Development-Disrupting Chitin Synthesis Inhibitor, Novaluron, Reprogramming the Chitin Degradation Mechanism of Red Palm Weevils
Abstract
1. Introduction
2. Results
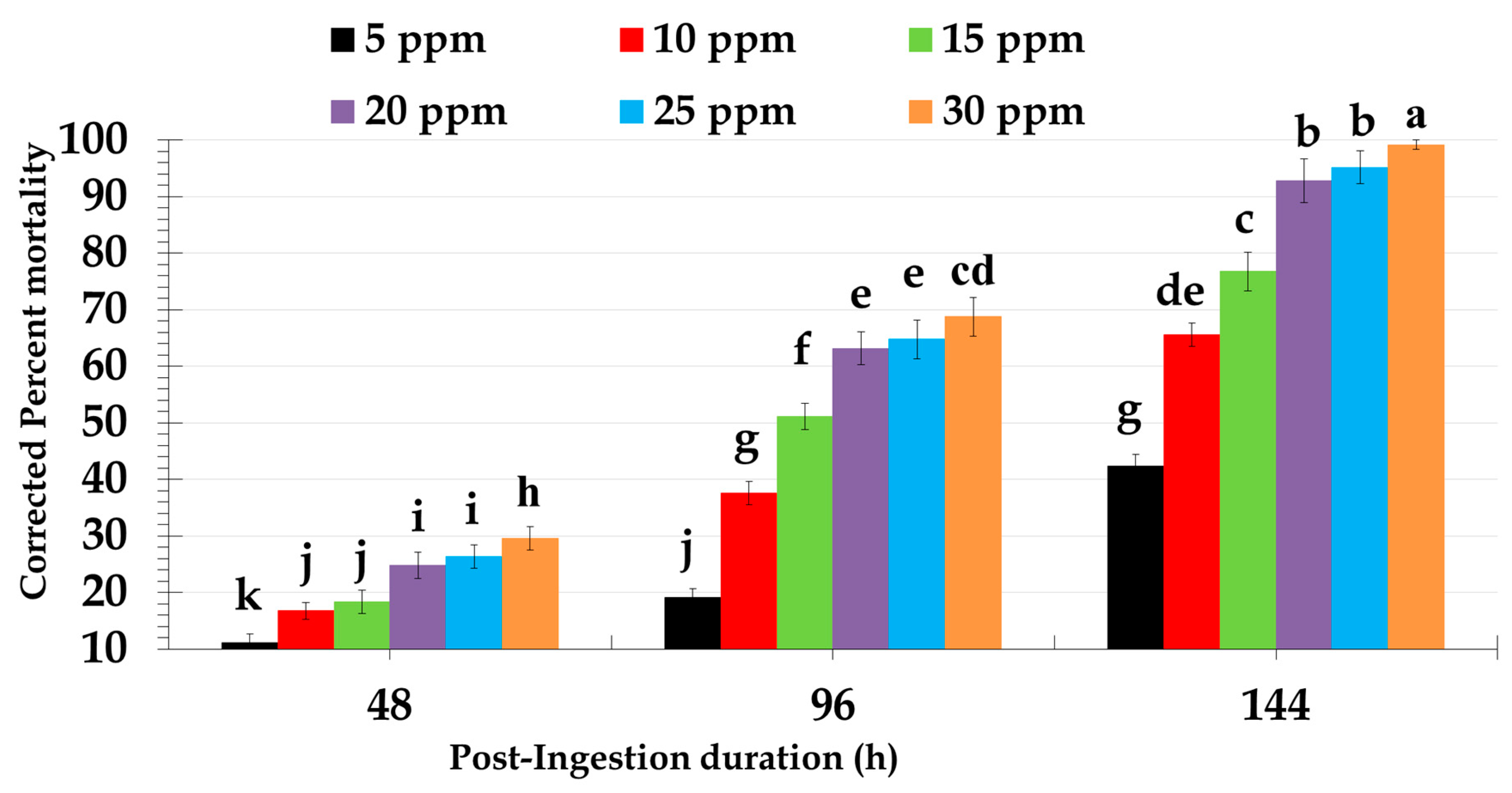
2.1. Biological Activity of Different Doses of Novaluron Against Red Palm Weevil Larvae
2.2. Nutritional Indices of Red Palm Weevil Larvae in Response to Different Doses of Novaluron
2.3. Sequence Annotations for Chitin Degradation Related Genes of Red Palm Weevils
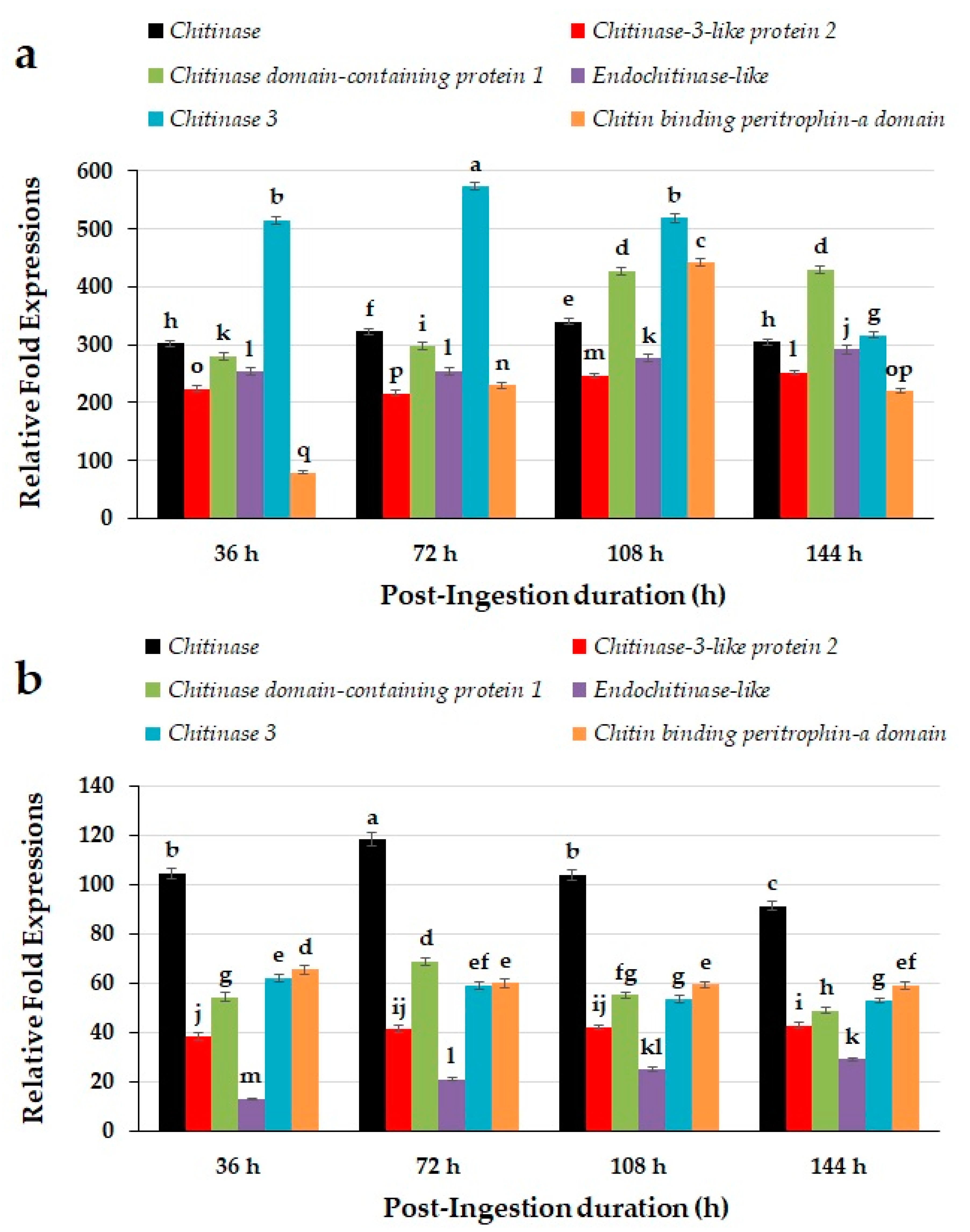
2.4. Quantitative Expression Patterns of Chitin Degradation Related Genes of Red Palm Weevils
3. Discussion
4. Materials and Methods
4.1. Rearing of Experimental Insects
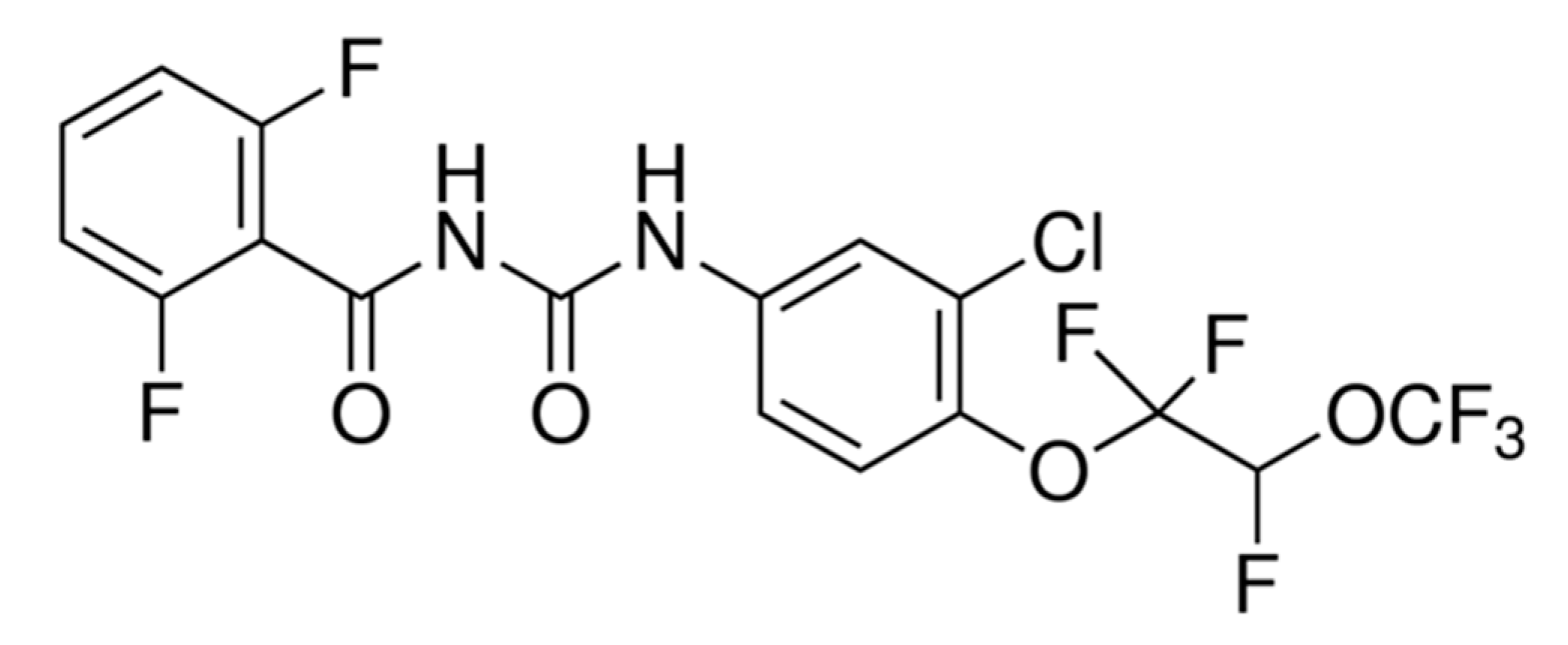
4.2. Chitin Synthesis Inhibitor
4.3. Biological Activity of Novaluron against Red Palm Weevil Larvae
