Biosynthesis of Isoprene Units in Euphorbia lathyris Laticifers vs. Other Tissues: MVA and MEP Pathways, Compartmentation and Putative Endophytic Fungi Contribution
Abstract
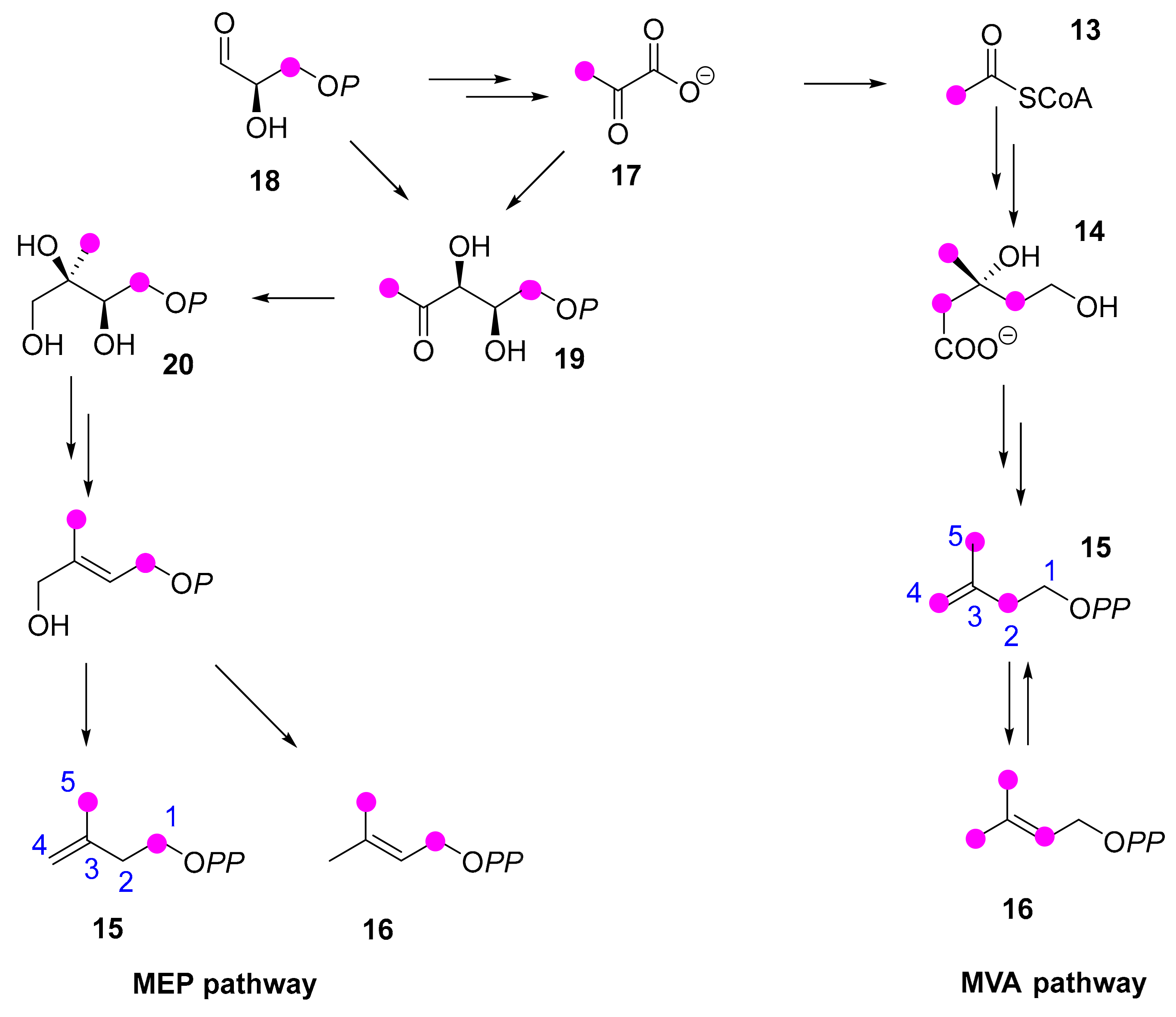
1. Introduction
2. Results
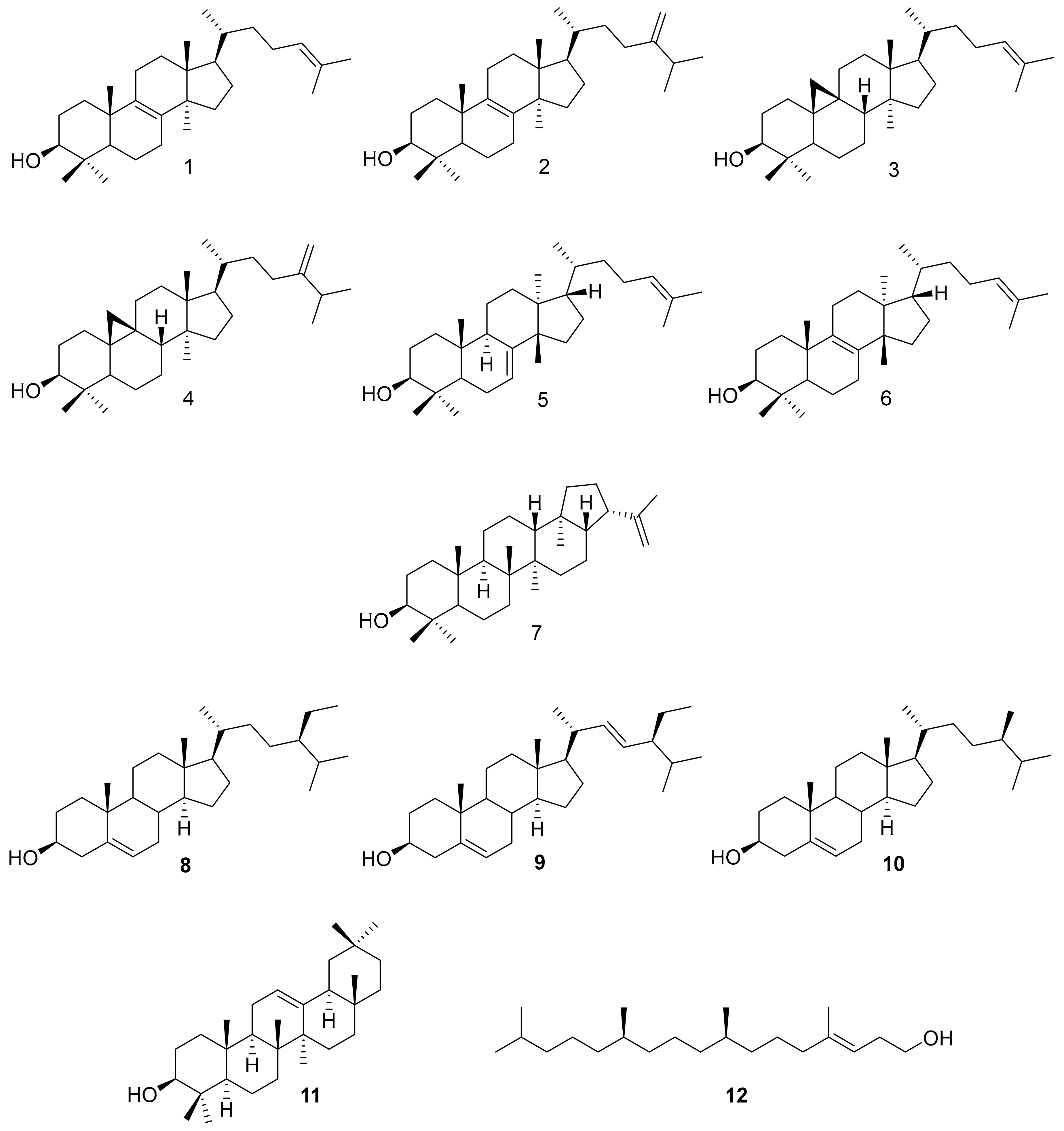
2.1. Latex Triterpenes and Other Isoprenoids in E. lathyris
2.1.1. Plant Material and Incorporation Conditions for Labeled Precursors
2.1.2. E. lathyris Isoprenoids
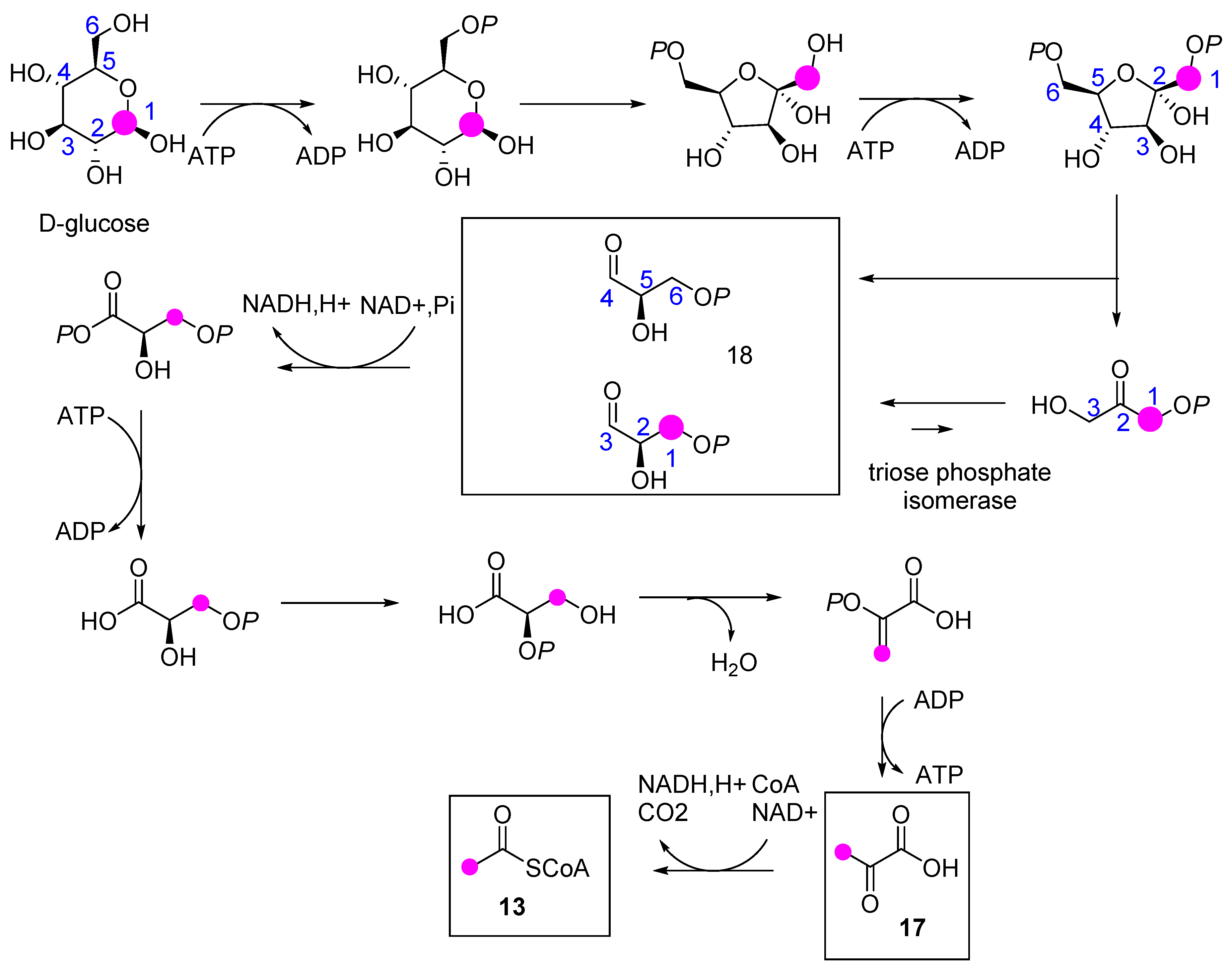
2.2. E. lathyris Labeling with (1-13C)Glucose
2.3. E. lathyris Plantlet Labeling with (1,6-13C2)Glucose
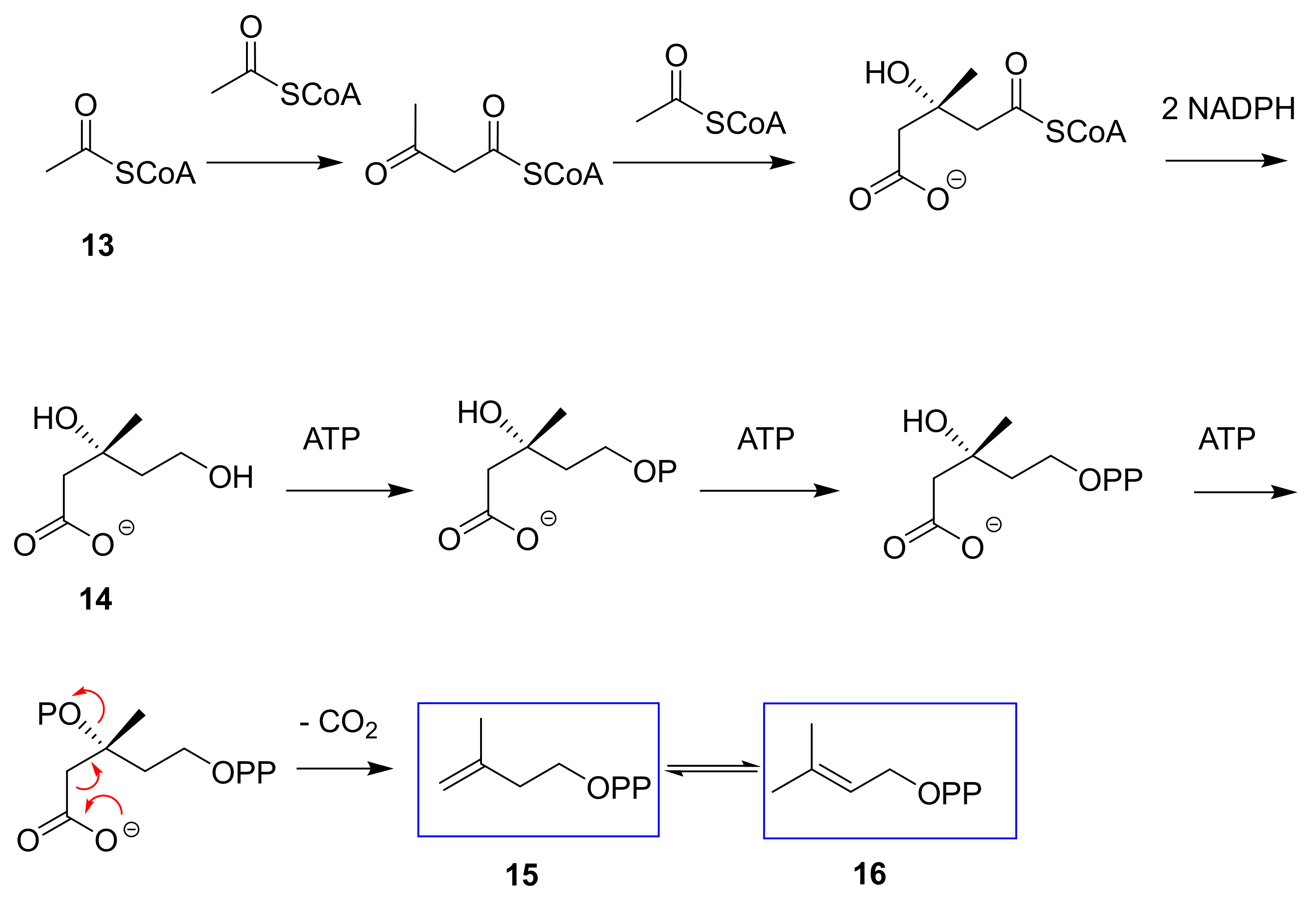
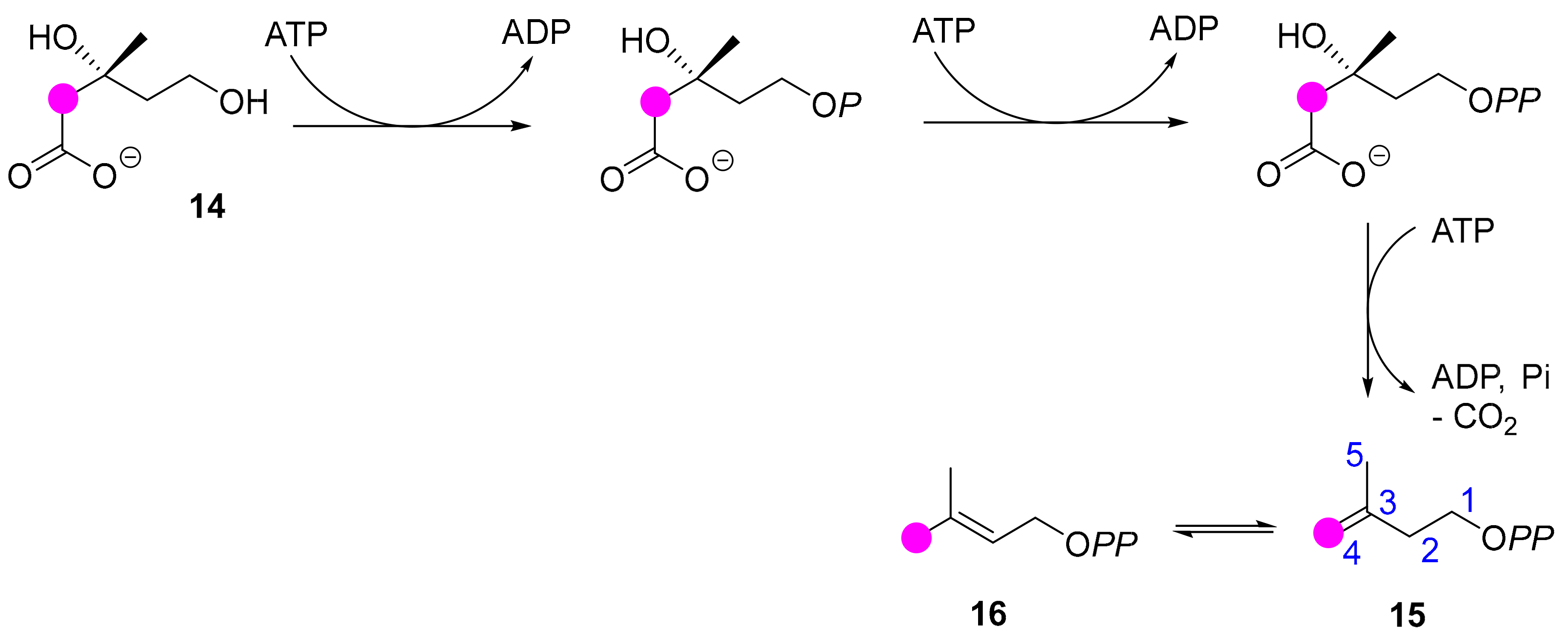
2.4. E. lathyris Labeling with(3R,S)-(2-13C)Mevalonate
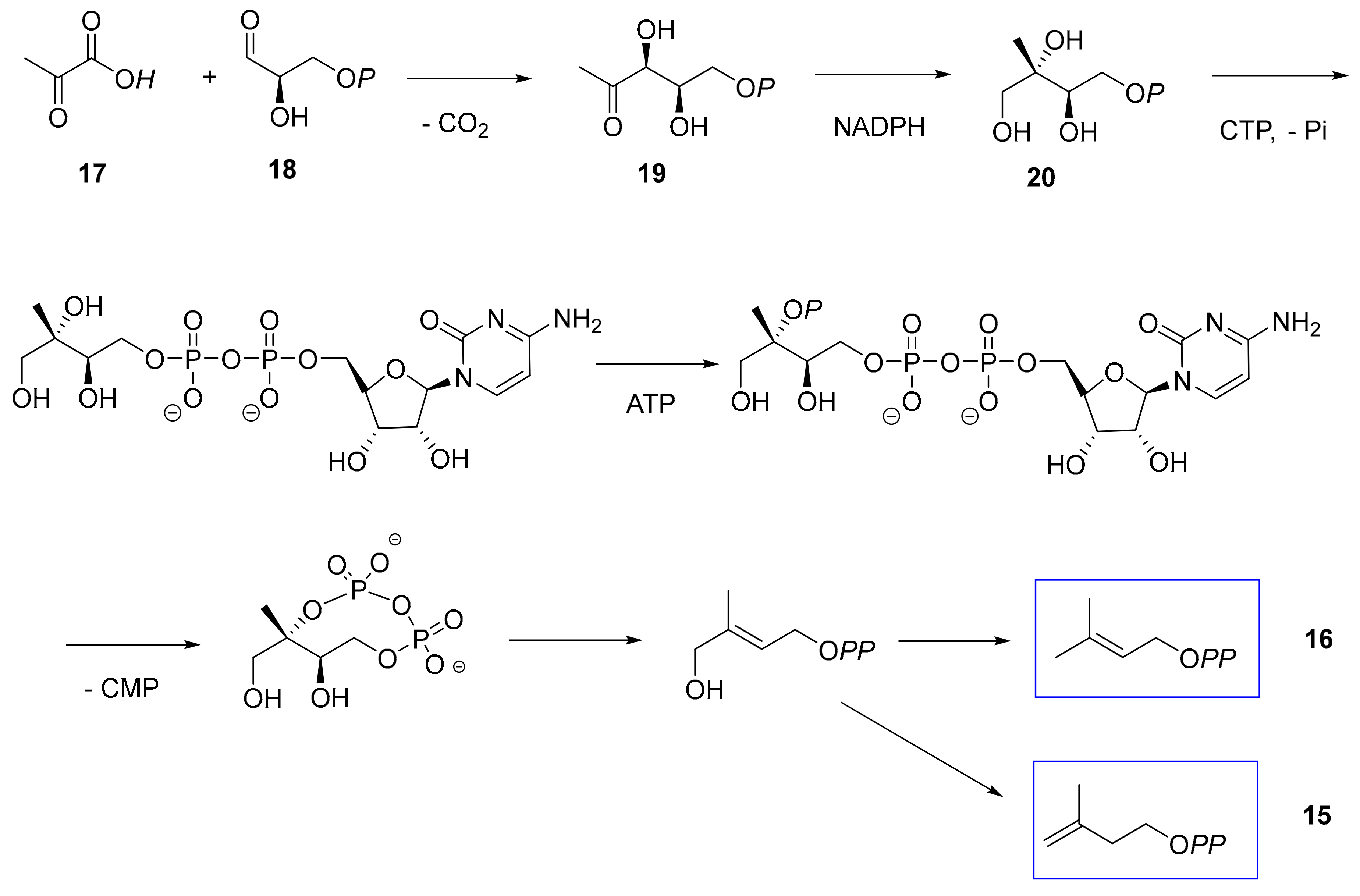
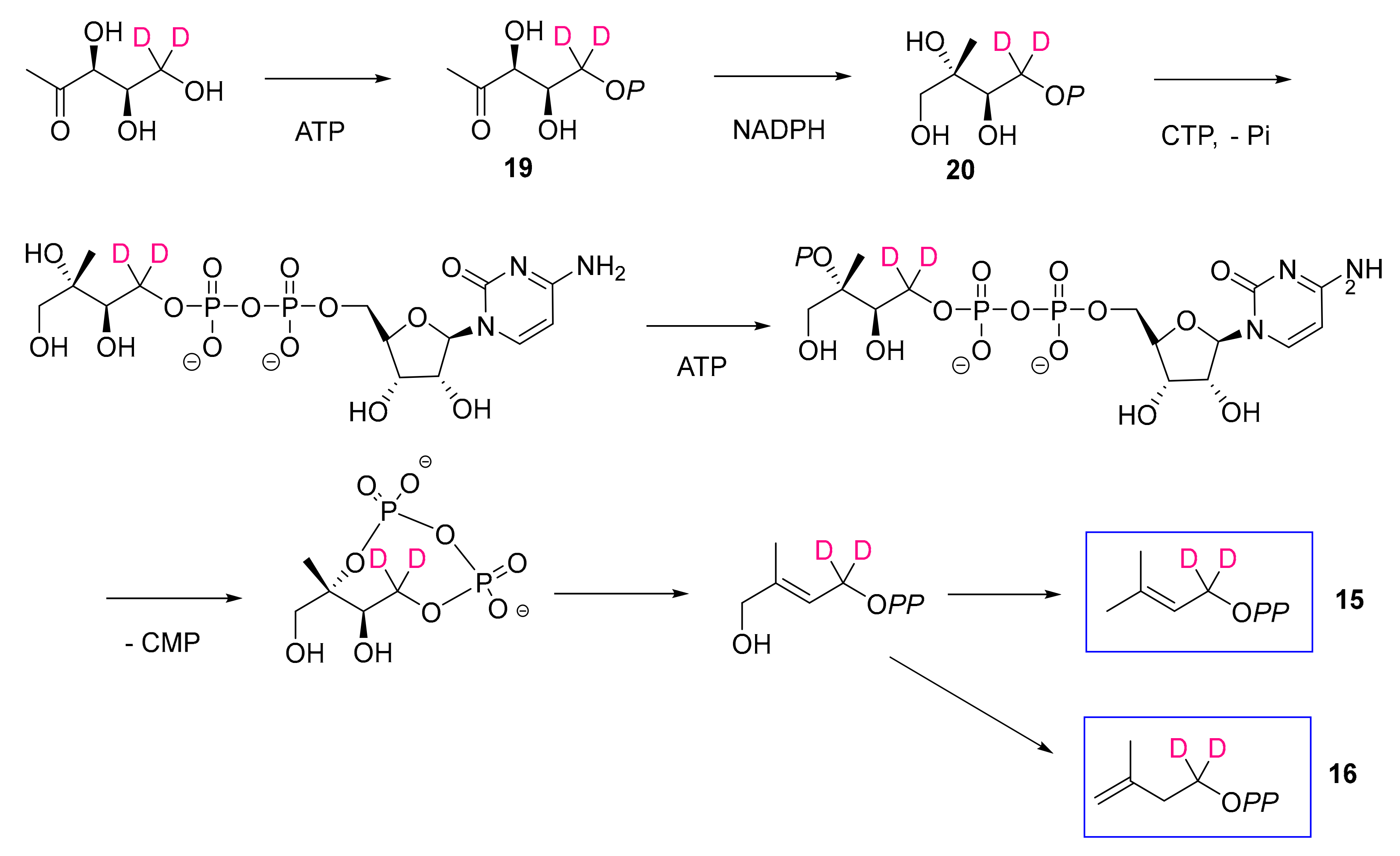
2.5. E. lathyris Labeling with (5,5-2H2)Deoxyxylulose
2.6. E. lathyris Isoprenoid Analysis by Natural Abundance IR GC-MS
3. Discussion and Conclusions
3.1. Latex Triterpenes are (Mainly) Synthesized via the MVA Pathway in E. lathyris
3.2. Compartmentation of Triterpenoid Biosynthesis and Possible Involvement of Endophytic Fungi for the Biosynthesis of Hopenol B
4. Materials and Methods
4.1. Plant Material
4.2. Analytical Methods
4.3. Isolation of Triterpenes and Sterols
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gas chromatography |
| GC-MS | Gas chromatography coupled to mass spectrometry |
| IR GC-MS | Isotope ratio GC-MS |
| IPP | Isopentenyl diphosphate |
| MEP | Methylerythritol phosphate |
| MS medium | Murashige-Skoog medium |
| MVA | Mevalonate |
| NMR | Nuclear magnetic resonance |
| TLC | Thin layer chromatography |
References
- Steinmann, V.W.; Porter, J.M. Phylogenetic relationships in Euphorbiae (Eupharbiaceae) based on ITS and ndhf sequence data. Ann. Missouri Bot. Gard. 2002, 89, 453–490. [Google Scholar] [CrossRef]
- Bruyns, P.V.; Mapaya, R.J.; Hedderson, T. A new subgeneric classification for Euphorbia (Euphorbiacea) in Southern Africa based on ITS and psbA-trnH sequence data. Taxon 2006, 55, 397–420. [Google Scholar] [CrossRef]
- Horn, J.W.; van Ee, B.W.; Morawetz, J.J.; Riina, R.; Steinmann, V.W.; Wurdack, K.J. Phylogenetics and the evolution of major structural characters in the giant genus Euphorbia, L. (Euphorbiaceae). Mol. Phylogenet. Evol. 2012, 63, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Mahlberg, P.G. Laticifers: An historical perspective. Bot. Rev. 1993, 59, 1–23. [Google Scholar] [CrossRef]
- Anton, R. Etude chimiotaxonomique sur le genre Euphorbia (Euphorbiacées). Ph.D. Thesis, Université Louis Pasteur, Strasbourg, France, 1974. [Google Scholar]
- Ourisson, G.; Rohmer, M.; Anton, R. From terpenes to sterols: Macroevolution and microevolution. Rec. Adv. Phytochem. 1979, 13, 131–162. [Google Scholar]
- Uppadhyay, R.R.; Hecker, E. Diterpene esters of the irritant and cocarcinogenic latex of Euphorbia marginata. Am. J. Bot. 1975, 55, 375–381. [Google Scholar]
