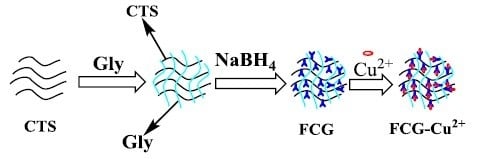
Efficient Removal of Copper Ion from Wastewater Using a Stable Chitosan Gel Material
Abstract
:1. Introduction
2. Results and Discussion
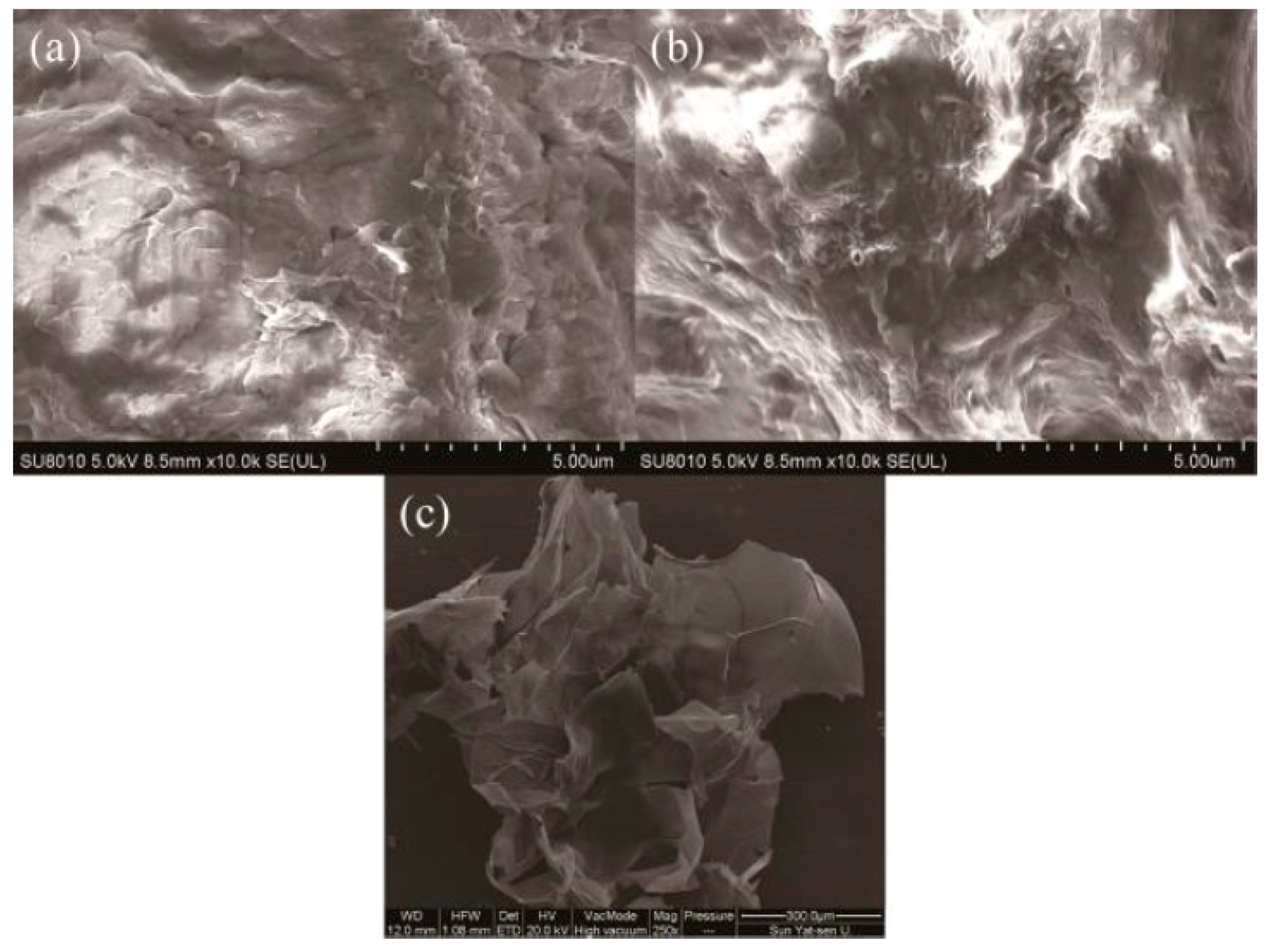
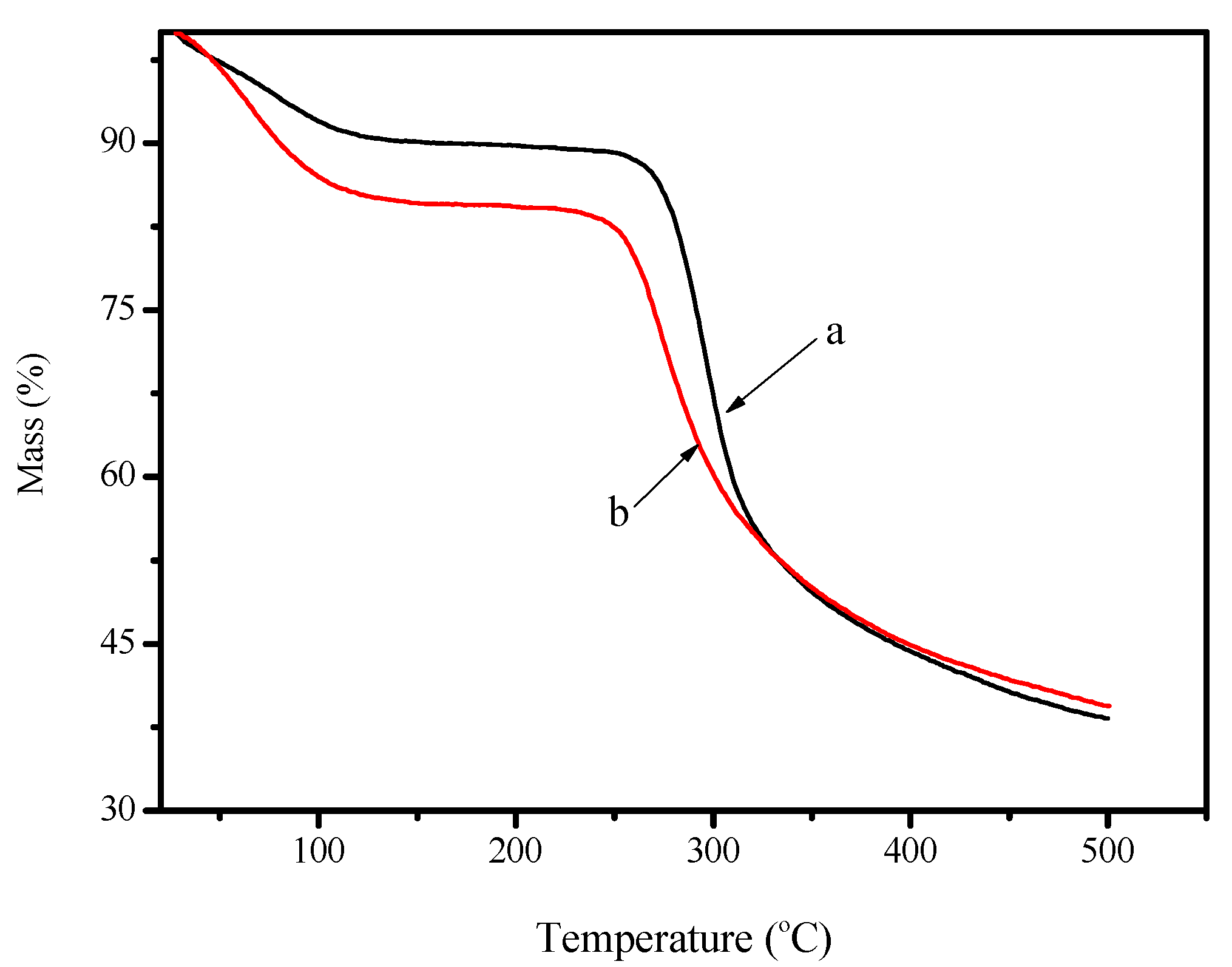
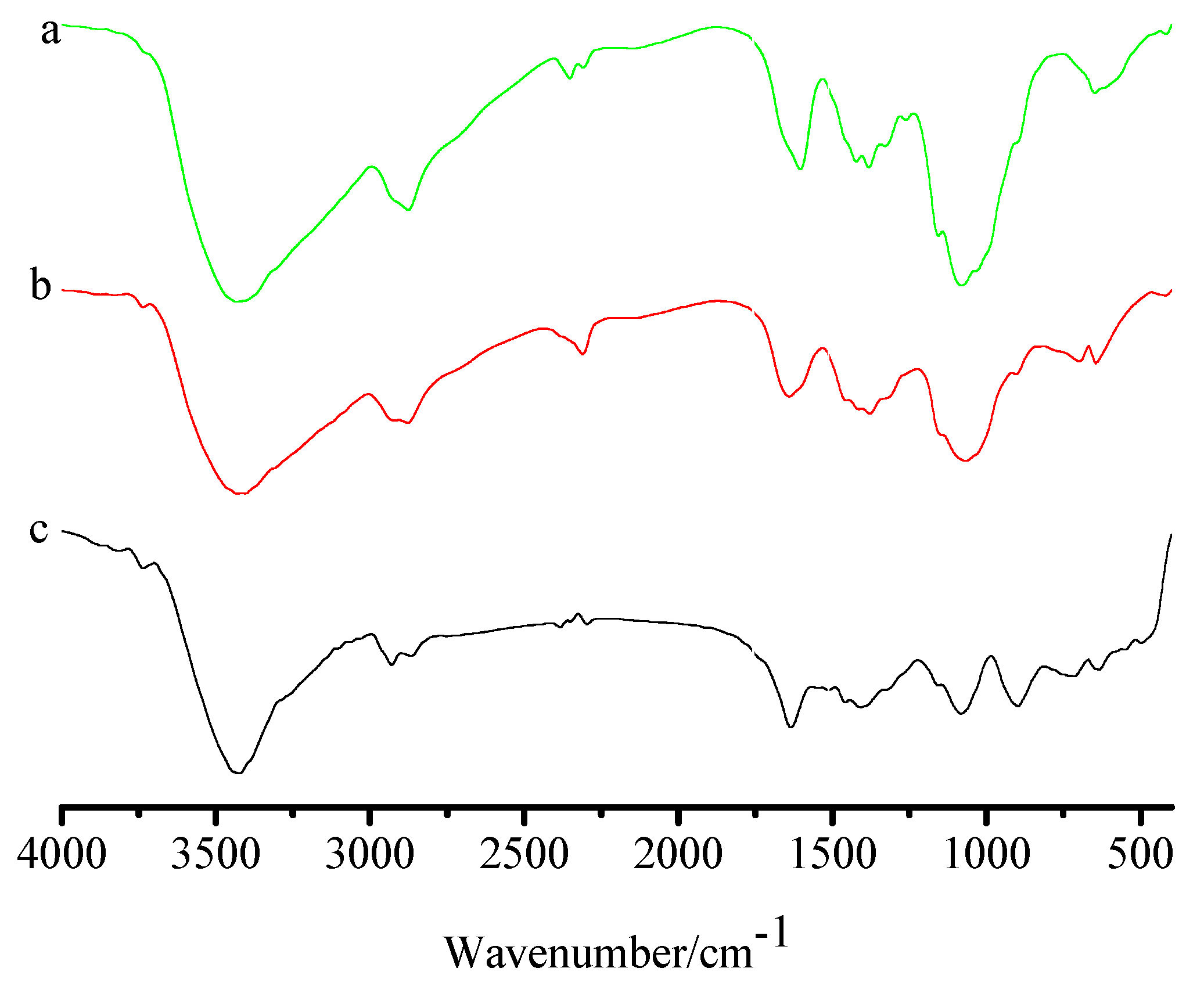
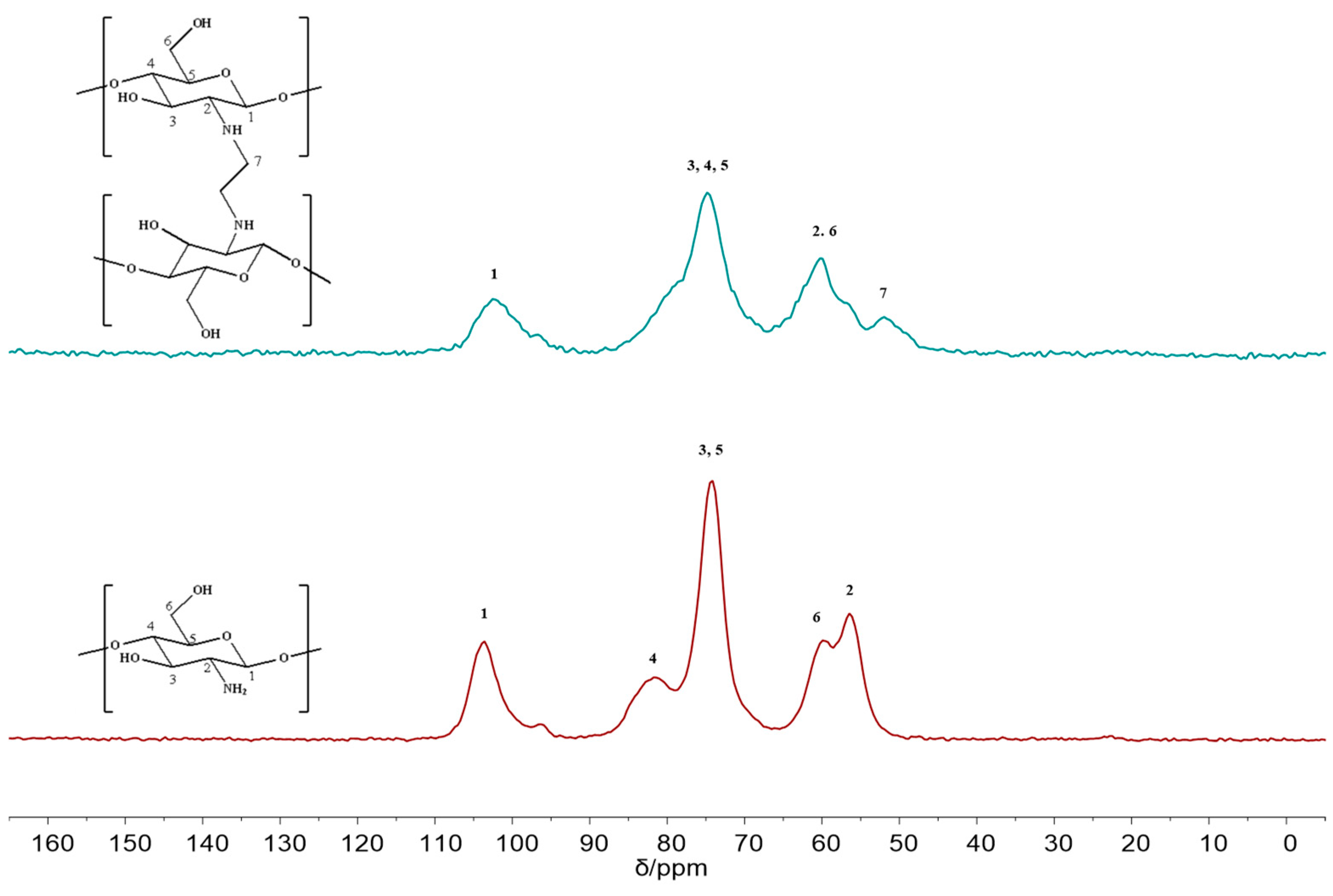
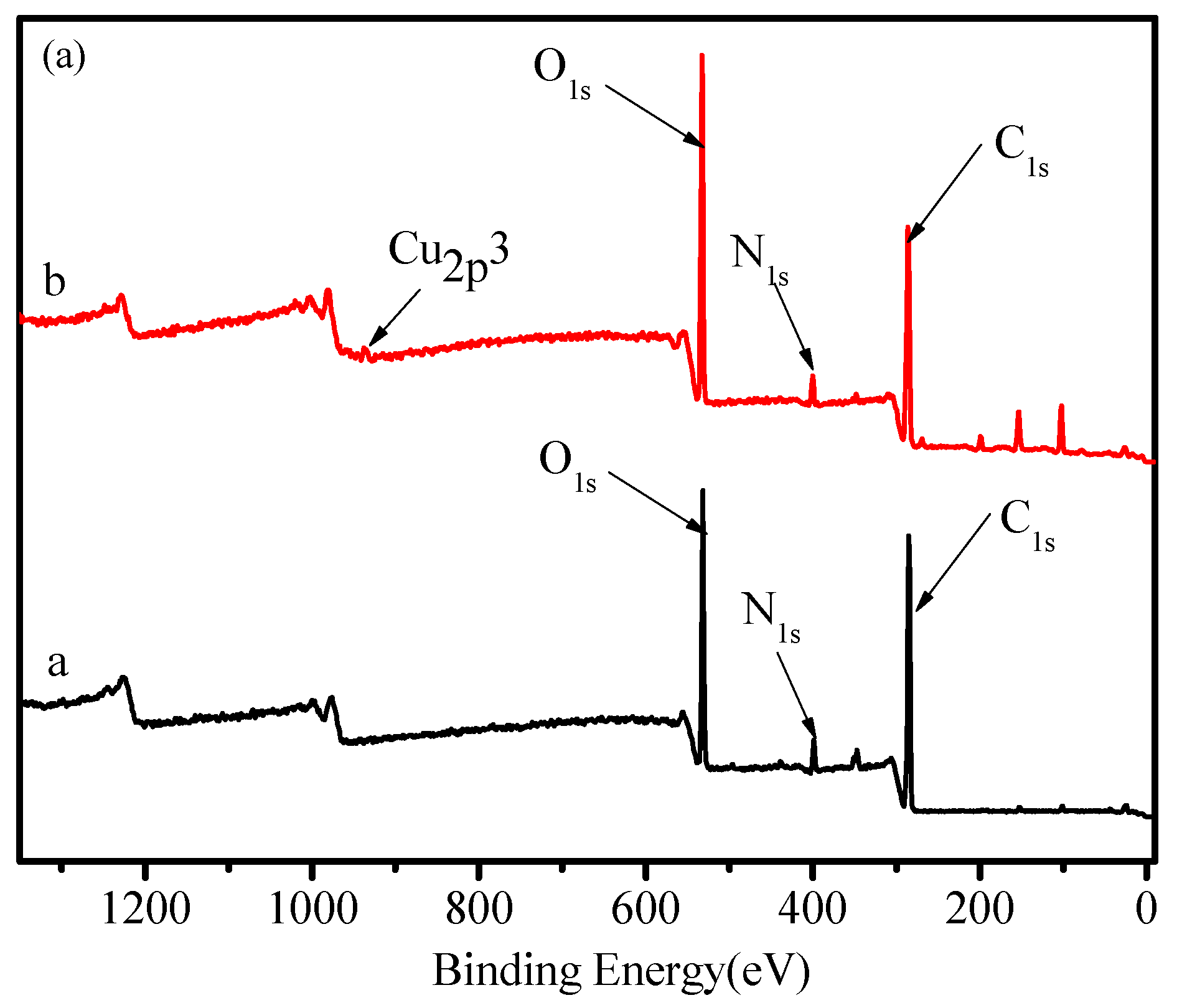
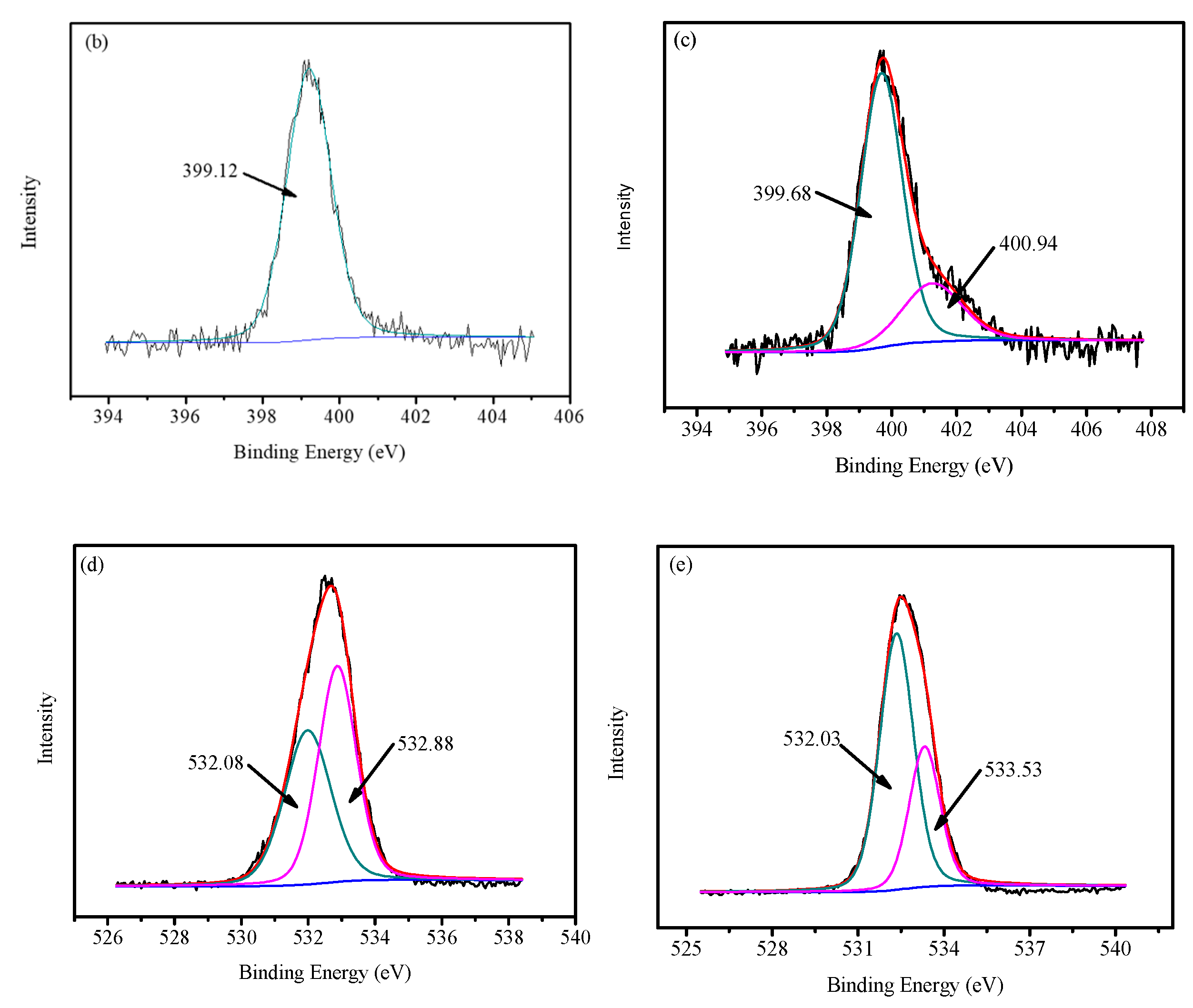
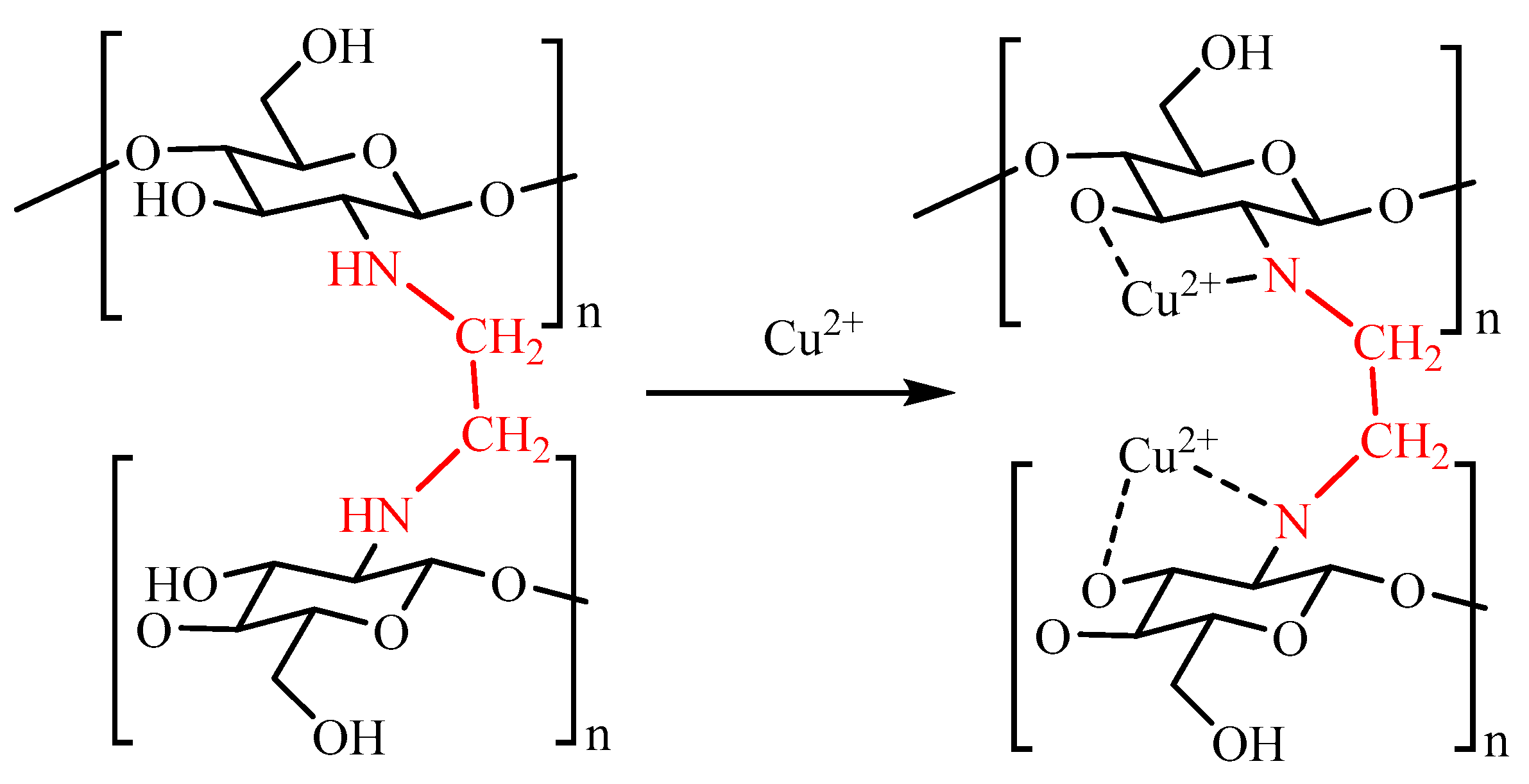
2.1. Characterization of FCG
2.2. Batch Adsorption Experiment
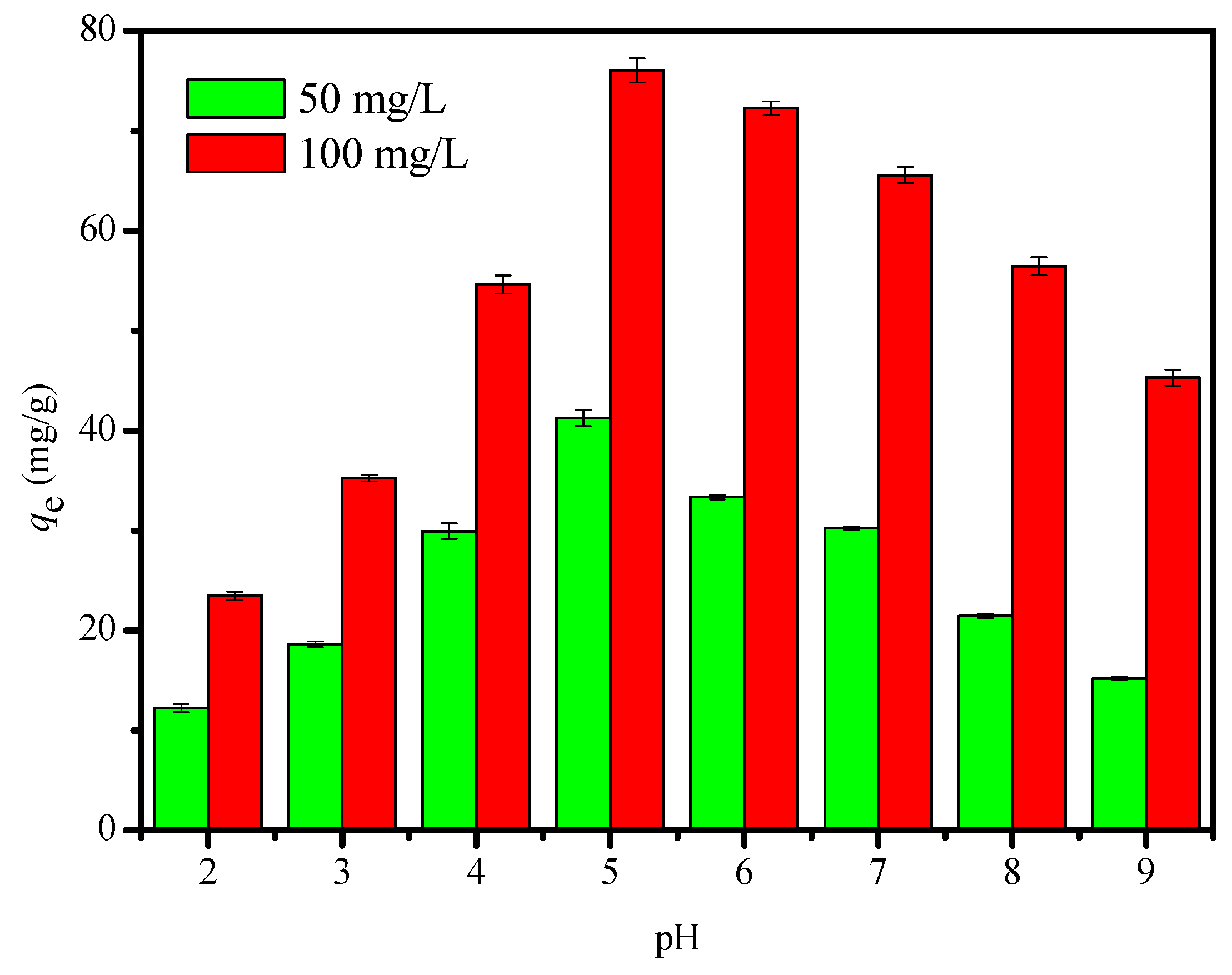
2.2.1. Effect of pH of the Solution
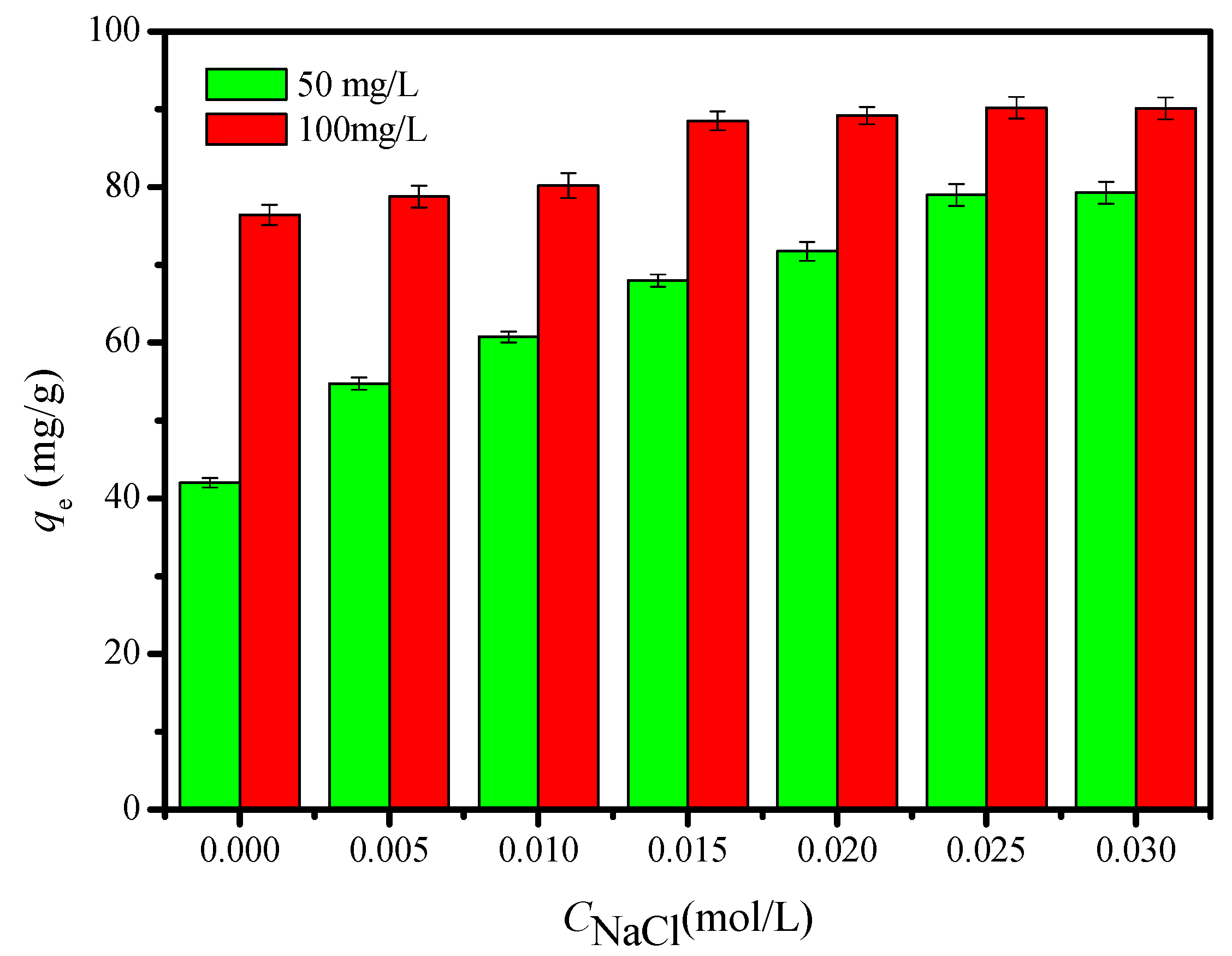
2.2.2. Effects of Ionic Strength
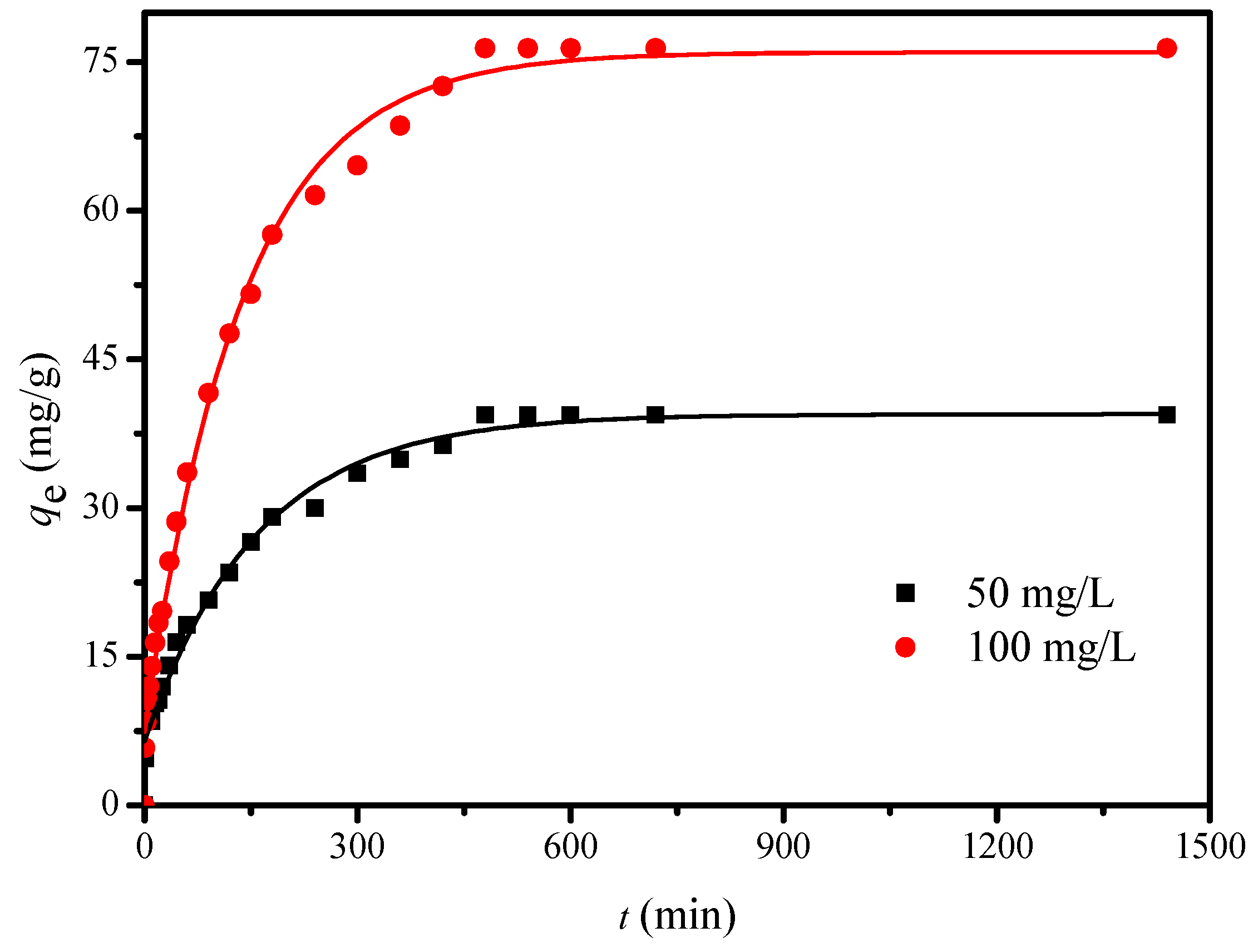
2.2.3. Adsorption Kinetics
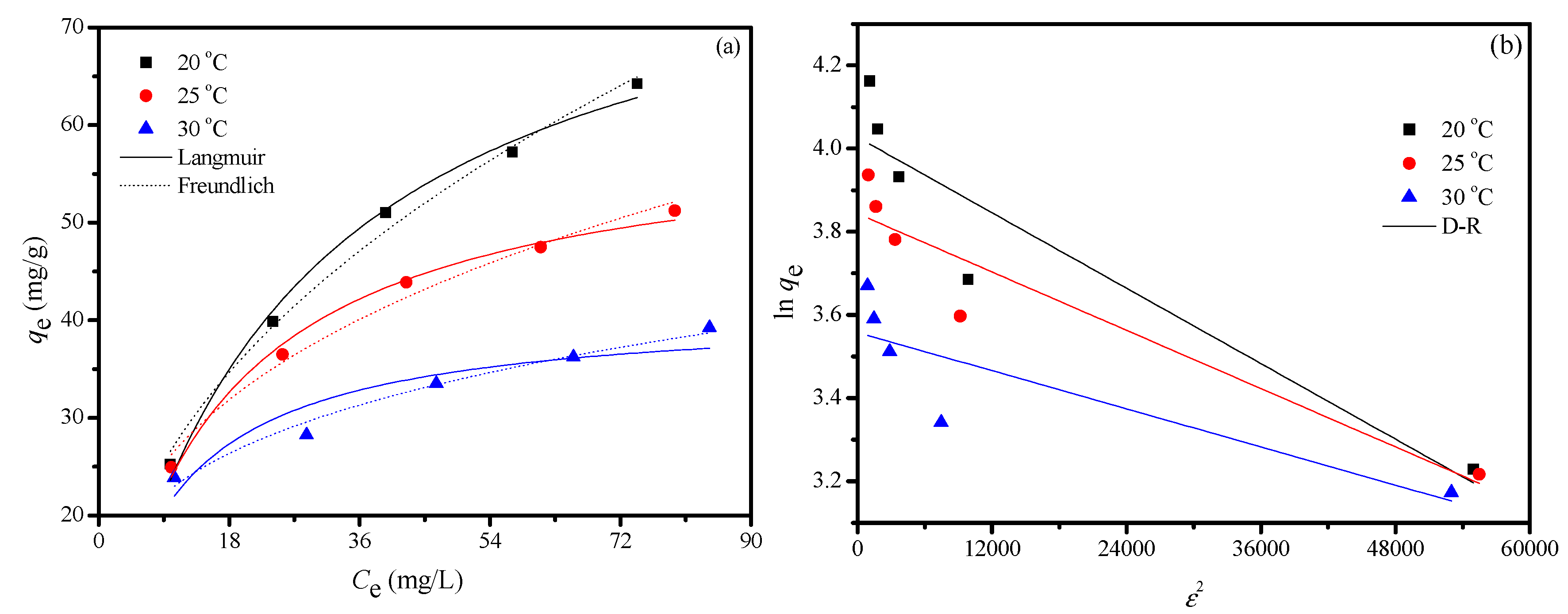
2.2.4. Adsorption Isotherm
2.2.5. Thermodynamic Study
2.2.6. Regeneration Tests
3. Materials and Methods
3.1. Adsorbent
3.2. Synthesis of FCG
3.3. Characterization of FCG
3.4. Adsorption Experiments of Cu2+ Ion
3.5. Kinetic Experiments
3.6. Adsorption Isotherm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Ali, B.M.; Wang, F.; Boukherroub, R.; Lei, W.; Xia, M. Phytic acid-doped polyaniline nanofibers-clay mineral for efficient adsorption of copper (II) ions. J. Colloid Interface Sci. 2019, 553, 688–698. [Google Scholar] [PubMed]