4.4. Negative Impacts of Novaluron on Red Palm Weevil Larval Development
4.5. Analysis of Sequences Encoding Genes of Chitin Degradation Mechanism of Red Palm Weevils
4.6. Regulation of Genes Encoding Chitin Degradation Mechanism of Red Palm Weevils
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Jabr, A.M.; Al-Ayied, H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013, 11, 456–463. [Google Scholar]
- Hussain, A.; Rizwan-ul-haq, M.; Al-jabr, A.M. Red palm weevil: Understanding the fungal disease mechanism and host defense. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex Research Center: Badajoz, Spain, 2013; pp. 1278–1286. [Google Scholar]
- Al-Ayedh, H. Evaluation of date palm cultivars for rearing the red date palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Florida Entomol. 2008, 91, 353–358. [Google Scholar] [CrossRef]
- Ávalos, J.; Soto, A. Study of chromatic attraction of the red palm weevil, Rhynchophorus ferrugineus using bucket traps. Bull. Insecto. 2015, 68, 1–8. [Google Scholar]
- Guarino, S.; Colazza, S.; Peri, E.; Bue, P.L.; Germanà, M.P.; Kuznetsova, T.; Gindin, G.; Soroker, V. Behaviour-modifying compounds for management of the red palm weevil (Rhynchophorus ferrugineus Oliver). Pest Manag. Sci. 2015, 71, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Paoli, F.; Dallai, R.; Cristofaro, M.; Arnone, S.; Francardi, V.; Roversi, P.F. Morphology of the male reproductive system, sperm ultrastructure and γ-irradiation of the red palm weevil Rhynchophorus ferrugineus Oliv. (Coleoptera: Dryophthoridae). Tissue Cell 2014, 46, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Aljabr, A.M. Toxicity and detoxification mechanism of black pepper and its major constituent in controlling Rhynchophorus ferrugineus Olivier (Curculionidae: Coleoptera). Neotrop. Entomol. 2017, 46, 685–693. [Google Scholar] [CrossRef]
- AlJabr, A.M.; Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H. Toxicity of Plant Secondary Metabolites Modulating Detoxification Genes Expression for Natural Red Palm Weevil Pesticide Development. Molecules 2017, 22, 169. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-haq, M.; AlJabr, A.M.; Al-Ayedh, H. Lethality of Sesquiterpenes Reprogramming Red Palm Weevil Detoxification Mechanism for Natural Novel Biopesticide Development. Molecules 2019, 24, 1648. [Google Scholar] [CrossRef]
- AlJabr, A.M.; Hussain, A.; Rizwan-ul-haq, M.; Al-Ayedh, H. Method for Controlling Growth of Red Palm Weevil US Patent No US009596851B1, 2017.