- Fürstenberger, G.; Berry, D.L.; Sorg, B.; Marks, F. Skin tumor promotion by phorbol esters is a two-stage process. Proc. Natl. Acad. Sci. USA 1981, 78, 7722–7726. [Google Scholar]
- Eschenmoser, A.; Ružička, L.; Jeger, O.; Arigoni, D. Zur Kenntnis der Triterpene. 190. Mitteilung. Eine stereochemische Interpretation der biogenetischen Isoprenregel bei den Triterpenen. Helv. Chim. Acta 1955, 38, 1890–1904. [Google Scholar] [CrossRef]
- Rohmer, M.; Bouvier, P.; Ourisson, G. Molecular evolution of biomembranes: Structural equivalents and phylogenetic equivalents of sterols. Proc. Natl. Acad. Sci. USA 1979, 76, 847–851. [Google Scholar] [CrossRef]
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Calvin, M. Hydrocarbons via photosynthesis. Energy Res. 1977, 1, 299–327. [Google Scholar] [CrossRef]
- Calvin, M. Chemistry, population, resources. Pure Appl. Chem. 1978, 50, 407–425. [Google Scholar] [CrossRef]
- Nemethy, E.K.; Otvos, J.W.; Calvin, M. Hydrocarbons from Euphorbia lathyris. Pure Appl. Chem. 1981, 53, 1101–1108. [Google Scholar] [CrossRef][Green Version]
- Nemethy, E.K.; Skrukrud, C.; Piazza, G.J.; Calvin, M. Terpenoid biosynthesis in Euphorbia latex. Biochim. Biophys. Acta 1983, 760, 343–349. [Google Scholar] [CrossRef]
- Bloch, K. Sterol molecule: Structure, biosynthesis and function. Steroids 1992, 57, 378–383. [Google Scholar] [CrossRef]
- Flesch, G.; Rohmer, M. Prokaryotic hopanoids: The biosynthesis of the bacteriohopane skeleton. Formation of isoprenic units from two distinct acetate pools and a novel type of carbo/carbon linkage between a triterpene and D-ribose. Eur. J. Biochem. 1988, 175, 405–411. [Google Scholar] [CrossRef]
- Rohmer, M.; Knani, M.; Simonin, P.; Sutter, B.; Sahm, H. Isoprenoid biosynthesis in bacteria – A novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 1993, 295, 517–524. [Google Scholar] [CrossRef]
- Schwarz, M.C. Terpen-Biosynthese in Gingko biloba: Eine überraschende Geschichte. Ph.D. Thesis, Nb. 10951. Eidgenössische Technische Hochschule, Zürich, Switzerland, 1994. [Google Scholar]
- Schwarz, M.C.; Arigoni, D. Ginkgolide biosynthesis. In Comprehensive Natural Product Chemistry: Isoprenoids including Carotenoids and Steroids; Cane, D.E., Ed.; Pergamon: Oxford, UK, 1996; Volume 2, pp. 367–400. [Google Scholar]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid. Res. 2013, 51, 95–148. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Hoeffler, J.F.; Meyer, O.; Tritsch, D.; Kagan, I.; Grosdemange-Billiard, C.; Rohmer, M.; Bach, T.J. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in Tobacco Bright Yellow-2 cells. J. Biol. Chem. 2003, 278, 26666–26675. [Google Scholar] [CrossRef]
- Ponsinet, G.; Ourisson, G. Biosynthèse in vitro des triterpènes dans le latex d’Euphorbia. Phytochemistry 1968, 7, 89–98. [Google Scholar] [CrossRef]
