progress with proso, pearl and other millets. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Tsang, C.-H.; Li, K.-T.; Hsu, T.-F.; Tsai, Y.-C.; Fang, P.-H.; Hsing, Y.-I.C. Broomcorn and foxtail millet were cultivated in Taiwan about 5000 years ago. Bot. Stud. 2017, 58, 3. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. food Sci. food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Gupta, S.M.; Arora, S.; Mirza, N.; Pande, A.; Lata, C.; Puranik, S.; Kumar, J.; Kumar, A. Finger Millet: A “Certain” Crop for an “Uncertain” Future and a Solution to Food Insecurity and Hidden Hunger under Stressful Environments. Fronti. Plant Sci. 2017, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Regina, A.; Rahman, S.; Li, Z.; Morell, M.K. Starch: Synthesis. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK; London, UK, 2016; pp. 181–189. [Google Scholar]
- Vamadevan, V.; Liu, Q. Starch: Starch Architecture and Structure. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 190–197. [Google Scholar]
- Zhu, F. Structure, physicochemical properties, and uses of millet starch. Food Res. Int. 2014, 64, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Afzal, M.; Okuno, K. Intraspecific differentiation and geographical distribution of Wx alleles for low amylose content in endosperm of foxtail millet, Setaria italica (L.) Beauv. Euphytica 1998, 102, 289–293. [Google Scholar] [CrossRef]
- Kuo, S.-M.; Chen, Y.-R.; Yin, S.-Y.; Ba, Q.-X.; Tsai, Y.-C.; Kuo, H.-J.W.; Lin, Y.-R. Waxy allele diversification in foxtail millet (Setaria italica) landraces of Taiwan. PLoS ONE 2019, 13. [Google Scholar] [CrossRef]
- Juliano, B.O.; Perez, C.M.; Blakeney, A.B.; Castillo, T.; Kongseree, N.; Laignelet, B.; Lapis, E.T.; Murty, V.V.S.; Paule, C.M.; Webb, B.D. International cooperative testing on the amylose content of milled rice. Starch—Stärke 1981, 33, 157–162. [Google Scholar] [CrossRef]
- Cozzolino, D. The use of the rapid visco analyser (RVA) in breeding and selection of cereals. J. Cereal Sci. 2016, 70, 282–290. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Tseng, M.-C.; Wu, Y.-P.; Lin, M.-Y.; Wei, F.-J.; Hwu, K.-K.; Hsing, Y.-I.; Lin, Y.-R. Genetic factors responsible for eating and cooking qualities of rice grains in a recombinant inbred population of an inter-subspecific cross. Mol. Breeding 2014, 34, 655–673. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical properties of starches from diverse rice cultivars varying in apparent amylose content and gelatinisation temperature combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low–glycemic index diets in the management of diabetes. Diabetes Care 2003, 26, 2261. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Robin, F. Slowly digestible starch – its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Englyst, H.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Hu, P.; Zhao, H.; Duan, Z.; Linlin, Z.; Wu, D. Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J. Cereal Sci. 2004, 40, 231–237. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Singh Sodhi, N.; Singh Gill, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Krishna Kumari, S.; Thayumanavan, B. Characterization of starches of proso, foxtail, barnyard, kodo, and little millets. Plant. Foods Hum. Nutr. 1998, 53, 47–56. [Google Scholar] [CrossRef]