- Meena, A.K.; Mishra, G.; Rai, P.; Rajagopal, C.; Nagar, P. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Doong, R.-A.; Tsai, C.-W.; Liao, C.-I. Coupled removal of bisphenol A and copper ion by titanate nanotubes fabricated at different calcination temperatures. Sep. Purif. Technol. 2012, 91, 81–88. [Google Scholar] [CrossRef]
- Johnson, P.D.; Watson, M.A.; Brown, J.; Jefcoat, I.A. Peanut hull pellets as a single use sorbent for the capture of Cu(II) from wastewater. Waste Manag. 2002, 22, 471–480. [Google Scholar] [CrossRef]
- Taki, M.; Iyoshi, S.; Ojida, A.; Hamachi, I.; Yamamoto, Y. Development of highly sensitive fluorescent probes for detection of intracellular copper(I) in living systems. J. Am. Chem. Soc. 2010, 132, 5938–5939. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Parlayıcı, S. Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ion. J. Hazard. Mater. 2009, 164, 982–986. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T. Ion-exchange of Pb2+, Cu2+, Zn2+, Cd2+, and Ni2+ ion from aqueous solution by Lewatit CNP 80. J. Hazard. Mater. 2007, 140, 299–307. [Google Scholar] [CrossRef]
- Yoo, H.; Kwak, S.-Y. Surface functionalization of PTFE membranes with hyperbranched poly(amidoamine) for the removal of Cu2+ ion from aqueous solution. J. Membrane Sci. 2013, 448, 125–134. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Evaluation of Cu2+, Co2+ and Ni2+ ion removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010, 160, 157–163. [Google Scholar] [CrossRef]