- Groeneveld, H.V.; Roelvink, P.W. Lipid synthesis in the laticifers of Euphorbia lathyris L. seedlings. Planta 1982, 154, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.J.; Holzwarth, J.A. Enhancement of terpenoid biosynthesis from mevalonate in a fraction of the latex from Euphorbia lathyris. Plant. Physiol. 1989, 89, 681–686. [Google Scholar] [CrossRef] [PubMed]
- La biosynthèse des stérols dans les tissus de tabac cultivés in vitro. Cinétique de formation des stérols et de leurs précurseurs. Eur. J. Biochem. 1969, 9, 526–533. [CrossRef] [PubMed]
- Broers, S.T.J. I. über die frühen Stufen der Biosynthese von Isoprenoiden in Escherichia coli. II. Beitrag zur Aufklärung der Biosynthese von Vitamin B12 in Propionibacterium shermanii. Ph.D. Thesis, Nb. 10978. Eidgenössische Technische Hochschule, Zürich, Switzerland, 1994. [Google Scholar]
- Ross, J.D.; Bradbeer, J.W. Concentrations of gibberellins in chilled hazel seeds. Nature 1968, 220, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.C.; Chang, J.L.L. Does gibberellic acid stimulate seed germination via amylase synthesis? Plant Physiol. 1972, 49, 441–442. [Google Scholar] [CrossRef][Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schaffstein, G. Untersuchungen an ungegliedreten Milchröhren. Beih. Bot. Centralbl. 1932, 48, 197–220. [Google Scholar]
- Koops, A.J.; Italaander, E.; Groeneveld, H.W. Triterpenoid biosynthesis in the seedlings of Euphorbia lathyris. Plant Sci. 1991, 74, 193–201. [Google Scholar] [CrossRef]
- Koops, A.J.; Baas, J.W.; Groeneveld, H.W. The composition of phytosterols, latex triterpenols and wax triterpenoids in the seedlings of Euphorbia lathyris L. Plant. Sci. 1991, 74, 185–191. [Google Scholar] [CrossRef]
- Giner, J.L.; Djerassi, C. Reinvestigation of the biosynthesis of lanosterol in Euphorbia lathyris. Phytochemistry 1985, 39, 333–335. [Google Scholar] [CrossRef]
- Koops, A.J.; Groeneveld, H.W. Mobilization of endosperm reserves and uptake of sucrose and valine by the cotyledons of Euphorbia lathyris L. J. Exp. Bot. 1990, 41, 1279–1286. [Google Scholar] [CrossRef]
- Koops, A.J.; Groeneveld, H.W. The quantitative contribution of sucrose and threonine to triterpenoid production in the etiolated seedlings of Euphorbia lathyris L. Plant. Physiol. 1991, 138, 162–167. [Google Scholar] [CrossRef]
- Sgraja, T.; Smith, T.K.; Hunter, W.N. Structure, substrate recognition and reactivity of Leishmania major mevalonate kinase. BMS Struct. Biol. 2007, 7, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Wungsintaweekul, J.; Herz, S.; Hecht, S.; Eisenreich, W.; Rohdich, F.; Bacher, A.; Zenk, M.H. Phosphorylation of 1-deoxy-d-xylulose by d-xylulokinase of Escherichia coli. Eur. J. Biochem. 