- Fujita, S.; Sugimoto, Y.; Yamashita, Y.; Fuwa, H. Physicochemical studies of starch from foxtail millet (Setaria italica Beauv.). Food Chem. 1996, 55, 209–213. [Google Scholar] [CrossRef]
- Annor, G.A.; Marcone, M.; Bertoft, E.; Seetharaman, K. Physical and molecular characterization of millet starches. Cereal Chem. 2014, 91, 286–292. [Google Scholar] [CrossRef]
- Huber, K.C.; BeMiller, J.N. Channels of maize and sorghum starch granules. Carbohydr. Polym. 2000, 41, 269–276. [Google Scholar] [CrossRef]
- Kasem, S.; Waters, D.L.E.; Rice, N.F.; Shapter, F.M.; Henry, R.J. The endosperm morphology of rice and its wild relatives as observed by scanning electron microscopy. Rice 2011, 4, 12–20. [Google Scholar] [CrossRef]
- Fannon, J.; Hauber, R.; BeMiller, J.N. Surface pores of starch granules. Cereal Chem. 1992, 69, 284–288. [Google Scholar]
- Zhang, L.; Zhao, L.; Zhang, J.; Cai, X.; Liu, Q.; Wei, C. Relationships between transparency, amylose content, starch cavity, and moisture of brown rice kernels. J. Cereal Sci. 2019, 90, 102854. [Google Scholar] [CrossRef]
- Huang, T.; Zhu, B.; Du, X.; Li, B.; Wu, X.; Wang, S. Study on gelatinization property and edible quality mechanism of rice. Starch—Stärke 2012, 64, 846–854. [Google Scholar] [CrossRef]
- Naguleswaran, S.; Vasanthan, T.; Hoover, R.; Bressler, D. The susceptibility of large and small granules of waxy, normal and high-amylose genotypes of barley and corn starches toward amylolysis at sub-gelatinization temperatures. Food Res. Int. 2013, 51, 771–782. [Google Scholar] [CrossRef]
- Sakamoto, S. Characteristics and ethnobotanical comparison of foxtail millet (Setaria italica P. Beauv.) samples from Southern Formosa and the Batan Islands. Bull. Am. Mus. Nat. Hist. 1978, 3, 682–708. [Google Scholar]
- Wang, L.Q.; Liu, W.J.; Xu, Y.; He, Y.Q.; Luo, L.J.; Xing, Y.Z.; Xu, C.G.; Zhang, Q. Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain. Theor. Appl. Genet. 2007, 115, 463–476. [Google Scholar] [CrossRef]
- Park, I.-M.; Ibáñez, A.M.; Zhong, F.; Shoemaker, C.F. Gelatinization and pasting properties of waxy and non-waxy rice starches. Starch—Stärke 2007, 59, 388–396. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists, 3rd ed.; Elsevier Science: New York, NY, USA, 2018. [Google Scholar]
- Li, K.; Zhang, T.; Sui, Z.; Narayanamoorthy, S.; Jin, C.; Li, S.; Corke, H. Genetic variation in starch physicochemical properties of Chinese foxtail millet (Setaria italica Beauv.). Int. J. Biol. Macromol. 2019, 133, 337–345. [Google Scholar] [CrossRef]
- Champagne, E.T.; Bett, K.L.; Vinyard, B.T.; McClung, A.M.; Barton, F.E., II; Moldenhauer, K.; Linscombe, S.; McKenzie, K. Correlation between cooked rice texture and rapid visco analyser measurements. Cereal Chem. 1999, 76, 764–771. [Google Scholar] [CrossRef]
- Zhao, R.; Bean, S.; Wu, X.; Wang, D. Assessing fermentation quality of grain sorghum for fuel ethanol production using Rapid Visco-Analyzer. Cereal Chem. 2008, 85, 830–836. [Google Scholar] [CrossRef]
