Volatiles of Black Pepper Fruits (Piper nigrum L.)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Essential Oils
3.2. Gas Chromatographic-Mass Spectral Analysis
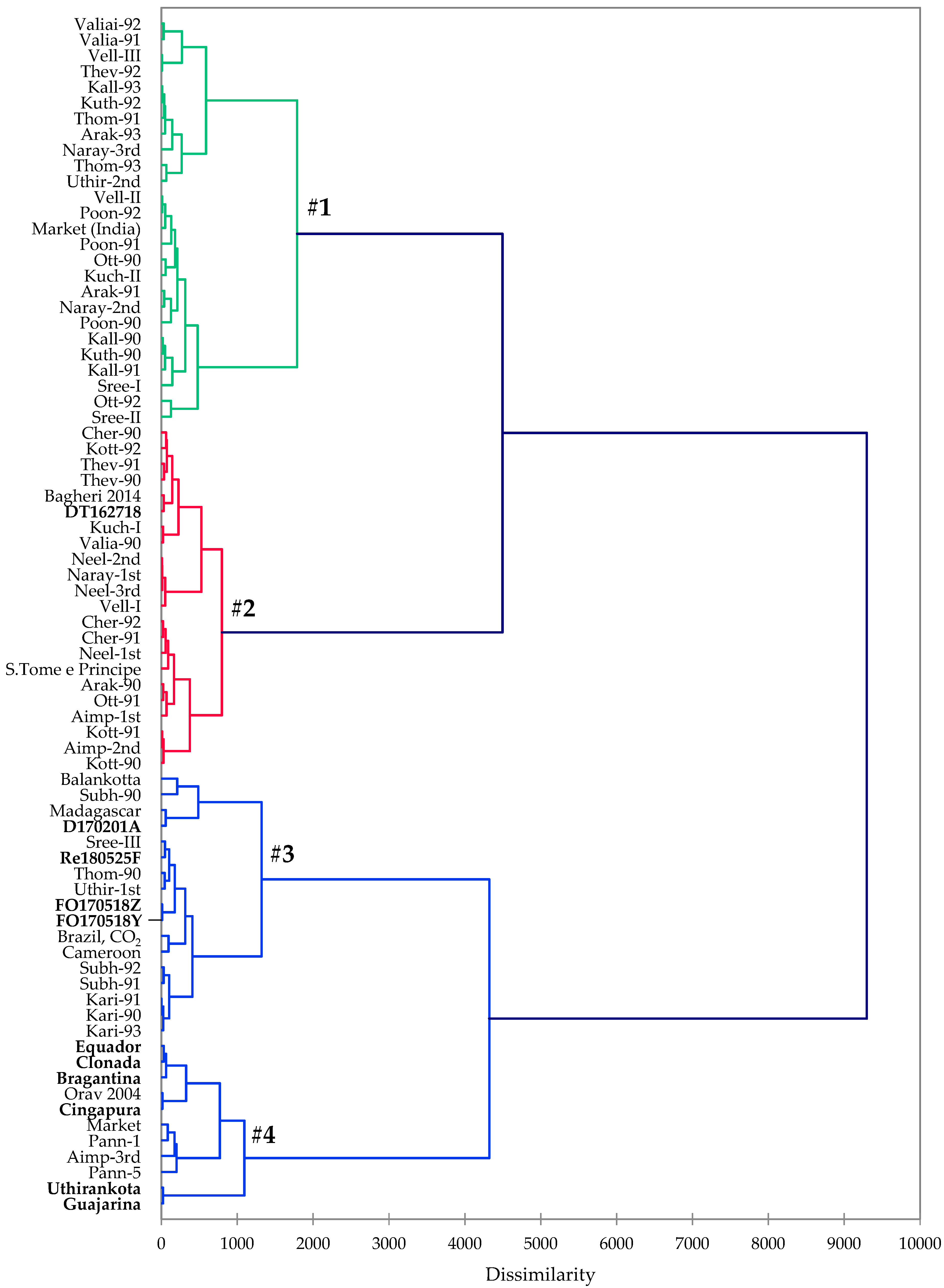
3.3. Hierarchical Cluster Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tchoumbougnang, F.; Dongmo, P.M.J.; Sameza, M.L.; Fombotioh, N.; Wouatsa, N.A.V.; Amvam, Z.P.H.; Menut, C. Comparative essential oils composition and insecticidal effect of different tissues of Piper capense L., Piper guineense Schum. et Thonn., Piper nigrum L. and Piper umbellatum L. grown in Cameroon. Afr. J. Biotechnol. 2009, 8, 424–431. [Google Scholar]
- Kapoor, I.P.S.; Singh, B.; Singh, G.; De Heluani, C.S.; De Lampasona, M.P.; Catalan, C.A.N. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum). J. Agric. Food Chem. 2009, 57, 5358–5364. [Google Scholar] [CrossRef]
- Orav, A.; Stulova, I.; Kailas, T.; Müürisepp, M. Effect of storage on the essential oil composition of Piper nigrum L. fruits of different ripening states. J. Agric. Food Chem. 2004, 52, 2582–2586. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Bagheri, H.; Bin Abdul Manap, M.Y.; Solati, Z. Antioxidant activity of Piper nigrum L. essential oil extracted by supercritical CO2 extraction and hydro-distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ramnik, S.; Narinder, S.; Saini, B.S.; Harwinder, S.R. In vitro antioxidant activity of pet ether extract of black pepper. Ind. J. Pharmacol. 2008, 40, 147–151. [Google Scholar]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M.P. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. J. Sci. Food Agric. 2004, 84, 1878–1884. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Umadevi, N.P.; Kuttan, R. Antioxidant, anti-inflammtory and antinociceptive properties of black pepper essential oil (Piper nigrum Linn). J. Essent. Oil Bear. Plants 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.H.; Kim, S.I.; Cho, M.H.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef]
- Hashim, S.; Aboobaker, V.S.; Madhubala, R.; Bhattacharya, R.K.; Rao, A.R. Modulatory effects of essential oils from spices on the formation of DNA adduct by aflatoxin B1 in vitro. Nutr. Cancer 1994, 21, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kristiniak, S.; Harpel, J.; Breckenridge, D.M.; Buckle, J. Black pepper essential oil to enhance intravenous catheter insertion in patients with poor vein visibility. J. Altern. Complement. Med. 2012, 18, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Ebihara, Ã.S.; Maruyama, Ã.M.; Kobayashi, M.; Itou, A.; Arai, H.; Sasaki, H. A Randomized trial of olfactory stimulation using black pepper oil in older people with swallowing dysfunction. J. Am. Geriatr. Soc. 2006, 54, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.E.; Behm, F.M. Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug Alcohol Depend. 1994, 34, 225–229. [Google Scholar] [CrossRef]