2001, 268, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hemmerlin, A.; Tritsch, D.; Hartmann, M.A.; Pacaud, K.; Hoeffler, J.F.; van Dorsselaer, A.; Rohmer, M.; Bach, T.J. A cytosolic Arabidopsis d-xylulose kinase catalyzes the phosphorylation of 1-deoxy- d-xylulose into a precursor of the plastidial isoprenoid pathway. Plant. Physiol. 2006, 142, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Hoeffler, J.F.; Hemmerlin, A.; Grosdemange-Billiard, C.; Bach, T.J.; Rohmer, M. Isoprenoid biosynthesis in higher plants and in E. coli: On the branching in the methylerythritol phosphate pathway and the independent biosynthesis of isopentenyl and dimethylallyl diphosphates. Biochem. J. 2002, 366, 573–583. [Google Scholar] [CrossRef]
- Zahnley, J.C.; Axelrod, B. d-xylulokinase and d-ribulokinase in higher plants. Plant. Physiol. 1965, 40, 372–378. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 133–149. [Google Scholar] [CrossRef]
- De Laeter, J.R.; Böhlke, J.K.; De Bièvre, P.; Hidaka, H.; Peiser, H.S.; Rosman, K.J.R.; Taylor, P.D.P. Atomic weights of the elements: Review 2000 (IUPAC technical support). Pure Appl. Chem. 2003, 75, 683–800. [Google Scholar] [CrossRef]
- Bodlenner, A.; Liu, W.; Hirsch, G.; Schaeffer, P.; Blumenberg, M.; Lendt, R.; Tritsch, D.; Michaelis, W.; Rohmer, M. C35 hopanoid side-chain biosynthesis: Reduction of ribosylhopane into bacteriohopanetetrol by a cell-free system derived from Methylobacterium organophilum. ChemBioChem 2015, 16, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Piel, J.; Donath, J.; Bandemer, K.; Boland, W. Mevalonate-independent biosynthesis of terpenoid volatiles in plants: Induced and constitutive emission of volatiles. Angew. Chem. Int. Ed. 1998, 37, 2478–2481. [Google Scholar] [CrossRef]
- Jux, A.; Gleisner, G.; Boland, W. Classification of terpenoids according to the methylerythritol or the mevalonate pathway with natural 13C/12C isotope ratios: Dynamic allocation or resources in induced plants. Angew. Chem. Int. Ed. 2001, 40, 2091–2093. [Google Scholar] [CrossRef]
- Sessions, A.L.; Burgoyne, T.W.; Schimmelmann, A.; Hayes, J.M. Fractionation of hydrogen isotopes in lipid biosynthesis. Org. Geochem. 1999, 30, 1193–1200. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Naraoka, H.; Poulson, S.R. Carbon and hydrogen fractionation during lipid biosynthesis in a higher plant (Cryptomeria japonica). Phytochemistry 2004, 65, 323–330. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Naraoka, H.; Poulson, S.R. Hydrogen and carbon isotopic fractionations of lipid biosynthesis among terrestrial (C3, C4 and CAM) and aquatic plants. Phytochemistry 2004, 65, 1369–1381. [Google Scholar] [CrossRef]
- Sessions, A.L. Seasonal changes in lipid D/H fractionation by Spartina alterniflora. Geochim. Cosmochim. Acta 2006, 70, 2153–2162. [Google Scholar] [CrossRef]
- Benveniste, P. Biosynthesis and accumulation of sterols. Annu. Rev. Plant. Biol. 2004, 55, 429–457. [Google Scholar] [CrossRef]
