Nanostructured and Photochromic Material for Environmental Detection of Metal Ions
Abstract
1. Introduction
2. Results
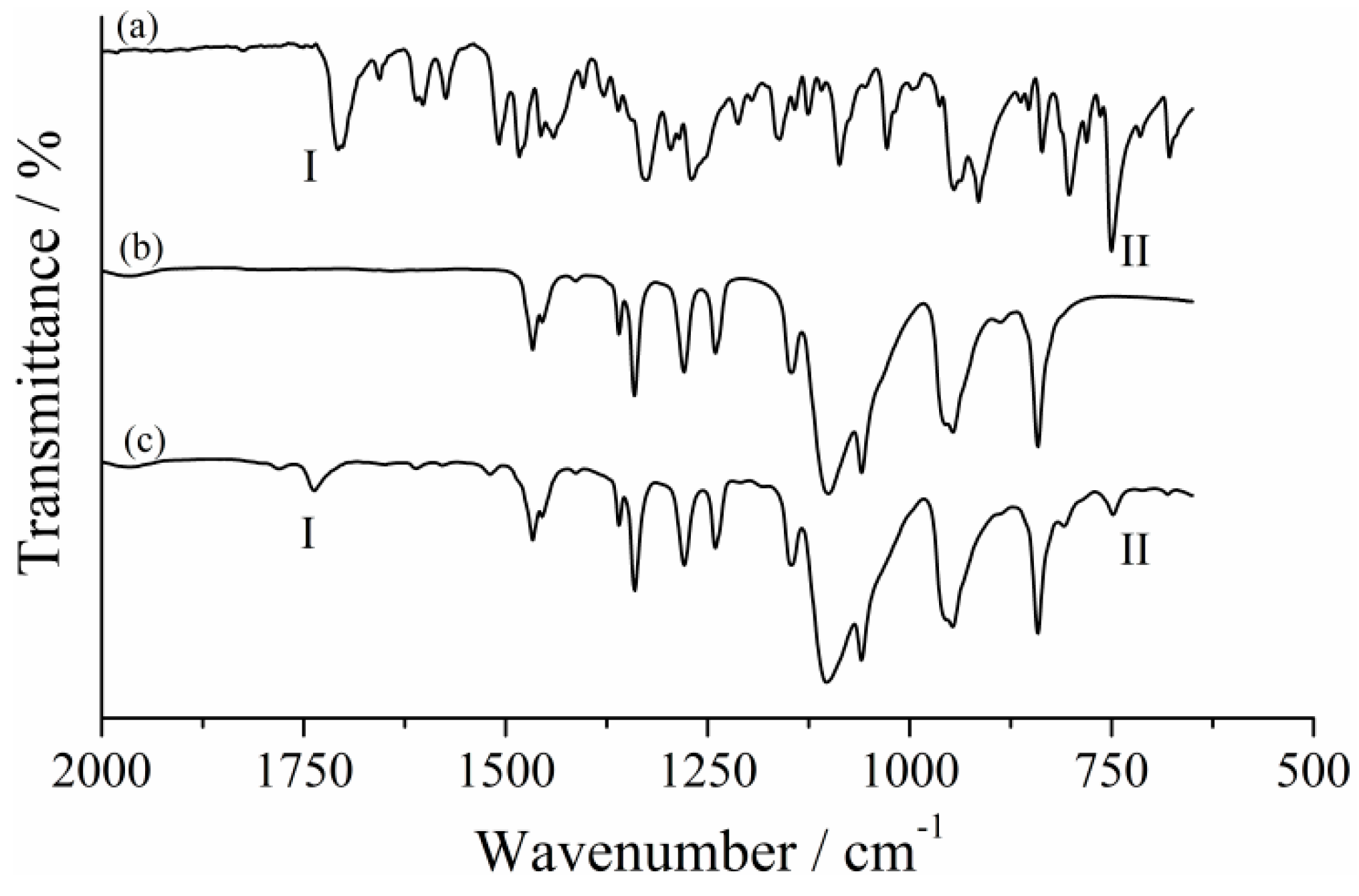
2.1. PEGSP2 Structural Characterization
2.2. Polymers (PEG 2000 and PEGSP2) Thermal Analysis Characterization
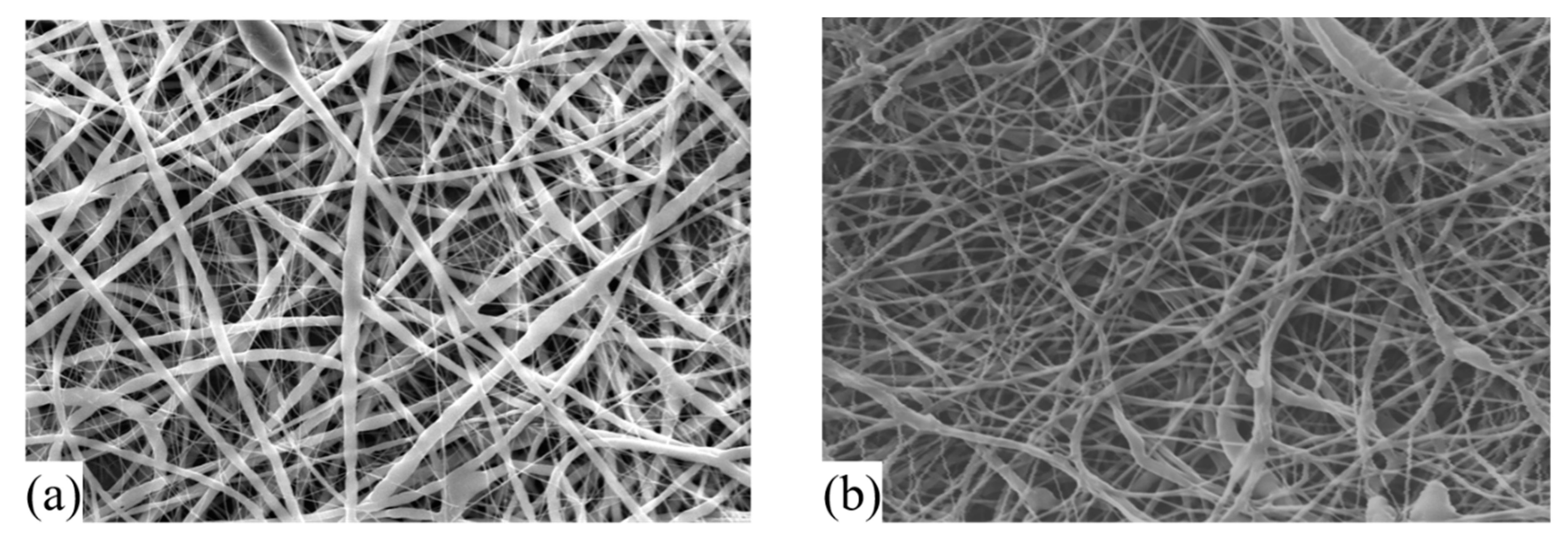
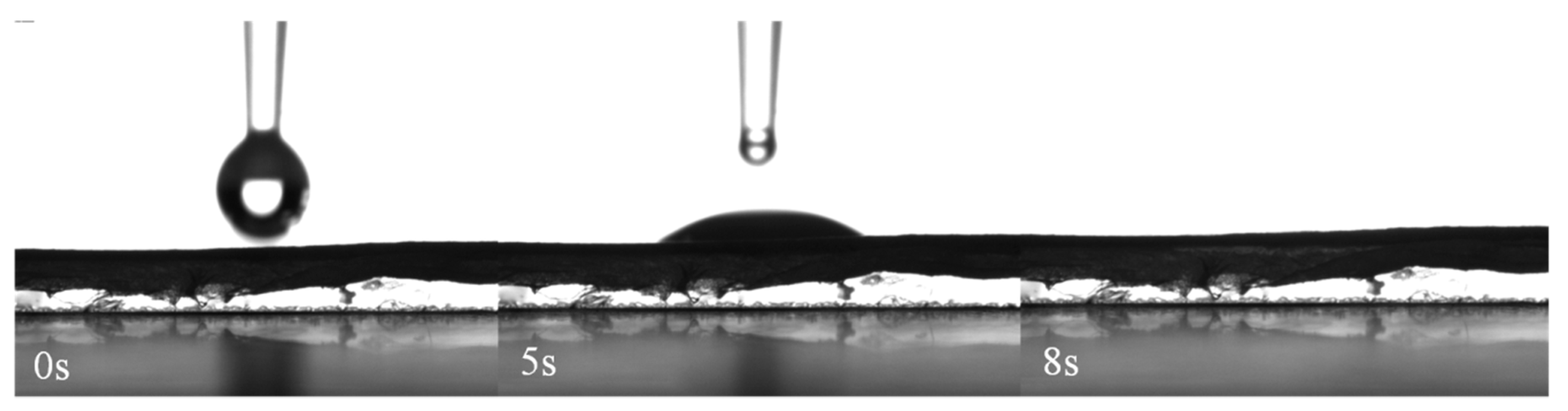
2.3. Electrospun Nanofibers Characterization
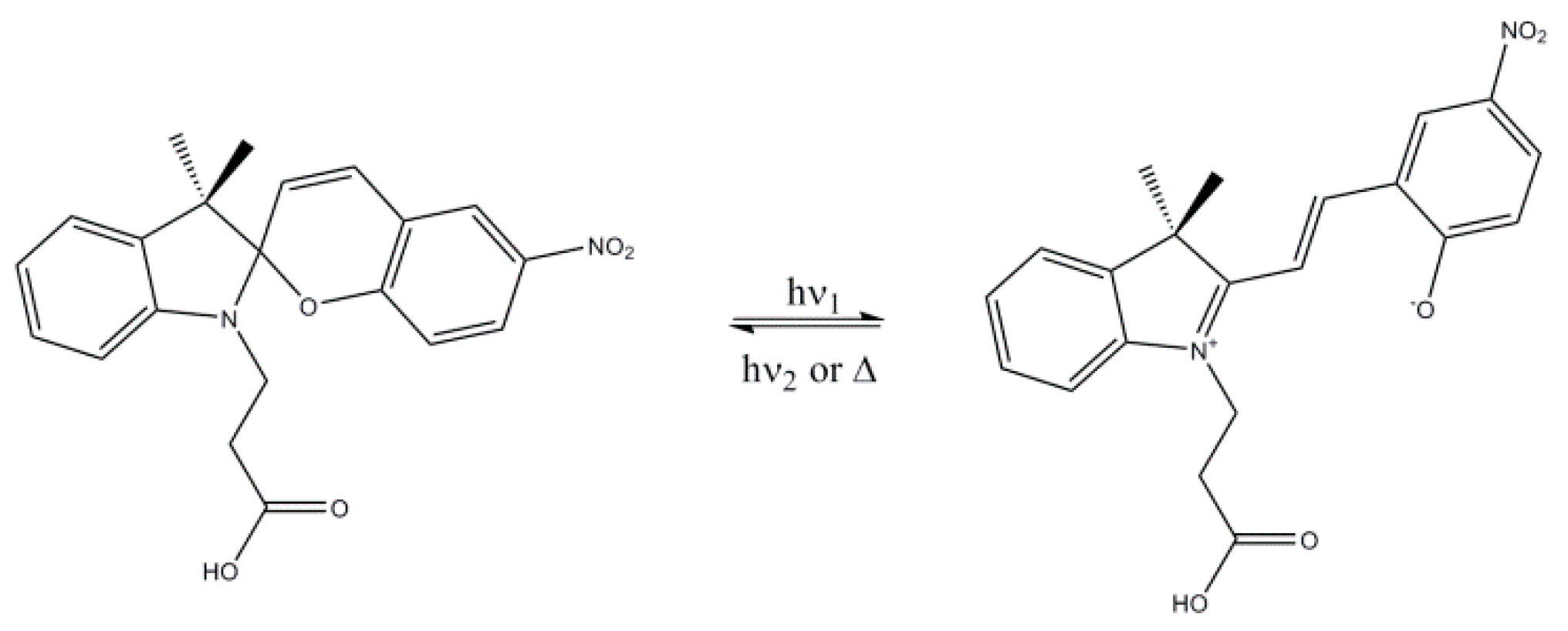
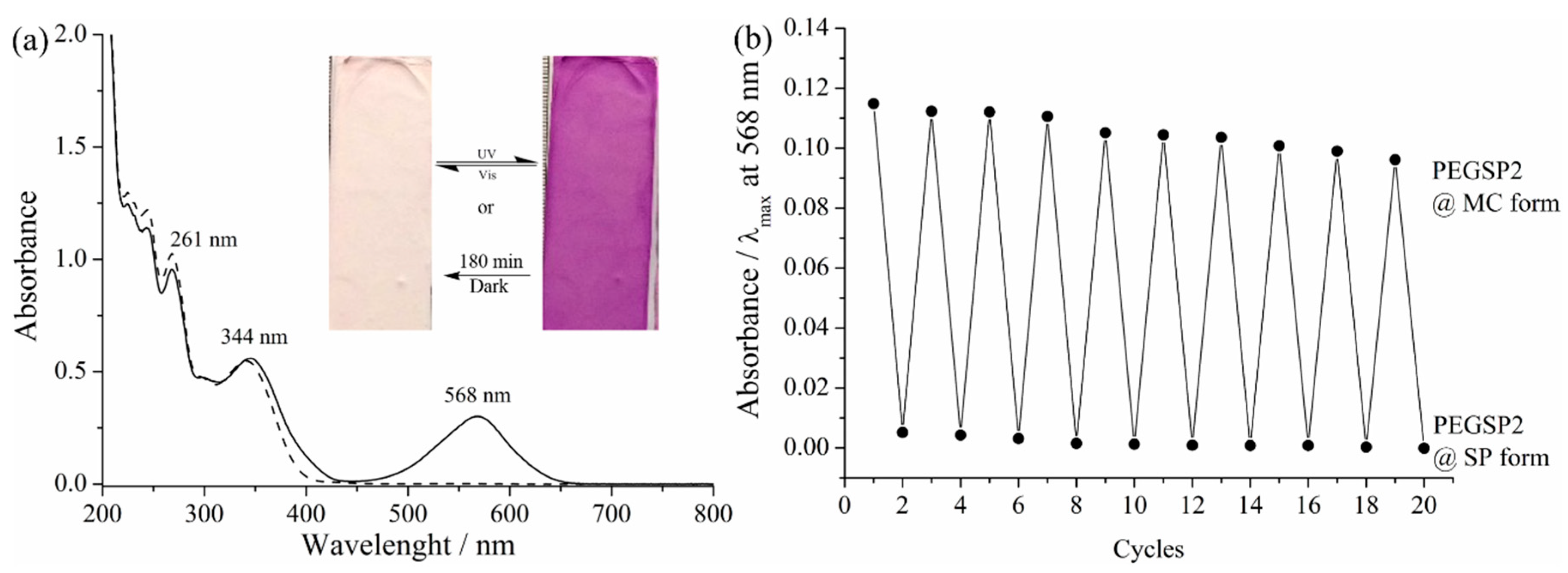
2.4. Photochromic Properties and Chemical Sensing of the ES Nanofibers
3. Materials and Methods
3.1. Polymer Modification
3.2. Electrospun Fibers
3.3. Experimental Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shang, J.; Lin, S.; Theato, P. Fabrication of color changeable CO2 sensitive nanofibers. Polym. Chem. 2017, 8, 7446–7451. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Patil, V.B. Room temperature ammonia gas sensing properties of polyaniline nanofibers. J. Mater. Sci. Mater. Electron. 2019, 30, 8371–8380. [Google Scholar] [CrossRef]
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Lakshmi, K.; Kadirvelu, K.; Mohan, P.S. Chemically modified electrospun nanofiber for high adsorption and effective photocatalytic decontamination of organophosphorus compounds. J. Chem. Technol. Biotechnol. 2019, 94, 3190–3200. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Glucose sensors based on electrospun nanofibers: A review Fiber-based Platforms for Bioanalytics. Anal. Bioanal. Chem. 2016, 408, 1285–1306. [Google Scholar] [CrossRef]