- Atwa, A.A.; Hegazi, E.M. Comparative susceptibilities of different life stages of the red palm weevil (Coleoptera: Curculionidae) treated by entomopathogenic nematodes. J. Econ. Entomol. 2014, 107, 1339–1347. [Google Scholar] [CrossRef]
- Sahito, H.A.; Mallah, N.A.; Kousar, T.; Kubar, W.A.; Shah, Z.H.; Jatoi, F.A.; Mangrio, W.M. Life table parameters of saw toothed grain beetle, Oryzaephilus surinamensis (L., 1758) on different varieties of stored date palm fruits infested under laboratory conditions. J. Entomol. Zool. Stud. 2017, 5, 95–99. [Google Scholar]
- Cito, A.; Mazza, G.; Strangi, A.; Benvenuti, C.; Barzanti, G.P.; Dreassi, E.; Turchetti, T.; Francardi, V.; Roversi, P.F. Characterization and comparison of Metarhizium strains isolated from Rhynchophorus ferrugineus. FEMS Microbiol. Lett. 2014, 355, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lo Verde, G.; Torta, L.; Mondello, V.; Caldarella, C.G.; Burruano, S.; Caleca, V. Pathogenicity bioassays of isolates of Beauveria bassiana on Rhynchophorus ferrugineus. Pest Manag. Sci. 2015, 71, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; AlJabr, A. Susceptibility and immune defence mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int. J. Mol. Sci. 2016, 17, 1518. [Google Scholar] [CrossRef]
- Al-Shawaf, A.M.; Al-Shagagh, A.A.; Al-Bakshi, M.M.; Al-Saroj, S.A.; Al-Badr, S.M.; Al-Dandan, A.M.; Abdallah, A. Ben Toxicity of some insecticides against red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Indian J. Plant Prot. 2010, 38, 13–16. [Google Scholar]
- Shar, M.; Rustamani, M.; Nizamani, S.; Bhutto, L. Red palm weevil (Rhynchophorus ferrugineus Olivier) infestation and its chemical control in Sindh province of Pakistan. African J. Agric. Res. 2012, 7, 1666–1673. [Google Scholar]
- Aljabr, A.M.; Rizwan-Ul-Haq, M.; Hussain, A.; Al-Mubarak, A.I.; Al-Ayied, H.Y. Establishing midgut cell culture from Rhynchophorus ferrugineus (Olivier) and toxicity assessment against ten different insecticides. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 296–303. [Google Scholar] [CrossRef]
- Cabello, T.P.; de la Peña, J.; Barranco, P.; Belda, J. Laboratory evaluation of imidacloprid and oxamyl against Rhynchophorus ferrugineus. Tests Agrochem. Cultiv. 1997, 18, 6–7. [Google Scholar]
- Al-Samarrie, A.I.; Abo-Akela, A. Distribution of injected pesticides in date palm trees. Agric. Biol. J. N. Am. 2011, 2, 1416–1426. [Google Scholar] [CrossRef]
- Al-Ayedh, H.; Hussain, A.; Rizwan-ul-Haq, M.; Al-Jabr, A.M. Status of insecticide resistance in field-collected populations of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Int. J. Agric. Biol. 2016, 18, 103–110. [Google Scholar] [CrossRef]
- Javed, S.; Ahmad, M.; Ahmad, M.; Abdin, M.; Hamid, R.; Khan, M.; Musarrat, J. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21. [Google Scholar] [CrossRef]
- Shahidi, F.; Abuzaytoun, R. Chitin, Chitosan, and Co-Products: Chemistry, Production, Applications, and Health Effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar]
- Cohen, E. Chitin. In Encyclopedia of Insects; Elsevier: Amsterdam, The Netherlands, 2009; pp. 156–157. [Google Scholar]
- Doucet, D.; Retnakaran, A. Insect Chitin. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 437–511. [Google Scholar]
- Merzendorfer, H. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef]
- Merzendorfer, H. Chitin synthesis inhibitors: old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea Chitin Synthesis Inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef]
- Fontoura, N.G.; Bellinato, D.F.; Valle, D.; Lima, J.B.P. The efficacy of a chitin synthesis inhibitor against field populations of organophosphate-resistant Aedes aegypti in Brazil. Mem. Inst. Oswaldo Cruz 2012, 107, 387–395. [Google Scholar] [CrossRef]