- Jin, M.L.; Lee, M.W.; Kim, O.T. Two cycloartenol synthases for phytosterol biosynthesis in Polygala tenuifolia Willd. Int. J. Mol. Sci. 2017, 18, 2426–2437. [Google Scholar] [CrossRef]
- Forestier, E.; Romero-Segura, C.; Pateraki, I.; Centeno, E.; Compagnon, V.; Preiss, M.; Berna, A.; Boronat, A.; Bach, T.J.; Darnet, S.; et al. Distinct triterpene synthases in the laticifers of Euphorbia lathyris. Sci. Rep. 2019, 9, 4840–4851. [Google Scholar] [CrossRef]
- Pale-Grosdemange, C.; Feil, C.; Rohmer, M.; Poralla, K. Occurrence of cationic intermediates and deficient control during the enzymatic cyclization of squalene to hopanoids. Angew. Chem. Int. Ed. 1998, 37, 2237–2240. [Google Scholar] [CrossRef]
- Connolly, J.D.; Hill, R.A. Dictionary of terpenoids; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Rohmer, M.; Bouvier-Navé, P.; Ourisson, G. Distribution of hopane triterpenes in prokaryotes. J. Gen. Microbiol. 1984, 130, 1137–1150. [Google Scholar]
- Marsili, A.; Morelli, I.; Bernardini, C.; Pacchiani, M. Constituents of some mosses. Phtytochemistry 1972, 11, 2003–2005. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Xue, J.; Myers, P.A.; Zhang, Z.; Tan, K.; Zhang, Z.; Xie, S. Occurrence of diploptene in moss species from the Dajiuhu peatland in southern China. Org. Geochem. 2010, 41, 321–324. [Google Scholar] [CrossRef]
- Gonzalez, A.; Betancor, C.; Hernandez, R.; Salazar, J.A. 6β,22-Dihydroxyhopane, a new triterpene from the fern Cheilanthes marantae. Phytrochemistry 1976, 15, 1996–1997. [Google Scholar] [CrossRef]
- Ageta, H.; Iwata, K.; Natori, S. Fern constituents: Adianene, filicene, 7-fernene, isofernene and diploptene. Triterpenoid hydrocarbons isolated from Adiantum monochlamys. Tetrahedron Lett. 1964, 3413–3418. [Google Scholar] [CrossRef]
- Inatumi, Y.; Inada, A.; Murata, H.; Nishi, M.; Nakanishi, T. Constituents of a fern, Diplazium subsinuatum. III. Four new hopane-triterpene lactone glycosides. Chem. Pharm. Bull. 2000, 48, 1930–1934. [Google Scholar] [CrossRef][Green Version]
- Corbett, R.E.; Cumming, S.D. Lichens and fungi. Part VII. Extractives from the lichen Sticta mougeotiana var. dissecta Del. J. Chem. Soc. C 1971, 955–960. [Google Scholar] [CrossRef]
- Ejiri, H.; Shibata, S. Zeorin from the mycobiont of Anaptychia hypoleuca. Phytochemistry 1974, 13, 2871. [Google Scholar] [CrossRef]
- Nicollier, G.; Tabacchi, R.; Gavin, J.; Breton, J.L.; Gonzalez, A.G. Triterpènes de la “mousse de chêne” (Evernia prunastri (l.) Ach.). Helv. Chim. Acta 1979, 62, 807–810. [Google Scholar] [CrossRef]
- van Eijk, G.W.; Roeijmans, H.J.; Seykens, D. Hopanoids from the entomogenous fungus Aschersonia aleyrodis. Tetrahedron Lett. 1986, 27, 2533–2534. [Google Scholar] [CrossRef]
- Isaka, M.; Hywel-Jones, N.; Sappan, M.; Mongkolsamrit, S.; Saidaengkham, S. Hopane triterpenes as chemotaxonomic markers for the scale insect pathogens Hypocrella s. lat. and Aschersonia. Mycol. Res. 2009, 113, 491–497. [Google Scholar] [CrossRef]