- Zhou, M.X.; Mendham, N.J. Predicting barley malt extract with a Rapid Viscoanalyser. J. Cereal Sci. 2005, 41, 31–36. [Google Scholar] [CrossRef]
- He, L.; Zhang, B.; Wang, X.; Li, H.; Han, Y. Foxtail millet: Nutritional and eating quality, and prospects for genetic improvement. Front. Agr. Sci. Eng. 2015, 2, 124–133. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Starch granule porosity and its changes by means of amylolysis. Int. Agrophysics 2007, 21, 107–113. [Google Scholar]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Tech. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Molla, M.M.; Wang, C.; Diao, X.; Shen, Q. In vitro starch digestibility and in vivo glycemic response of foxtail millet and its products. Food Funct. 2016, 7, 372–379. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Narayanan, J.; Sanjeevi, V.; Rohini, U.; Trueman, P.; Viswanathan, V. Postprandial glycaemic response of foxtail millet dosa in comparison to a rice dosa in patients with type 2 diabetes. Indian J. Med. Res. 2016, 144, 712–717. [Google Scholar]
- Ring, S.G.; Gee, J.M.; Whittam, M.; Orford, P.; Johnson, I.T. Resistant starch: Its chemical form in foodstuffs and effect on digestibility in vitro. Food Chem. 1988, 28, 97–109. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Zhang, W.; Bi, J.; Yan, X.; Wang, H.; Zhu, C.; Wang, J.; Wan, J. In vitro measurement of resistant starch of cooked milled rice and physico-chemical characteristics affecting its formation. Food Chem. 2007, 105, 462–468. [Google Scholar] [CrossRef]
- Anacleto, R.; Badoni, S.; Parween, S.; Butardo, V.M., Jr.; Misra, G.; Cuevas, R.P.; Kuhlmann, M.; Trinidad, T.P.; Mallillin, A.C.; Acuin, C.; et al. Integrating a genome-wide association study with a large-scale transcriptome analysis to predict genetic regions influencing the glycaemic index and texture in rice. Plant. Biotechnol. J. 2019, 17, 1261–1275. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Bean, S.R.; Funnell, D.L.; Graybosch, R.A. Rapid Iodine Staining Techniques for Identifying the Waxy Phenotype in Sorghum Grain and Waxy Genotype in Sorghum Pollen. Crop. Sci. 2004, 44, 764–767. [Google Scholar] [CrossRef]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
Sample Availability: Samples of the foxtail millet powder are available from the authors. |


| Endosperm phenotype | ||||
|---|---|---|---|---|
| Trait | Waxy | Low-AC | Non-waxy | |
| No. of accessions | 48 | 21 | 23 | |
| AC (%) | Range | 0.7–3.2 | 3.7–8.6 | 8.7–16.9 |
| Mean | 1.7 c | 7.21 b | 12.07 a | |
| PKV (cP) | Range | 1641.5–2574.5 | 940–2706 | 942–2555 |
| Mean | 2051.76 a | 2143.60 a | 1931.32 a | |
| HPV (cP) | Range | 908-1190 | 634–1115 | 654–1023 |
| Mean | 1033.17 a | 888.0 a | 853.25 b | |
| BDV (cP) | Range | 677–1641.5 | 306–1720.5 | 288.5–1724 |
| Mean | 1018.59 ab | 1255.6 a | 1078.07 b | |
| CPV (cP) | Range | 1183.5–1768 | 1498–2922 | 1734–3117.5 |
| Mean | 1403.75 c | 1888.35 b | 2258.76 a | |
| SBV (cP) | Range | 291–1136.5 | 476–2243.5 | 682.5–2491 |
| Mean | 427.2 c | 1108.8 b | 1555.99 a | |
| PaT(℃) | Range | 72.15–77.32 | 74.68–79 | 73.58–80.23 |
| Mean | 74.67 b | 76.36 a | 76.69 a | |