- Xue, R.; Behera, P.; Xu, J.; Viapiano, M.S.; Lannutti, J.J. Sensors and Actuators B: Chemical Polydimethylsiloxane core—Polycaprolactone shell nanofibers as biocompatible, real-time oxygen sensors. Sens. Actuators B Chem. 2014, 192, 697–707. [Google Scholar] [CrossRef]
- Deniz, E.; Tomasulo, M.; Cusido, J.; Sortino, S.; Raymo, F.M. Fast and stable photochromic oxazines for fluorescence switching. Langmuir 2011, 27, 11773–11783. [Google Scholar] [CrossRef]
- Li, B.D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Khatri, Z.; Ali, S.; Khatri, I.; Mayakrishnan, G.; Hun, S.; Kim, I. UV-responsive polyvinyl alcohol nanofibers prepared by electrospinning. Appl. Surf. Sci. 2015, 342, 64–68. [Google Scholar] [CrossRef]
- Yoon, J.; Chae, S.K.; Kim, J.M. Colorimetric sensors for volatile organic compounds (VOCs) based on conjugated polymer-embedded electrospun fibers. J. Am. Chem. Soc. 2007, 129, 3038–3039. [Google Scholar] [CrossRef] [PubMed]
- Thornton, B.T.E.; Harrison, A.; Pham, A.L.; Castano, C.E.; Tang, C. Polyaniline-Functionalized Nanofibers for Colorimetric Detection of HCl Vapor. ACS Omega 2018, 3, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Drew, C.; Lee, S.H.; Senecal, K.J.; Kumar, J.; Samuelson, L.A. Electrospun Nanofibrous Membranes for Highly Sensitive Optical Sensors. Nano Lett. 2002, 2, 1273–1275. [Google Scholar] [CrossRef]