- Wang, W.L.; Liu, P.P.; Zhang, Y.P.; Tao, H.W.; Gu, Q.Q.; Zhu, W.M. 2-Hydroxydiplopterol, a new cytotoxic pentacyclic triterpenoid from the halotolerant fungus Aspergillus variecolor B-17. Arch. Pharm. Res. 2009, 32, 1211–1214. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; López-Dias, C.; Landa, B.B. The hidden habit of the entomopathogenic fungus Beauveria bassiana. First demonstration of vertical plant transmission. PLoS ONE 2014, 9, e89278. [Google Scholar] [CrossRef]
- Egan, J.M.; Kaur, A.; Kellog, J.J.; Oberlies, N.H.; Cech, N.B. Antimicrobial fungal endophytes from the botanical medicine goldenseal (Hydrastis canadensis). Phytochem. Lett. 2016, 17, 219–225. [Google Scholar] [CrossRef]
- Parsa, S.; García-Lemos, A.; Castillo, K.; Ortiz, V.; López-Lavalle, L.A.B.; Braun, J.; Vega, F. Fungal endophytes in germinated seeds of the common bean, Phaseolus vulgaris. Fungal Biol. 2016, 120, 783–790. [Google Scholar] [CrossRef]
- de Azevedo Silva, Liotti, R.G.; de Araújo Boiei, A.P.; de Molo Reis, E.; Borges Silva Passos, M.; dos Santos, E.L.; Moreira Sampaio, O.; Januário, A.H.; Bassi Branco, C.L.; Ferreira da Silva, G.; Furtado de Mendonça, E.A.; et al. Diversity of cultivable fungal endophytes in Paulinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS ONE 2018, 13, e0195874. [Google Scholar] [CrossRef]
- Shen, X.Y.; Chang, Y.L.; Cai, C.J.; Fan, L.; Gao, J.; Hou, C.L. Diversity and antimicrobial activity of culturable endophytic fungi isolated from Moso bamboo seeds. PLoS ONE 2014, 9, e95838. [Google Scholar] [CrossRef]
- Kamdem, R.S.T.; Wang, H.; Wafo, P.; Ebrahim, W.; Özkaya, F.C.; Makhloufi, G.; Janiak, C.; Sureechatchiyan, P.; Kassack, M.U.; Lin, W.; et al. Induction of new metabolites from the endophytic fungus Bionectria sp. through bacterial co-culture. Fitoterapia 2018, 124, 132–136. [Google Scholar] [CrossRef]
- Hodgson, S.; de Cates, C.; Hodgson, J.; Morley, N.J.; Sutton, B.C.; Gange, A.C. Vertical transmission of fungal endophytes is widespread in forbs. Ecol. Evol. 2014, 4, 1199–1208. [Google Scholar] [CrossRef]
- Wiewióra, B.; Zurek, G.; Pańka, D. Is the vertical transmission of Neotyphodium lolii in perennial ryegrass the only possible way to the spread of endophytes? PLoS ONE 2015, 10, e0117231. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asal, S.; Lee, I.J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant. Sci. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, M.; Hyde, E.R.; Dorsey, B.L.; La Val, T.P.; Mullen, M.; Yoo, J.; Knight, R.; Baum, M.M. Euphorbia plant latex is inhabited by diverse microbial communities. Am. J. Bot. 2015, 102, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Aitzetmüller, K.; Guaraldo-Gonçalvez, L.A. Dynamic impregnation of silica stationary phases for the argentation chromatography of lipids. J. Chromatogr. A 1990, 519, 349–358. [Google Scholar]