| PeT(min) | Range | 6.93–9 | 6.93–8.93 | 6.97–8.8 |
| Mean | 7.37 b | 8.34 a | 8.21 a | |
| Accession* | AC (%) | Starch Content (%) | RDS (%) | SDS (%) | RS (%) | HI | eGI |
|---|---|---|---|---|---|---|---|
| 387 | 14.4 ± 3.6 bc | 72.4 ± 1.3 b | 20.9 ± 1.8 bc | 37.4 ± 9.0 abc | 41.7 ± 7.3 bcd | 119.7 ± 15.4 cd | 104.9 ± 8.4 cd |
| 419 | 16.0 ± 1.1 b | 67.4 ± 1.7 b | 18.2 ± 2.5 bcd | 30.6 ± 3.3 bcd | 51.2 ± 2.1 d | 124.6 ± 3.1 cd | 107.6 ± 1.7 cd |
| 467 | 16.9 ± 0.2 ab | 73.1 ± 4.2 b | 13.1 ± 1.1 de | 42.2 ± 2.8 ab | 44.7 ± 1.8 bcd | 137.8 ± 5.5 bc | 114.9 ± 3.0 bc |
| 445 | 7.7 ± 2.0 d | 71.1 ± 2.2 b | 17.1 ± 1.0 bcd | 32.2 ± 3.4 abcd | 50.7 ± 2.6 b | 141.2 ± 17.3 abc | 116.7 ± 9.5 abc |
| 478 | 6.0 ± 0.7 d | 69.9 ± 4.4 b | 17.6 ± 1.1 bcd | 33.8 ± 0.1 abc | 48.6 ± 1.2 bc | 134.9 ± 5.6 bc | 113.3 ± 3.1 bc |
| 434 | 1.5 ± 0.4 e | 67.6 ± 4.7 b | 26.8 ± 1.9 a | 36.3 ± 4.3 abc | 36.8 ± 3.6 d | 165.9 ± 7.0 a | 130.3 ± 3.9 a |
| 436 | 1.3 ± 0.1 e | 70.6 ± 3.7 b | 20.8 ± 0.4 bc | 38.5 ± 0.5 abc | 40.7 ± 0.4 cd | 158.5 ± 6.9 ab | 126.2 ± 3.8 ab |
| HY4 | 1.9 ± 0.8 e | 71.4 ± 2.5 b | 20.2 ± 1.4 bc | 42.7 ± 2.8 a | 37.1 ± 1.7 d | 154.9 ± 0.4 ab | 124.3 ± 0.2 ab |
| LC3 | 1.9 ± 0.0 e | 65.9 ± 0.8 b | 21.7 ± 2.1 ab | 43.1 ± 3.9 a | 35.2 ± 4.9 d | 164.9 ± 5.8 a | 129.7 ± 3.2 a |
| IR8 | 20.7 ± 0.7 a | 90.5 ± 2.6 a | 11.2 ± 0.6 e | 21.0 ± 2.6 d | 67.9 ± 2.5 a | 77.8 ± 6.2 e | 82.4 ± 3.4 e |
| TN11 | 12.2 ± 0.9 c | 91.1 ± 3.0 a | 18.0 ± 1.3 cd | 24.2 ± 0.7 d | 57.8 ± 0.5 a | 100.0 ± 0.0 de | 94.6 ± 0.0 de |
| Hung-No | 1.4 ± 0.2 e | 88.9 ± 2.3 a | 20.4 ± 2.7 bc | 27.3 ± 3.6 cd | 52.3 ± 0.9 b | 112.6 ± 3.5 c | 101.5 ± 1.9 c |
| Parameter | RDS | SDS | RS |
|---|---|---|---|
| Starch content | −0.50 | −0.15 | 0.38 |
| AC | −0.55 | −0.40 | 0.59 * |
| PKV | −0.13 | −0.47 | 0.41 |
| HPV | 0.02 | −0.23 | 0.16 |
| BDV | −0.15 | −0.47 | 0.42 |
| CPV | −0.56 * | −0.50 | 0.67 * |
| SBV | −0.56 * | −0.46 | 0.64 * |
| PeT | −0.78 ** | −0.54 | 0.82 *** |
| PaT | −0.64 * | −0.39 | 0.63 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.-Y.; Kuo, S.-M.; Chen, Y.-R.; Tsai, Y.-C.; Wu, Y.-P.; Lin, Y.-R. Genetic Variation of Physicochemical Properties and Digestibility of Foxtail Millet (Setaria italica) Landraces of Taiwan. Molecules 2019, 24, 4323. https://doi.org/10.3390/molecules24234323
Yin S-Y, Kuo S-M, Chen Y-R, Tsai Y-C, Wu Y-P, Lin Y-R. Genetic Variation of Physicochemical Properties and Digestibility of Foxtail Millet (Setaria italica) Landraces of Taiwan. Molecules. 2019; 24(23):4323. https://doi.org/10.3390/molecules24234323
Chicago/Turabian StyleYin, Song-Yu, Shu-Meng Kuo, Yu-Ru Chen, Yuan-Ching Tsai, Yong-Pei Wu, and Yann-Rong Lin. 2019. "Genetic Variation of Physicochemical Properties and Digestibility of Foxtail Millet (Setaria italica) Landraces of Taiwan" Molecules 24, no. 23: 4323. https://doi.org/10.3390/molecules24234323
APA StyleYin, S.-Y., Kuo, S.-M., Chen, Y.-R., Tsai, Y.-C., Wu, Y.-P., & Lin, Y.-R. (2019). Genetic Variation of Physicochemical Properties and Digestibility of Foxtail Millet (Setaria italica) Landraces of Taiwan. Molecules, 24(23), 4323. https://doi.org/10.3390/molecules24234323