- Cordell, B.; Buckle, J. The effects of aromatherapy on nicotine craving on a U.S. campus: A small comparison study. J. Altern. Complement. Med. 2013, 19, 1–5. [Google Scholar] [CrossRef]
- Mori, M.; Ikeda, N.; Kato, Y.; Minamino, M.; Watabe, K. Inhibition of elastase activity by essential oils in vitro. J. Cosmet. Dermatol. 2003, 1, 183–187. [Google Scholar] [CrossRef]
- Liu, Y.; Yadev, V.R.; Aggarwal, B.B.; Nair, M.G. Inhibitory effects of black pepper (Piper nigrum) extracts and compounds on human tumor cell proliferation, cyclooxygenase enzymes, lipid peroxidation and nuclear transcription factor-kappa-B. Nat. Prod. Commun. 2010, 5, 1253–1257. [Google Scholar] [CrossRef]
- Costa, R.; Machado, J.; Abreu, C. Evaluation of analgesic properties of Piper nigrum essential oil: A randomized, double-blind, placebo-controlled study. World J. Tradit. Chin. Med. 2016, 2, 60–64. [Google Scholar] [CrossRef]
- Liu, L.; Song, G.; Hu, Y. GC-MS analysis of the essential oils of Piper nigrum L. and Piper longum L. Chromatographia 2007, 66, 785–790. [Google Scholar] [CrossRef]
- Ferreira, S.R.S.; Nikolov, Z.L.; Doraiswamy, L.K.; Meireles, M.A.A.; Petenate, A.J. Supercritical fluid extraction of black pepper (Piper nigrum L.) essential oil. J. Supercrit. Fluids 1999, 14, 235–245. [Google Scholar] [CrossRef]
- Zacharia, T.J.; Gopalam, A. Nature, production and quality of essential oils of pepper, ginger, turmeric, cardamom and tree spices. Indian Perfum. 1987, 31, 188–205. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Möllenbeck, S.; König, T.; Schreier, P.; Schwab, W.; Rajaonarivony, Ã.J.; Ranarivelo, L. Chemical composition and analyses of enantiomers of essential oils from Madagascar. Flav. Fragr. J. 1997, 12, 63–69. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.; Vila, R.; Tomi, F.; Cañigueral, S.; Casanova, J.; Proença Da Cunha, A.; Adzet, T. Essential oils from four Piper species. Phytochemistry 1998, 49, 2019–2023. [Google Scholar] [CrossRef]
- Nirmala Menon, A.; Padmakumari, K.P.; Jayalekshmy, A. Essential oil composition of four major cultivars of black pepper (Piper nigrum L.) III. J. Essent. Oil Res. 2003, 15, 155–157. [Google Scholar] [CrossRef]
- Nirmala Menon, A.; Padmakumari, K.P. Studies on essential oil composition of cultivars of black pepper (Piper nigrum L.)-V. J. Essent. Oil Res. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- Nirmala Menon, A.; Padmakumari, K.P. Essential oil composition of four major cultivars of black pepper (Piper nigrum L.)-IV. J. Essent. Oil Res. 2005, 17, 206–208. [Google Scholar] [CrossRef]