- Wang, W.-B.; Huang, D.-J.; Kang, Y.-R.; Wang, A.-Q. One-step in situ fabrication of a granular semi-IPN hydrogel based on chitosan and gelatin for fast and efficient adsorption of Cu2+ ion. Colloid Surf. B 2013, 106, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Anbinder, P.S.; Macchi, C.; Amalvy, J.; Somoza, A. A study of the structural changes in a chitosan matrix produced by the adsorption of copper and chromium ions. Carbohydr. Polym. 2019, 222, 114987. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-step fabrication of graphene oxide enhanced magnetic composite gel for highly efficient dye adsorption and catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, C.; Chu, L.; Tang, Y.; Luo, S. Rapid and efficient treatment of wastewater with high-concentration heavy metals using a new type of hydrogel-based adsorption process. Bioresour. Technol. 2016, 219, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Chiou, M.-S.; Ho, P.-Y.; Li, H.-Y. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dye. Pigment. 2004, 60, 69–84. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Fajardo, A.R.; Muniz, E.C.; Tambourgi, E.B. Superabsorbent hydrogel based on modified polysaccharide for removal of Pb2+ and Cu2+ from water with excellent performance. J. Appl. Polym. Sci. 2007, 105, 2903–2909. [Google Scholar] [CrossRef]