- Ongun, M.Z.; Ertekin, K.; Gocmenturk, M.; Ergun, Y.; Suslu, A. Copper ion sensing with fluorescent electrospun nanofibers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Syu, J.H.; Cheng, Y.K.; Hong, W.Y.; Wang, H.P.; Lin, Y.C.; Meng, H.F.; Zan, H.W.; Horng, S.F.; Chang, G.F.; Hung, C.H.; et al. Electrospun fibers as a solid-state real-time zinc ion sensor with high sensitivity and cell medium compatibility. Adv. Funct. Mater. 2013, 23, 1566–1574. [Google Scholar] [CrossRef]
- Terra, I.A.A.; Mercante, L.A.; Andre, R.S.; Correa, D.S. Fluorescent and colorimetric electrospun nanofibers for heavy-metal sensing. Biosensors 2017, 7, 61. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fi ber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.A. Synthesis of functional polymers by post-polymerization modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef]
- Günay, K.A.; Theato, P.; Klok, H.A. Standing on the shoulders of hermann staudinger: Post-polymerization modification from past to present. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1–28. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Rini, M.; Holm, A.K.; Nibbering, E.T.J.; Fidder, H. Ultrafast UV-mid-IR investigation of the ring opening reaction of a photochromic spiropyran. J. Am. Chem. Soc. 2003, 125, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Heiligman-Rim, R.; Hirshberg, Y.; Fischer, E. Photochromism in Spiropyrans. Part I-V. J. Phys. Chem. 1962, 66, 2470–2477. [Google Scholar] [CrossRef]