- Mamatha, B.S.; Prakash, M.; Nagarajan, S.; Bhat, K.K. Evaluation of the flavor quality of pepper (Piper nigrum L.) cultivars by GC-MS, electronic nose and sensory analysis techniques. J. Sens. Stud. 2008, 23, 498–513. [Google Scholar] [CrossRef]
- Nirmala Menon, A.; Padmakumari, K.P.; Jayalekshmy, A.; Gopalakrishnan, M.; Narayanan, C.S. Essential oil composition of four popular Indian cultivars of black pepper (Piper nigrum L.). J. Essent. Oil Res. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P.; Jayalekshmy, A. Essential oil composition of cultivars of four major cultivars of black pepper (Piper nigrum L.). J. Essent. Oil Res. 2002, 14, 84–86. [Google Scholar] [CrossRef]
Sample Availability: Essential oil samples are not available from the authors. |
| RI a | RI b | Compound | D170201A | FO170518Y | FO170518Z | Re180525F | DT162718 |
|---|---|---|---|---|---|---|---|
| 921 | 921 | Tricyclene | --- | --- | --- | --- | tr c |
| 924 | 924 | α-Thujene | 0.1 | 0.1 | 0.2 | 0.9 | 0.6 |
| 932 | 932 | α-Pinene | 28.7 | 12.9 | 15.2 | 5.1 | 11.1 |
| 945 | 945 | α-Fenchene | --- | --- | --- | --- | tr |
| 954 | 946 | Camphene | 1.0 | 0.6 | 0.6 | 0.1 | 0.3 |
| 969 | 969 | Sabinene | 0.2 | 0.4 | 0.7 | 6.9 | 13.9 |
| 974 | 974 | β-Pinene | 15.3 | 12.6 | 13.4 | 9.1 | 15.1 |
| 988 | 988 | Myrcene | 2.6 | 2.7 | 3.0 | 2.1 | 1.3 |
| 1002 | 1002 | α-Phellandrene | 2.2 | 2.5 | 3.4 | 2.3 | 0.6 |
| 1003 | 1003 | p-Mentha-1(7),8-diene | tr | --- | --- | --- | --- |
| 1008 | 1008 | δ-3-Carene | 9.0 | 11.7 | 12.8 | 11.7 | 10.4 |
| 1017 | 1014 | α-Terpinene | 0.1 | --- | 0.1 | 0.2 | 0.1 |
| 1020 | 1020 | p-Cymene | 0.3 | 0.8 | 0.4 | 1.1 | 0.3 |
| 1022 | 1022 | o-Cymene | --- | 0.1 | --- | 0.1 | --- |
| 1025 | 1025 | β-Phellandrene | 0.2 | --- | --- | 1.4 | 0.9 |
| 1026 | 1026 | 1,8-Cineole | 0.2 | 0.3 | 0.4 | --- | tr |
| 1029 | 1024 | Limonene | 19.5 | 18.2 | 18.2 | 17.4 | 15.1 |
| 1032 | 1032 | (Z)-β-Ocimene | tr | --- | --- | --- | --- |
| 1044 | 1044 | (E)-β-Ocimene | 0.4 | 0.2 | 0.3 | 0.1 | 0.1 |
| 1054 | 1054 | γ-Terpinene | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 |
| 1069 | 1065 | cis-Sabinene hydrate | --- | --- | --- | 0.1 | 0.1 |
| 1086 | 1086 | Terpinolene | 0.5 | 0.8 | 0.9 | 0.5 | 0.2 |
| 1095 | 1095 | Linalool | 0.6 | 0.4 | 0.3 | 0.5 | 0.3 |
| 1100 | 1098 | trans-Sabinene hydrate | --- | --- | --- | --- | 0.1 |
| 1135 | 1135 | trans-Pinocarveol | --- | --- | --- | --- | tr |
| 1140 | 1140 | cis-β-Terpineol | --- | 0.2 | --- | --- | --- |
| 1141 | 1141 | Camphor | 0.1 | 0.1 | tr | --- | --- |
| 1174 | 1174 | Terpinen-4-ol | 0.1 | --- | --- | 0.3 | 0.2 |
| 1186 | 1186 | α-Terpineol | 0.3 | --- | 0.2 | --- | 0.1 |