- Rohmer, M.; Brand, R.D. Les stérols et leurs précurseurs chez Astasia longa Pringsheim. Eur. J. Biochem. 1972, 36, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.; Hemmerlin, A.; Bach, T.J.; Rohmer, M. Mevalonate-derived isopentenyl diphosphate is the precursor of ubiquinone prenyl side-chain in tobacco BY-2 cells. Biochem. J. 1998, 331, 615–621. [Google Scholar] [CrossRef]
- Disch, A.; Schwender, J.; Müller, C.; Lichtenthaler, H.K.; Rohmer, M. Distribution of the mevalonate and glyceraldehyde phosphate/pyruvate pathways in unicellular algae and the cyanobacterium Synechocystis PCC6714. Biochem. J. 1998, 333, 381–388. [Google Scholar] [CrossRef]
- Rieley, G. Derivatization of organic compounds prior to gas chromatographic-combustion-isotope ratio mass spectrometric analysis: Identification of isotope fractionation processes. Analyst 1994, 119, 915–919. [Google Scholar] [CrossRef]
- Abe, I.; Rohmer, M. Enzymic cyclization of 2,3-dihydrosqualene and squalene 2,3-epoxides by squalene cyclases: From pentacyclic to tetracyclic triterpenes. J. Chem. Soc. Perkin Trans. I 1994, 783–791. [Google Scholar] [CrossRef]
- Rohmer, M.; Anding, C.; Ourisson, G. Non-specific hopanoid biosynthesis by a cell-free system from Acetobacter pasteurianum. Eur. J. Biochem. 1990, 112, 541–547. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Amounts 1 | ||||
|---|---|---|---|---|
| Number of Analyses | Triterpene Fraction | Sterol Fraction | ||
| Whole plantlets from surface sterilized seeds | 3 | 1.9 | 0.6 | |
| Whole plantlets grown in plant pots | 2 | 1.5 | 0.8 | |
| Adult plants grown outdoor (>80 cm size) | aerial parts | 3 | 7.5 | 1.0 |
| roots | 3 | traces, <0.1 | 0.7 | |
| latex | 4 | 20% 2 | traces |
| Plantlets 1 | Adult Plants 2 | Latex 3 | |
|---|---|---|---|
| Lanosterol 1 | 18 | 18 | 25 |
| Butyrospermol 5 | 23 | 23 | 23 |
| Cycloartenol 3 | 17 | 16 | 18 |
| 24-Methylene cycloartanol 4 | 29 | 29 | 29 |
| Hopenol B 7 | 7 | 9 | 6 |
| Other triterpenes | 5 | 5 | 3 |
| Isoprenoid | C-1 | C-2 | C-3 | C-4 | C-5 | SAM 2 |
|---|---|---|---|---|---|---|
| 24-Methylene cycloartanol (4) 3 | 1.1 | 1.5 | 1.1 | 1.5 | 1.6 | 1.1 |
| 24-Methylene cycloartanol (4) | 1.2 | 2.6 | 1.1 | 2.6 | 2.9 | 2.5 |
| Cycloartenol (3) | 1.3 | 3.6 | 1.1 | 3.7 | 3.7 | - |
| Lanosterol (1) | 1.3 | 3.5 | 1.3 | 3.4 | 3.4 | - |
| Butyrospermol (5) | 1.2 | 3.1 | 1.3 | 3.3 | 2.9 | - |
| Hopenol B (7) | 1.5 | 2.7 | 1.4 | 2.9 | 3.3 | - |
| Sitosterol (8) | 1.6 | 2.4 | 1.4 | 2.4 | 2.4 | 2.0 |
| Phytol (12) | 2.0 | 1.1 | 1.3 | 1.2 | 2.2 | - |
| C-1 | C-2 | C-3 | C-4 | C-5 | SAM 2 | |
|---|---|---|---|---|---|---|
| 24-Methylene cycloartanol (4) | 1.6 | 3.7 | 1.5 | 3.9 | 4.2 | 3.6 |
| Cycloartenol (3) | 1.8 | 4.9 | 1.7 | 5.3 | 5.2 | - |
| Lanosterol (1) | 1.4 | 4.5 | 1.6 | 4.8 | 4.7 | - |