- Wojtyk, J.T.C.; Wasey, A.; Kazmaier, P.M.; Hoz, S.; Buncel, E. Thermal reversion mechanism of n-functionalized merocyanines to spiropyrans: A solvatochromic, solvatokinetic, and semiempirical study. J. Phys. Chem. A 2000, 104, 9046–9055. [Google Scholar] [CrossRef]
- Rubinson, K.A.; Meuse, C.W. Deep hydration: Poly(ethylene glycol) Mw 2000–8000 da probed by vibrational spectrometry and small-angle neutron scattering and assignment of Δg° to individual water layers. Polymer 2013, 54, 709–723. [Google Scholar] [CrossRef]
- Dust, J.M.; Fang, Z.H.; Harris, J.M. Proton NMR Characterization of Poly(ethylene Glycols) and Derivatives. Macromolecules 1990, 23, 3742–3746. [Google Scholar] [CrossRef]
- . Miguez, F.B.; Reis, I.F.; Dutra, L.P.; Silva, I.M.S.; Verano-Braga, T.; Lopes, J.F.; De Sousa, F.B. Electronic investigation of light-induced reversible coordination of Co(II)/spiropyran complex. Dyes Pigments 2019, 171, 107757. [Google Scholar] [CrossRef]
- Tyer, N.W.; Becker, R.S. Photochromic Spiropyrans. I. Absorption Spectra and Evaluation of the π-Electron Orthogonality of the Constituent Halves. J. Am. Chem. Soc. 1970, 92, 1289–1294. [Google Scholar] [CrossRef]