| 1328 | 1330 | Bicycloelemene | 0.1 | --- | --- | 0.1 | tr |
| 1335 | 1335 | δ-Elemene | 1.1 | 2.9 | 2.9 | 0.9 | 1.0 |
| 1348 | 1345 | α-Cubebene | tr | 0.1 | 0.1 | 0.3 | 0.1 |
| 1369 | 1369 | Cyclosativene d | 0.2 | --- | --- | 0.1 | 0.1 |
| 1373 | 1373 | α-Ylangene | --- | 0.1 | 0.1 | --- | --- |
| 1374 | 1374 | Isoledene | --- | 0.2 | 0.2 | --- | --- |
| 1376 | 1374 | α-Copaene | 0.1 | 0.2 | 0.2 | 3.1 | 2.0 |
| 1387 | 1387 | β-Cubebene | --- | --- | --- | 0.2 | 0.2 |
| 1389 | 1389 | β-Elemene | 1.0 | 1.8 | 1.4 | 1.1 | 0.2 |
| 1408 | 1408 | (Z)-β-Caryophyllene | --- | --- | --- | --- | tr |
| 1409 | 1408 | α-Gurjunene | 0.1 | 0.1 | 0.1 | 0.2 | tr |
| 1417 | 1417 | (E)-β-Caryophyllene | 8.7 | 18.3 | 15.2 | 25.6 | 21.6 |
| 1430 | 1430 | β-Copaene | --- | 0.1 | --- | 0.1 | 0.1 |
| 1436 | 1434 | γ-Elemene | 0.1 | 0.1 | 0.1 | --- | --- |
| 1437 | 1437 | α-Guaiene | 0.1 | 0.6 | 0.3 | 0.4 | tr |
| 1448 | 1448 | cis-Murrola-3,5-diene | --- | --- | --- | --- | tr |
| 1452 | 1452 | α-Humulene | 0.9 | 1.8 | 1.3 | 1.6 | 0.7 |
| 1461 | 1461 | cis-Cadina-1(6),4-diene | --- | --- | --- | --- | tr |
| 1475 | 1475 | trans-Cadina-1(6),4-diene | tr | 0.1 | 0.4 | --- | tr |
| 1484 | 1484 | Germacrene D | 2.0 | 2.5 | 3.0 | 0.2 | 0.1 |
| 1490 | 1489 | β-Selinene | 1.3 | 2.0 | 1.9 | 1.5 | 0.1 |
| 1493 | 1493 | trans-Muurola-4(14),5-diene | --- | --- | --- | --- | 0.1 |
| 1496 | 1496 | Viridiflorene | --- | --- | --- | 0.1 | --- |
| 1498 | 1498 | α-Selinene | 1.0 | 1.5 | 1.5 | 1.3 | --- |
| 1500 | 1500 | α-Muurolene | --- | 0.1 | 0.1 | 0.2 | 0.3 |
| 1500 | 1500 | Bicyclogermacrene | --- | --- | --- | --- | 0.3 |
| 1501 | 1501 | epi-Zonarene | 0.1 | --- | --- | --- | --- |
| 1505 | 1505 | β-Bisabolene | tr | 0.3 | --- | 0.1 | 0.7 |
| 1505 | 1505 | (E,E)-α-Farnesene | --- | --- | 0.1 | --- | --- |
| 1514 | 1514 | Cubebol | tr | --- | --- | 0.3 | 0.1 |
| 1518 | 1521 | α-Panasinsen | tr | 0.1 | 0.1 | --- | --- |
| 1521 | 1521 | trans-Calamenene | --- | --- | --- | tr | tr |
| 1523 | 1522 | δ-Cadinene | 0.1 | 0.1 | 0.2 | 0.8 | 0.7 |
| 1533 | 1533 | trans-Cadina-1,4-diene | --- | --- | --- | --- | tr |
| 1548 | 1548 | α-Elemol | tr | --- | --- | --- | --- |
| 1561 | 1559 | Germacrene B | 0.1 | 0.2 | 0.1 | --- | --- |
| 1561 | 1561 | (E)-Nerolidol | --- | --- | --- | --- | tr |
| 1577 | 1577 | Spathulenol | tr | --- | --- | 0.1 | tr |
| 1582 | 1582 | Caryophyllene oxide | 0.2 | 1.4 | 0.3 | 0.8 | 0.5 |
| 1608 | 1608 | Humulene epoxide II | --- | 0.1 | --- | --- | --- |
| 1618 | 1618 | 1,10-di-epi-Cubenol | 0.1 | --- | --- | --- | --- |
| 1626 | 1629 | iso-Spathulenol | 0.3 | --- | --- | 0.3 | 0.1 |
| 1639 | 1644 | allo-Aromadendrene epoxide | tr | --- | --- | --- | --- |
| 1640 | 1640 | τ-Muurolol | --- | --- | --- | 0.1 | tr |