- Sivagangi Reddy, N.; Madhusudana Rao, K.; Sudha Vani, T.J.; Krishna Rao, K.S.V.; Lee, Y.I. Pectin/poly(acrylamide-co-acrylamidoGlycolic acid) pH sensitive semi-IPN hydrogels: Selective removal of Cu2+ and Ni2+ modeling and kinetic studies. Desalin. Water Treat. 2016, 57, 6503–6514. [Google Scholar] [CrossRef]
- Teong, L.; Hanafiah, M.A.K.M.; Ngah, W.W. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar]
- Jiang, X.; Sun, Y.; Liu, L.; Wang, S.; Tian, X. Adsorption of C.I. reactive blue 19 from aqueous solutions by porous particles of the grafted chitosan. Chem. Eng. J. 2014, 235, 151–157. [Google Scholar] [CrossRef]
- Awual, M.R.; Eldesoky, G.E.; Yaita, T.; Naushad, M.; Shiwaku, H.; Alothman, Z.A.; Suzuki, S. Schiff based ligand containing nano-composite adsorbent for optical copper(II) ions removal from aqueous solutions. Chem. Eng. J. 2015, 279, 639–647. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.; Wei, Y.; Sarhan, A. Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. J. Hazard. Mater. 2010, 177, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Nitrate removal from aqueous solutions by cross-linked chitosan beads conditioned with sodium bisulfate. J. Hazard. Mater. 2009, 166, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Li, M.M.; Ye, H.; Zhao, B.X. Effective removal of heavy metal ion Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211, 366–372. [Google Scholar] [CrossRef]
- Prashanth, K.H. Solid state structure of chitosan prepared under different N-deacetylating conditions. Carbohydr. Polym. 2002, 50, 27–33. [Google Scholar] [CrossRef]