- Xu, Q.-Y.; Meng, Q.-W.; Shi, J.-F.; Deng, P.; Guo, W.-C.; Li, G.-Q. Novaluron ingestion causes larval lethality and inhibits chitin content in Leptinotarsa decemlineata fourth-instar larvae. Pestic. Biochem. Physiol. 2017, 143, 173–180. [Google Scholar] [CrossRef]
- Ghoneim, K.; Al-Dali, A.; Abdel-Ghaffar, A. Effectiveness of lufenuron (CGA-184699) and diofenolan (CGA-59205) on the general body metabolism of the red palm weevil, Rhynchophorus ferrugineus (Curculionidae: Coleoptera). Pakistan J. Biol. Sci. 2003, 6, 1125–1129. [Google Scholar]
- El-Bokl, M.M.; Baker, R.F.A.; El-Gammal, H.L.; Mahmoud, M.Z. Biological and histopathological effects of some insecticidal agents against red palm weevil Rhynchophorus ferrugineus. Egypt. Acad. J. Biol. Sci. 2010, 1, 7–22. [Google Scholar] [CrossRef]
- El-Bokl, M.; Bakr, R.; El-Gammal, H.; Mahmoud, M. Gonadal ultrastructure effects of some Insecticidal agents against Red Palm weevil Rhynchophorus ferrugineus. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 12–19. [Google Scholar]
- Rodriguez-Saona, C.; Wanumen, A.; Salamanca, J.; Holdcraft, R.; Kyryczenko-Roth, V. Toxicity of insecticides on various life stages of two tortricid Pests of cranberries and on a non-target predator. Insects 2016, 7, 15. [Google Scholar] [CrossRef]
- Ishaaya, I.; Kontsedalov, S.; Horowitz, A.R. Novaluron (Rimon), a novel IGR: Potency and cross-resistance. Arch. Insect Biochem. Physiol. 2003, 54, 157–164. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Ahmed, S.; Al-Jabr, A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 2015, 60, 849–859. [Google Scholar] [CrossRef]
- Martinez, D.S.; Freire, M.d.; Mazzafera, P.; Araujo-Júnior, R.T.; Bueno, R.D.; Macedo, M.L. Insecticidal effect of labramin, a lectin-like protein isolated from seeds of the beach apricot tree, Labramia bojeri, on the Mediterranean flour moth, Ephestia kuehniella. J. Insect Sci. 2012, 12, 62. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ahmed, S. Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci. 2009, 16, 511–517. [Google Scholar] [CrossRef]
- Koul, O.; Singh, G.; Singh, R.; Singh, J.; Daniewski, W.M.; Berlozecki, S. Bioefficacy and mode-of-action of some limonoids of salannin group from Azadirachta indica A. Juss and their role in a multicomponent system against lepidopteran larvae. J. Biosci. 2004, 29, 409–416. [Google Scholar] [CrossRef]
- Silva, L.B.; Silva, W.; Macedo, M.L.R.; Peres, M.T.L.P. Effects of Croton urucurana extracts and crude resin on Anagasta kuehniella (Lepidoptera: Pyralidae). Brazilian Arch. Biol. Technol. 2009, 52, 653–664. [Google Scholar] [CrossRef]
- Yang, W.-J.; Xu, K.-K.; Zhang, R.-Y.; Dou, W.; Wang, J.-J. Transcriptional Regulation of a Chitinase Gene by 20-Hydroxyecdysone and Starvation in the Oriental Fruit Fly, Bactrocera dorsalis. Int. J. Mol. Sci. 2013, 14, 20048–20063. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S. Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 2010, 67, 201–216. [Google Scholar] [CrossRef]
- Shen, Z.; Jacobs-Lorena, M. Characterization of a novel gut-specific chitinase gene from the human malaria vector Anopheles gambiae. J. Biol. Chem. 1997, 272, 28895–28900. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, G.; Cao, X.; Chen, Y.-R.; Muthukrishnan, S.; Jiang, H.; Blissard, G.W.; Kanost, M.R.; Wang, P. Overview of chitin metabolism enzymes in Manduca sexta: Identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem. Mol. Biol. 2015, 62, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, C.; Buschman, L.L.; Chen, M.-S.; Muthukrishnan, S.; Zhu, K.Y. A gut-specific chitinase gene essential for regulation of chitin content of peritrophic matrix and growth of Ostrinia nubilalis larvae. Insect Biochem. Mol. Biol. 2010, 40, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Statistix Statistix 8.1 Tallahassee; Analytical Software: Orlando, FL, USA, 2003.
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS Institute SAS User’s Guide: Statistics; SAS Institute: Cary, NC, USA, 2000.