| 1644 | 1644 | α-Muurolol (=δ-Cadinol) | --- | --- | --- | 0.3 | 0.1 |
| 1651 | 1651 | Pogostol | 0.1 | --- | --- | --- | --- |
| 1652 | 1652 | α-Cadinol | 0.1 | --- | 0.1 | --- | --- |
| 1660 | 1660 | Selin-11-en-4α-ol | tr | --- | --- | --- | --- |
| 1685 | 1685 | Germacra-4(15),5,10(14)-trien-1α-ol | 0.5 | --- | --- | --- | --- |
| Monoterpene hydrocarbons | 80.1 | 63.5 | 69.2 | 59.2 | 69.9 | ||
| Oxygenated monoterpenoids | 1.2 | 1.0 | 0.9 | 0.8 | 0.7 | ||
| Sesquiterpene hydrocarbons | 17.0 | 33.3 | 29.2 | 37.7 | 28.2 | ||
| Oxygenated sesquiterpenoids | 1.3 | 1.5 | 0.4 | 1.9 | 0.8 | ||
| Total identified | 99.7 | 99.3 | 99.7 | 99.5 | 99.7 |
| RI a | RI b | Compound | Bragantina | Cingapura | Clonada | Equador | Guajarina | Uthirankota |
|---|---|---|---|---|---|---|---|---|
| 921 | 924 | α-Thujene | 0.2 | --- | 0.2 | 0.6 | 0.7 | 1.4 |
| 929 | 932 | α-Pinene | 9.2 | 6.8 | 8.0 | 7.4 | 11.3 | 10.3 |
| 965 | 969 | Sabinene | --- | 0.1 | 0.5 | --- | --- | --- |
| 973 | 974 | β-Pinene | 33.6 | 20.3 | 29.2 | 29.2 | 45.6 | 48.0 |
| 987 | 988 | Myrcene | 2.5 | 2.5 | 3.0 | 3.4 | 0.1 | --- |
| 1001 | 1002 | α-Phellandrene | --- | 0.4 | 0.3 | 0.8 | --- | --- |
| 1007 | 1008 | δ-3-Carene | --- | 14.3 | 9.3 | 4.5 | --- | --- |
| 1012 | 1014 | α-Terpinene | 0.0 | --- | --- | --- | 0.7 | 1.4 |
| 1018 | 1020 | p-Cymene | --- | 0.1 | --- | --- | --- | --- |
| 1025 | 1024 | Limonene | 38.1 | 31.1 | 36.5 | 30.8 | 29.7 | 24.3 |
| 1042 | 1044 | (E)-β-Ocimene | tr c | tr | tr | 0.1 | --- | 0.3 |
| 1054 | 1054 | γ-Terpinene | 0.5 | 0.1 | 0.2 | 0.7 | 1.1 | 2.0 |
| 1061 | 1065 | cis-Sabinene hydrate | 0.2 | --- | 0.1 | 0.6 | 0.4 | 0.8 |
| 1084 | 1086 | Terpinolene | 0.3 | 0.8 | 0.5 | 0.4 | 0.2 | 0.4 |
| 1094 | 1095 | Linalool | 0.6 | 1.6 | 1.1 | 3.4 | 1.7 | 1.2 |
| 1116 | 1118 | cis-p-Menth-2-en-1-ol | 0.1 | --- | tr | 0.2 | 0.2 | 0.3 |
| 1133 | 1136 | trans-p-Menth-2-en-1-ol | 0.1 | --- | --- | 0.1 | 0.1 | 0.1 |
| 1174 | 1174 | Terpinen-4-ol | 2.3 | 0.1 | 0.8 | 2.9 | 4.2 | 5.6 |
| 1186 | 1186 | α-Terpineol | 0.5 | 0.3 | 0.4 | 0.2 | 0.2 | 0.2 |
| 1196 | 1194 | Myrtenol | --- | 0.1 | --- | --- | --- | --- |
| 1208 | 1214 | Linalyl formate | --- | 0.1 | --- | --- | --- | --- |
| 1222 | 1227 | Nerol | 0.1 | --- | --- | 0.1 | --- | --- |
| 1334 | 1335 | δ-Elemene | tr | 0.4 | tr | 0.1 | --- | --- |
| 1344 | 1346 | α-Terpinyl acetate | 0.1 | --- | --- | --- | --- | --- |
| 1373 | 1374 | α-Copaene | 0.4 | 0.6 | --- | --- | --- | --- |
| 1389 | 1389 | β-Elemene | 0.1 | 0.4 | 0.4 | 0.4 | --- | tr |
| 1410 | 1411 | cis-α-Bergamotene | --- | --- | --- | --- | tr | --- |
| 1416 | 1417 | (E)-β-Caryophyllene | 6.9 | 14.8 | 6.2 | 6.3 | 0.7 | 2.5 |
| 1430 | 1432 | trans-α-Bergamotene | --- | --- | --- | --- | 0.1 | --- |