- Amsden, B.G.; Sukarto, A.; Knight, D.K.; Shapka, S.N. Methacrylated glycol chitosan as a photopolymerizable biomaterial. Biomacromolecules 2007, 8, 3758–3766. [Google Scholar] [CrossRef]
- De Angelis, A.A.; Capitani, D.; Crescenzi, V. Synthesis and 13C CP-MAS NMR characterization of a new chitosan-based polymericnetwork. Macromolecules 1998, 31, 1595–1601. [Google Scholar] [CrossRef]
- Brigante, M.; Avena, M. Biotemplated synthesis of mesoporous silica for doxycycline removal. Effect of pH, temperature, ionic strength and Ca2+ concentration on the adsorption behaviour. Micropor. Mesopor. Mat. 2016, 225, 534–542. [Google Scholar] [CrossRef]
- Fiorentin, L.D.; Trigueros, D.E.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Pereira, N.C.; Barros, S.T.; Santos, O.A. Biosorption of reactive blue 5G dye onto drying orange bagasse in batch system: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 163, 68–77. [Google Scholar] [CrossRef]
- Lee, M.-S.; Ahn, J.-G.; Ahn, J.-W. Recovery of copper, tin and lead from the spent nitric etching solutions of printed circuit board and regeneration of the etching solution. Hydrometallurgy 2003, 70, 23–29. [Google Scholar] [CrossRef]
- Wang, C.P.; Wu, J.Z.; Sun, H.W.; Wang, T.; Liu, H.B.; Chang, Y. Adsorption of Pb(II) ion from aqueous solutions by tourmaline as a novel adsorbent. Ind. Eng. Chem. Res. 2011, 50, 8515–8523. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Li, W.; Zhong, Z.; Xu, J.; Ren, J.; Ma, Z. Adsorption behavior of heavy metal ions from aqueous solution by soy protein hollow microspheres. Ind. Eng. Chem. Res. 2013, 52, 11036–11044. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, J.-S.; Zhang, B.; Wang, J.-H.; Zhao, Y.-F.; Liu, J.-D. Preparation and characterization of silane coupling agent modified halloysite for Cr(VI) removal. Ind. Eng. Chem. Res. 2011, 50, 10246–10252. [Google Scholar] [CrossRef]
- Ngah, W.W.; Kamari, A.; Koay, Y. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int. J. Boil. Macromol. 2004, 34, 155–161. [Google Scholar] [CrossRef]
- Jayakumar, R.; Rajkumar, M.; Freitas, H.; Kumar, P.S.; Nair, S.; Furuike, T.; Tamura, H. Bioactive and metal uptake studies of carboxymethyl chitosan-graft-d-glucuronic acid membranes for tissue engineering and environmental applications. Int. J. Boil. Macromol. 2009, 45, 135–139. [Google Scholar] [CrossRef]
- Tan, S.; Wang, Y.; Peng, C.; Tang, Y. Synthesis and adsorption properties for metal ions of crosslinked chitosan acetate crown ethers. J. Appl. Polym. Sci. 1999, 71, 2069–2074. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R. Adsorptive removal of copper ions with highly porous chitosan/cellulose acetate blend hollow fiber membranes. J. Membr. Sci. 2006, 284, 313–322. [Google Scholar] [CrossRef]