Sample Availability: Samples of the compounds, Novaluron are not available from the authors. |
| Novaluron Dose | Efficacy of Conversion of Digested Food (%) | Efficacy of Conversion of Ingested Food (%) | Approximate Digestibility (%) |
|---|---|---|---|
| 30 ppm | 6.572 e | 4.946 f | 75.363 a |
| 25 ppm | 8.558 e | 6.302 e | 73.753 a |
| 20 ppm | 11.240 d | 7.758 d | 69.055 b |
| 15 ppm | 12.616 d | 8.336 d | 66.085 c |
| 10 ppm | 17.682 c | 11.392 c | 64.437 c |
| 5 ppm | 29.630 b | 17.440 b | 58.910 d |
| Control | 38.164 a | 19.570 a | 51.299 e |
| No | Annotation | Accession Number | Length (bp) | Expect Value |
|---|---|---|---|---|
| 1 | Chitinase | MK 904556 | 1984 | 1 × 10−127 |
| 2 | Chitinase-3-like protein 2 | MK 904557 | 3239 | 1 × 10−172 |
| 3 | Chitinase domain-containing protein 1 | MK 904558 | 4216 | 3 × 10−169 |
| 4 | Endochitinase-like | MK 904560 | 1946 | 0.0 |
| 5 | Chitinase 3 | MK 904561 | 6006 | 0.0 |
| 6 | Chitin binding peritrophin-a domain | MK 904562 | 508 | 8 × 10−71 |
| Gene | Product Length | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|---|
| Chitinase | 73 bp | MK 904556 | ACCTCTCTACGGCAGGACTT | AGCATCTGATACCGCAGCTC |
| Chitinase-3-like protein 2 | 85 bp | MK 904557 | TCCGGTTTTCGACATGGAGG | CGTGAGGCTCTGTTCGTCAT |
| Chitinase domain-containing protein 1 | 88 bp | MK 904558 | GTGAAGCGTTTCGCCAACAT | GCGAGGCACTAACTACGTACA |
| Endochitinase-like | 99 bp | MK 904560 | TCCGAACCAGTTTCCACCAG | AGTGGACGAGGGTTTTGGTC |
| Chitinase 3 | 94 bp | MK 904561 | GCTTCTCACCACCATCCGAA | AGGTGGCTTTTCATCGTCGT |
| Chitin binding peritrophin-a domain | 96 bp | MK 904562 | GGGGCCCTTTTCGATGCTAA | AGACGGGGTTGACCTTGAAC |
| Β-Actin | 74 bp | KM 438517 | TCTATGAAGGTTACGCCCTGC | GAGGTAGTCGGTCAAGTCACG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; AlJabr, A.M.; Al-Ayedh, H. Development-Disrupting Chitin Synthesis Inhibitor, Novaluron, Reprogramming the Chitin Degradation Mechanism of Red Palm Weevils. Molecules 2019, 24, 4304. https://doi.org/10.3390/molecules24234304
Hussain A, AlJabr AM, Al-Ayedh H. Development-Disrupting Chitin Synthesis Inhibitor, Novaluron, Reprogramming the Chitin Degradation Mechanism of Red Palm Weevils. Molecules. 2019; 24(23):4304. https://doi.org/10.3390/molecules24234304
Chicago/Turabian StyleHussain, Abid, Ahmed Mohammed AlJabr, and Hassan Al-Ayedh. 2019. "Development-Disrupting Chitin Synthesis Inhibitor, Novaluron, Reprogramming the Chitin Degradation Mechanism of Red Palm Weevils" Molecules 24, no. 23: 4304. https://doi.org/10.3390/molecules24234304
APA StyleHussain, A., AlJabr, A. M., & Al-Ayedh, H. (2019). Development-Disrupting Chitin Synthesis Inhibitor, Novaluron, Reprogramming the Chitin Degradation Mechanism of Red Palm Weevils. Molecules, 24(23), 4304. https://doi.org/10.3390/molecules24234304