| Butyrospermol (5) | 1.8 | 4.8 | 1.7 | 5.0 | 4.3 | - |
| Hopenol B (7) | 2.1 | 6.3 | 2.0 | 5.9 | 6.6 | - |
| Sitosterol (8) | 1.9 | 4.4 | 2.0 | 4.5 | 4.0 | 3.1 |
| Phytol (12) | 2.8 | 2.1 | 2.2 | 2.2 | 3.1 | - |
| Isoprenoid | C-1 | C-2 | C-3 | C-4 | C-5 | SAM 2 |
|---|---|---|---|---|---|---|
| 24-Methylene cycloartanol (4) | 1.7 | 4.3 | 1.7 | 4.5 | 4.2 | 3.5 |
| Cycloartenol (3) | 1.9 | 5.5 | 1.8 | 5.7 | 6.0 | - |
| Lanosterol (1) | 1.8 | 5.0 | 1.8 | 5.2 | 5.5 | - |
| Butyrospermol (5) | 2.0 | 4.7 | 1.7 | 5.1 | 5.1 | - |
| Hopenol B (7) | 2.3 | 6.9 | 2.1 | 7.2 | 7.4 | - |
| Sitosterol (8) | 1.3 | 3.2 | 1.3 | 3.4 | 3.4 | 2.4 |
| Phytol (12) | 4.3 | 2.6 | 2.8 | 2.9 | 4.7 | - |
| C-1 | C-2 | C-3 | C-4 | C-5 | SAM 2 | |
|---|---|---|---|---|---|---|
| Incorporation of mevalonolactone | ||||||
| 24-Methylene cycloartanol (4) | 1.1 | 1.2 | 1.1 | 1.6 | 1.2 | 1.4 |
| Lanosterol (1) | 1.1 | 1.1 | 1.1 | 1.0 | 1.1 | - |
| Butyrospermol (5) | 1.2 | 1.2 | 1.3 | 1.3 | 1.2 | - |
| Sitosterol (8) | 0.9 | 0.9 | 1.0 | 2.9 | 1.0 | 1.4 |
| Phytol (12) | 1.3 | 1.2 | 1.3 | 1.5 | 1.2 | - |
| Incorporation of mevalonate | ||||||
| 24-Methylene cycloartanol (4) | 1.1 | 1.2 | 1.1 | 4.3 | 1.2 | 1.3 |
| Cycloartenol (3) | 1.1 | 1.1 | 1.2 | 4.6 | 1.2 | - |
| Lanosterol (1) | 1.1 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Butyrospermol (5) | 1.1 | 1.1 | 1.1 | 1.1 | 1.3 | - |
| Hopenol B (7) | 1.0 | 1.1 | 1.3 | 1.3 | 1.3 | - |
| Sitosterol (8) | 1.0 | 1.1 | 1.1 | 5.7 | 1.1 | 1.0 |
| Phytol (12) | 1.1 | 1.1 | 1.1 | 1.4 | 1.1 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastaldo, C.; Lipko, A.; Motsch, E.; Adam, P.; Schaeffer, P.; Rohmer, M. Biosynthesis of Isoprene Units in Euphorbia lathyris Laticifers vs. Other Tissues: MVA and MEP Pathways, Compartmentation and Putative Endophytic Fungi Contribution. Molecules 2019, 24, 4322. https://doi.org/10.3390/molecules24234322
Gastaldo C, Lipko A, Motsch E, Adam P, Schaeffer P, Rohmer M. Biosynthesis of Isoprene Units in Euphorbia lathyris Laticifers vs. Other Tissues: MVA and MEP Pathways, Compartmentation and Putative Endophytic Fungi Contribution. Molecules. 2019; 24(23):4322. https://doi.org/10.3390/molecules24234322
Chicago/Turabian StyleGastaldo, Clément, Agata Lipko, Estelle Motsch, Pierre Adam, Philippe Schaeffer, and Michel Rohmer. 2019. "Biosynthesis of Isoprene Units in Euphorbia lathyris Laticifers vs. Other Tissues: MVA and MEP Pathways, Compartmentation and Putative Endophytic Fungi Contribution" Molecules 24, no. 23: 4322. https://doi.org/10.3390/molecules24234322
APA StyleGastaldo, C., Lipko, A., Motsch, E., Adam, P., Schaeffer, P., & Rohmer, M. (2019). Biosynthesis of Isoprene Units in Euphorbia lathyris Laticifers vs. Other Tissues: MVA and MEP Pathways, Compartmentation and Putative Endophytic Fungi Contribution. Molecules, 24(23), 4322. https://doi.org/10.3390/molecules24234322