| 1431 | 1434 | γ-Elemene | --- | --- | --- | 1.2 | --- | 0.2 |
| 1450 | 1454 | (E)-β-Farnesene | --- | --- | --- | --- | tr | --- |
| 1452 | 1452 | α-Humulene | 0.4 | 0.9 | 0.5 | 0.4 | --- | 0.1 |
| 1478 | 1484 | Germacrene D | --- | tr | --- | 0.1 | tr | --- |
| 1490 | 1493 | α-Zingiberene | --- | --- | --- | --- | tr | --- |
| 1491 | 1493 | epi-Cubebol | 0.3 | --- | --- | --- | --- | --- |
| 1492 | 1489 | β-Selinene | 0.1 | 0.5 | 1.0 | 0.2 | --- | 0.1 |
| 1493 | 1499 | Curzerene | --- | --- | --- | 0.6 | --- | 0.2 |
| 1505 | 1505 | β-Bisabolene | 0.5 | 0.1 | 0.2 | 0.4 | 0.1 | --- |
| 1513 | 1514 | Cubebol | 0.3 | 0.1 | tr | --- | --- | --- |
| 1521 | 1522 | δ-Cadinene | 0.4 | 0.3 | --- | --- | --- | --- |
| 1527 | 1529 | (E)-γ-Bisabolene | --- | --- | --- | --- | tr | --- |
| 1546 | 1548 | α-Elemol | --- | tr | 0.1 | 0.2 | 1.6 | tr |
| 1554 | 1559 | Germacrene B | --- | --- | --- | 0.1 | --- | --- |
| 1562 | 1561 | (E)-Nerolidol | --- | --- | --- | 1.6 | --- | --- |
| 1577 | 1577 | Spathulenol | --- | 0.1 | --- | --- | --- | --- |
| 1582 | 1582 | Caryophyllene oxide | 0.5 | 1.0 | 0.4 | 1.6 | 0.1 | 0.6 |
| 1588 | 1596 | Fokienol | --- | 0.2 | --- | --- | --- | --- |
| 1607 | 1608 | Humulene epoxide II | --- | --- | --- | 0.3 | --- | --- |
| 1610 | 1602 | Ledol | --- | --- | tr | --- | --- | --- |
| 1624 | 1632 | α-Acorenol | --- | --- | --- | --- | --- | 0.1 |
| 1625 | 1627 | 1-epi-Cubenol | 0.2 | --- | --- | --- | --- | --- |
| 1630 | 1630 | Muurola-4,10(14)-dien-1β-ol | --- | 1.1 | --- | 0.8 | --- | --- |
| 1636 | 1639 | Caryophylla-4(12),8(13)-dien-5β-ol | --- | 0.2 | --- | --- | --- | --- |
| 1640 | 1640 | τ-Murrolol | 0.3 | --- | --- | --- | --- | --- |
| 1644 | 1644 | α-Muurolol (=δ-Cadinol) | 1.3 | tr | tr | --- | --- | --- |
| 1649 | 1649 | β-Eudesmol | --- | --- | --- | --- | 0.1 | --- |
| 1656 | 1658 | neo-Intermedeol | --- | tr | 0.2 | --- | --- | --- |
| 1668 | 1668 | 14-Hydroxy-9-epi-(E)-caryophyllene | --- | 0.1 | --- | 0.1 | --- | --- |
| 1677 | 1679 | Khusinol | --- | 0.2 | --- | --- | --- | --- |
| 1682 | 1685 | α-Bisabolol | --- | tr | --- | --- | 1.1 | --- |
| 1714 | 1713 | 14-Hydroxy-α-humulene | --- | --- | --- | 0.2 | --- | --- |
| 1727 | 1728 | iso-Longifolol | --- | tr | --- | --- | --- | --- |
| 1763 | 1762 | β-Acoradienol | --- | --- | --- | 0.3 | --- | --- |
| 1930 | 1929 | Musk ambrette | --- | 0.2 | --- | --- | --- | --- |
| Monoterpene hydrocarbons | 84.4 | 76.6 | 87.6 | 77.9 | 89.5 | 88.0 | ||
| Oxygenated monoterpenoids | 3.8 | 2.2 | 2.4 | 7.3 | 6.7 | 8.2 | ||
| Sesquiterpene hydrocarbons | 8.9 | 17.8 | 8.3 | 9.7 | 0.8 | 3.0 | ||
| Oxygenated sesquiterpenoids | 2.9 | 3.0 | 0.6 | 5.0 | 2.9 | 0.7 | ||