- Kubinyi, M.; Varga, O.; Baranyai, P.; Kállay, M.; Mizsei, R.; Tárkányi, G.; Vidóczy, T. Metal complexes of the merocyanine form of nitrobenzospyran: Structure, optical spectra, stability. J. Mol. Struct. 2011, 1000, 77–84. [Google Scholar] [CrossRef]
- Minkin, V.I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Versatility of polyethylene glycol (PEG) in designing solid-solid phase change materials (PCMs) for thermal management and their application to innovative technologies. J. Mater. Chem. A 2017, 5, 18379–18396. [Google Scholar] [CrossRef]
- Simões, M.C.R.; Cragg, S.M.; Barbu, E.; De Sousa, F.B. The potential of electrospun poly(methyl methacrylate)/polycaprolactone core–sheath fibers for drug delivery applications. J. Mater. Sci. 2019, 54, 5712–5725. [Google Scholar] [CrossRef]
- Narayanan, G.; Shen, J.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Aliphatic polyester nanofibers functionalized with cyclodextrins and cyclodextrin-guest inclusion complexes. Polymers 2018, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, Y.; Wang, J.; Dong, C.; Han, H. Selective sensing Ca2+ with a spiropyran-based fluorometric probe. Luminescence 2019, 34, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Sahoo, P.R.; Kumar, S. Colorimetric and Fluorescence-Based Detection of Mercuric Ion Using a Benzothiazolinic Spiropyran. Chemosensors 2019, 7, 35. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Dai, X.; Li, H.; Yu, S.; Meng, W. A New Spiropyran-Based Sensor for Colorimetric and Fluorescent Detection of Divalent Cu2+ and Hg2+ Ions and Trivalent Ce3+, Cr3+ and Al3+ Ions. J. Fluoresc. 2019, 29, 569–575. [Google Scholar] [CrossRef]