- Ngah, W.W.; Endud, C.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Kumar, B.N.; Prathima, B.; Subbarao, Y.; Jyothi, N.V.V. A novel green synthesis of Fe3O4 magnetic nanorods using Punica Granatum rind extract and its application for removal of Pb(II) from aqueous environment. Arab. J. Chem. 2019, 12, 588–596. [Google Scholar] [CrossRef]
- Jang, L.; Nguyen, D.; Geesey, G. Selectivity of alginate gel for Cu vs Co. Water Res. 1995, 29, 307–313. [Google Scholar] [CrossRef]
- Gupta, V.; Rastogi, A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: Kinetics and equilibrium studies. J. Hazard. Mater. 2008, 152, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Han, H.; Du, C.; Luo, Z.; Wang, Y. Facile synthesis of melamine-based porous polymer networks and their application for removal of aqueous mercury ions. Polymer 2010, 51, 6193–6202. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Liu, B.; Lv, X.; Meng, X.; Yu, G.; Wang, D. Removal of Pb(II) from aqueous solution using dithiocarbamate modified chitosan beads with Pb(II) as imprinted ions. Chem. Eng. J. 2013, 220, 412–419. [Google Scholar] [CrossRef]
- Hao, Y.-M.; Man, C.; Hu, Z.-B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef]
- López, E.; Soto, B.; Arias, M.; Nunez, A.; Rubinos, D.; Barral, M.T. Adsorbent properties of red mud and its use for wastewater treatment. Water Res. 1998, 32, 1314–1322. [Google Scholar] [CrossRef]
- Lee, S.M.; Davis, A.P. Removal of Cu(II) and Cd(II) from aqueous solution by seafood processing waste sludge. Water Res. 2001, 35, 534–540. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Adsorbent | Adsorption Capacity (mg/g) | Refs. |