| Total identified | 99.9 | 99.5 | 99.0 | 99.9 | 99.8 | 99.9 |
| Compound | α-Thujene | α-Pinene | Camphene | Sabinene | β-Pinene | Myrcene | α-Phellandrene | δ-3-Carene | p-Cymene | Limonene | β-Phellandrene | (E)-β-Ocimene | Terpinolene | Linalool | δ-Elemene | α-Cubebene | α-Copaene | β-Elemene | β-Caryophyllene | α-Guaiene | α-Humulene | Germacrene D | β-Selinene | α-Selinene | β-Bisabolene | α-Farnesene | δ-Cadinene | Elemol | Caryophyllene oxide |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balankotta [29] | 0 | 20.9 | 0 | 0 | 0 | 13.5 | 0 | 11.7 | 8.2 | 25.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brazil, CO2 [20] | 1 | 4.1 | 0 | 11.6 | 2.6 | 7.7 | 1.9 | 14.4 | 1.8 | 19.8 | 0.4 | 0 | 0.7 | 0.4 | 0.8 | 0 | 1.3 | 1.3 | 21.8 | 0 | 1.5 | 0 | 2.5 | 3.1 | 0 | 0 | 0.5 | 0 | 0 |
| Subh-90 [26] | 0.1 | 7 | 0.2 | 0.5 | 7.6 | 7.9 | 0.1 | 19 | 2.3 | 22.7 | 0 | 0 | 0.1 | 0.5 | 0 | 0.1 | 0.9 | 0.2 | 7.6 | 0.1 | 0.3 | 0 | 0.1 | tr | 1.6 | 0 | 0.1 | 0.7 | 6 |
| Subh-91 [26] | 0.1 | 3.2 | 0.2 | 0.2 | 8 | 6.7 | 3.5 | 23.4 | 0.9 | 19.5 | 0 | 0.1 | 0 | 0.6 | 0 | 0.3 | 1.7 | 0.2 | 15.5 | 0 | 0.4 | 0 | 0.1 | 0.1 | 2.8 | 0 | 0 | 0.8 | 0.4 |
| Subh-92 [26] | 0.1 | 4.7 | 0.1 | 0.2 | 9.6 | 4.3 | 3.8 | 20.8 | 0.6 | 18.3 | 0 | 0 | 0 | 0.5 | 0 | 0.1 | 1.7 | 0.1 | 21.3 | 0 | 0.4 | 0 | 0.1 | 0.1 | 3.1 | 0 | 0 | 0.8 | 3 |
| Cameroon [1] | 1.8 | 5.6 | 0.1 | 11.2 | 6.7 | 2.5 | 4.5 | 18.5 | 0.7 | 14.7 | 0 | 0.1 | 1.2 | 0.7 | 1.7 | 0.2 | 1.4 | 1.3 | 12.8 | 0 | 1.3 | 0.2 | 0 | 2.2 | tr | 0 | 0.6 | 0 | 0 |
| Sree-III [27] | tr | 4.3 | 0.2 | 0.2 | 10.2 | 5.5 | 3 | 11.1 | 0.5 | 20.1 | 0 | 0.1 | 0.1 | 0.2 | 0 | tr | 1.5 | 0.1 | 23.1 | 0 | 0.4 | 0 | tr | tr | 2.5 | 0 | 0 | 0.6 | 0.3 |
| Uthir-1st [28] | 0.2 | 14.6 | 0.4 | 0.3 | 9.3 | 4.3 | 7.4 | 8.5 | 1.3 | 19.5 | 0 | 0 | 0 | 0.1 | 0 | tr | 0.9 | 0.2 | 25.1 | 0 | tr | 0 | tr | 0 | 0 | 0 | 0.1 | tr | 0.6 |
| Kari-90 [30] | 0.1 | 5.4 | 0.2 | 0.2 | 15.2 | 0 | 3.3 | 20.3 | 0.7 | 20.1 | 0 | 0 | 0 | 0.5 | 0 | 1.9 | 0 | 0.1 | 19.8 | 0 | 0.4 | 0 | 0.1 | 0.1 | 2.5 | 0 | 0.1 | 0.8 | 0.4 |
| Kari-91 [30] | tr | 5 | 0.1 | 0 | 14.3 | 0.8 | 2.8 | 21 | 0.6 | 19.7 | 0 | tr | 0 | tr | 0 | 2.2 | 0 | 0 | 20.6 | 0 | tr | 0 | 0.1 | 0.1 | 2.9 | 0 | 0.2 | 0.9 | 0.4 |
| Kari-93 [30] | 0.1 | 5.3 | 0.1 | 0.6 | 14.1 | 0.9 | 2.9 | 17.8 | 0.9 | 19.6 | 0 | 0.2 | 0.2 | 0.5 | 0 | 1.5 | 0 | 0.1 | 25.6 | 0 | 0.4 | 0 | 0.1 | 0.1 | 2.7 | 0 | 0 | 0.8 | 0.5 |
| Madagascar [24] | 0 | 25.4 | 0.8 | 0 | 15.7 | 0 | 0 | 10.8 | 1 | 21 | 0 | 0 | 0 | 0.6 | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thom-90 [29] | 0.8 | 12.9 | 0.3 | 3.8 | 6.4 | 6.3 | 2.2 | 12.6 | 0.6 | 16.4 | 0 | 0.3 | 0.1 | 0.6 | 0 | 1.3 | 0.9 | 0 | 23.5 | 0 | tr | 0 | 0.1 | 0.3 | 0 | 0 | tr | 0 | 0.8 |