- Filley, J.; Ibrahim, M.A.; Nimlos, M.R.; Watt, A.S.; Blake, D.M. Magnesium and calcium chelation by a bis-spiropyran. J. Photochem. Photobiol. A Chem. 1998, 117, 193–198. [Google Scholar] [CrossRef]
- Shao, N.; Wang, H.; Gao, X.; Yang, R.; Chan, W. Spiropyran-based fluorescent anion probe and its application for urinary pyrophosphate detection. Anal. Chem. 2010, 82, 4628–4636. [Google Scholar] [CrossRef]
- Natali, M.; Soldi, L.; Giordani, S. A photoswitchable Zn (II) selective spiropyran-based sensor. Tetrahedron 2010, 66, 7612–7617. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.S.A.; Saif, M.; Attia, M.S.; Abo-Aly, M.M.; Mobarez, S.N. Lanthanide complexes of spiropyran photoswitch and sensor: Spectroscopic investigations and computational modelling. Photochem. Photobiol. Sci. 2018, 17, 221–230. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, F.B.; Guerreiro, J.D.T.; Ma, M.; Anderson, D.G.; Drum, C.L.; Sinisterra, R.D.; Langer, R. Photo-response behavior of electrospun nanofibers based on spiropyran-cyclodextrin modified polymer. J. Mater. Chem. 2010, 20, 9910–9917. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

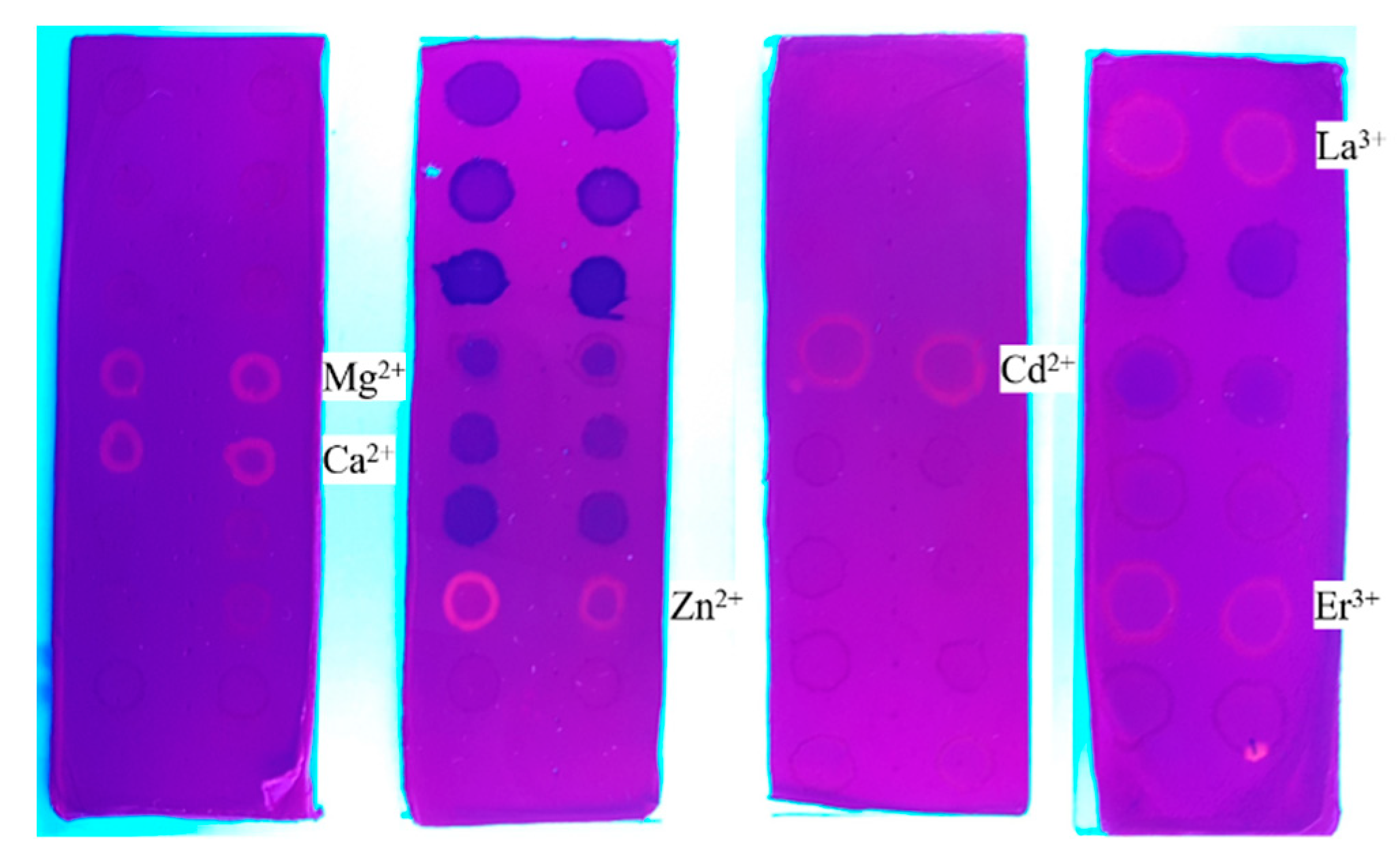
| Metal (Mn+) | Minimum Concentration for Detection (mmol L−1) | Spectral Effect |
|---|---|---|