|---|---|---|
| Thiourea-modified chitosan microspheres | 66.70 | [11] |
| Chitosan/PVA beads | 47.90 | [36] |
| Carboxymethyl chitosan-graft-d-glucuronic acid membranes | 70.21 | [37] |
| Chitosan acetate crown ether | 23.90 | [38] |
| Chitosan/cellulose composite | 53.50 | [39] |
| Glutaraldehyde-chitosan | 59.61 | [40] |
| Chitosan/SiO2/Fe3O4 | 31.72 | [41] |
| FCG | 75.40 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Chai, Y.; Zeng, L.; Gao, Z.; Zhang, J.; Ji, H. Efficient Removal of Copper Ion from Wastewater Using a Stable Chitosan Gel Material. Molecules 2019, 24, 4205. https://doi.org/10.3390/molecules24234205
Yang Z, Chai Y, Zeng L, Gao Z, Zhang J, Ji H. Efficient Removal of Copper Ion from Wastewater Using a Stable Chitosan Gel Material. Molecules. 2019; 24(23):4205. https://doi.org/10.3390/molecules24234205
Chicago/Turabian StyleYang, Zujin, Yuxin Chai, Lihua Zeng, Zitao Gao, Jianyong Zhang, and Hongbing Ji. 2019. "Efficient Removal of Copper Ion from Wastewater Using a Stable Chitosan Gel Material" Molecules 24, no. 23: 4205. https://doi.org/10.3390/molecules24234205
APA StyleYang, Z., Chai, Y., Zeng, L., Gao, Z., Zhang, J., & Ji, H. (2019). Efficient Removal of Copper Ion from Wastewater Using a Stable Chitosan Gel Material. Molecules, 24(23), 4205. https://doi.org/10.3390/molecules24234205