| Mg2+ | 0.692 | hypochromic |
| Ca2+ | 0.163 | hyperchromic |
| Cr3+ | 0.024 | hypochromic |
| Mn2+ | 0.037 | hyperchromic |
| Fe3+ | 0.009 | hypochromic |
| Fe2+ | 0.016 | hypochromic |
| Co2+ | 0.040 | hyperchromic |
| Ni2+ | 0.055 | hyperchromic |
| Cu2+ | 0.025 | hypochromic |
| Zn2+ | 0.037 | hypochromic |
| Cd2+ | 0.156 | hypochromic |
| La3+ | 1.250 | hypochromic |
| Ce3+ | 0.117 | hyperchromic |
| Nd3+ | 1.130 | hypochromic |
| Eu3+ | 2.602 | hypochromic |
| Er3+ | 1.804 | hypochromic |
| Yb3+ | 0.147 | hypochromic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, R.C.L.; Alexis, F.; De Sousa, F.B. Nanostructured and Photochromic Material for Environmental Detection of Metal Ions. Molecules 2019, 24, 4243. https://doi.org/10.3390/molecules24234243
Machado RCL, Alexis F, De Sousa FB. Nanostructured and Photochromic Material for Environmental Detection of Metal Ions. Molecules. 2019; 24(23):4243. https://doi.org/10.3390/molecules24234243
Chicago/Turabian StyleMachado, Raphael C. L., Frank Alexis, and Frederico B. De Sousa. 2019. "Nanostructured and Photochromic Material for Environmental Detection of Metal Ions" Molecules 24, no. 23: 4243. https://doi.org/10.3390/molecules24234243
APA StyleMachado, R. C. L., Alexis, F., & De Sousa, F. B. (2019). Nanostructured and Photochromic Material for Environmental Detection of Metal Ions. Molecules, 24(23), 4243. https://doi.org/10.3390/molecules24234243