| Market [29] | 2.4 | 10 | 0 | 0 | 24.4 | 15.2 | 0 | 0 | 0 | 26.5 | 0 | 0 | 0 | 0 | 0 | 3.5 | 0 | 0 | 2.4 | 0 | 0 | 0 | 0 | 0 | 0 | 4.6 | 0 | 0 | 6.2 |
| Pann-1 [29] | 3 | 7.7 | 0 | 0 | 21.2 | 13.8 | 1.3 | 3.4 | 0 | 21.1 | 0 | 0 | 0 | 0 | 0 | 2.2 | 0 | 0 | 10.6 | 0 | 0 | 0 | 0 | 0 | 0 | 5.9 | 0 | 0 | 0 |
| Pann-5 [29] | 2.8 | 7.1 | 0 | 0 | 22.3 | 12.3 | 0 | 2.3 | 0 | 20.3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 17.8 | 0 | 0 | 0 | 0 | 0 | 0 | 16.7 | 0 | 0 | 0 |
| Aimp-3rd [28] | 2.3 | 6.6 | 0.2 | 0 | 23.9 | 11.1 | 0.4 | 0 | 0.2 | 21 | 0 | 0.1 | 0.1 | 0.2 | 0 | tr | 0.4 | 1 | 16.4 | 0 | 1.1 | 0 | 0.3 | 0.7 | 0 | 0 | 0 | 9.6 | 1.2 |
| Orav 2004 [3] | 0.2 | 7.3 | 0.2 | 1.4 | 19 | 2.6 | 2.2 | 10.6 | 0.5 | 29.7 | 0.3 | 0 | 0.6 | 2.1 | 0 | 1.6 | 0 | 0 | 14 | 0 | 0 | tr | 0.3 | 0.3 | 0.2 | 0.2 | 0.6 | 0.4 | 0.8 |
| Bagheri 2014 [5] | 1.4 | 8 | 0.3 | 13.2 | 9.7 | 1.2 | 1.6 | 8.6 | 0.9 | 15 | 0 | 0 | 0.2 | 0.6 | |||||||||||||||
| Thev-90 [26] | 1.1 | 3.8 | tr | 10.5 | 8.3 | 0 | 0.7 | 5.3 | 0.3 | 13.7 | 0 | tr | 0.1 | 0.4 | |||||||||||||||
| Thev-91 [26] | 1.2 | 5.2 | tr | 16.2 | 8.7 | 0 | 0.6 | 5.5 | 0.5 | 18 | 0 | 0.2 | 0.1 | 0.2 | |||||||||||||||
| Thev-92 [26] | 0.5 | 1.9 | tr | 4.5 | 3.7 | 1.6 | 0.9 | 4.8 | 0.2 | 8.3 | 0 | 0.1 | tr | 0.3 | |||||||||||||||
| Poon-90 [26] | 1.5 | 5.1 | 0.2 | 4.5 | 11.7 | 6.6 | 1.4 | 2.1 | 0.4 | 15.8 | 0 | 12 | 0.1 | 0.8 | |||||||||||||||
| Poon-91 [26] | 0.8 | 4.9 | 0.1 | 2.3 | 10.2 | 7.2 | 1.2 | 2.1 | 6.2 | 15.2 | 0 | 0.2 | 0.1 | 0.5 | |||||||||||||||
| Poon-92 [26] | 0.8 | 3 | 0.1 | 7.8 | 6 | 4.1 | 1.5 | 7.3 | 0 | 14.9 | 0 | tr | 0.1 | 0.5 | |||||||||||||||
| Valia-90 [26] | 1.1 | 6.3 | 0.3 | 17.1 | 0 | 0.2 | 0.7 | 0 | 0.7 | 18.6 | 0 | 0 | 0.1 | 0.1 | |||||||||||||||
| Valia-91 [26] | 1.1 | 4.6 | 0.4 | 15.9 | tr | 0.2 | 2.1 | 10.5 | 0.3 | 15.9 | 0 | tr | 0.2 | 0.1 | |||||||||||||||
| Valiai-92 [26] | 0.8 | 2.9 | 0.3 | 12.9 | 0 | 0.1 | 1.6 | 8.7 | 0 | 12.9 | 0 | 0 | 0.1 | 0.1 | |||||||||||||||
| S.Tome e Principe [25] | 1.4 | 5.7 | 0.1 | 16.5 | 10.7 | 2 | 0.7 | 1.7 | 0.2 | 18.8 | 2.9 | 0.5 | 0.4 | 1.1 | |||||||||||||||
| Market (India) [2] | 0.8 | 4.5 | 0.1 | 5.9 | 7.9 | 1 | 0.6 | 4.4 | 1.2 | 13.2 | 0 | tr | 0.1 | 0.5 | |||||||||||||||
| Sree-I [27] | tr | 5.5 | 0.2 | 4.3 | 11.2 | 0 | 7.7 | 0.1 | 1.5 | 22.1 | 0 | tr | 0 | 0.5 | |||||||||||||||
| Sree-II [27] | 1.5 | 3.3 | 0.1 | 4.6 | 0 | 9.6 | 0 | 0.1 | 1.5 | 20.5 | 0 | 0.2 | 0.2 | 0.6 | |||||||||||||||
| Kuch-I [27] | tr | 5.4 | tr | 13.3 | 0 | 0 | 0 | 0.4 | 0 | 14.5 | 0 | 0 | 0.1 | 0.4 | |||||||||||||||
| Kuch-II [27] | 0.7 | 2.3 | 0.1 | 6.7 | 5.2 | 5.2 | 6.2 | 0.5 | 2 | 17.5 | 0 | 0.2 | 0.1 | 0.4 | |||||||||||||||
| Vell-I [27] | 1.7 | 3.6 | 0.2 | 18.8 | 10.9 | 0 | 0.2 | tr | 0.2 | 19.8 | 0 | 0 | 0.1 | 0.4 | |||||||||||||||
| Vell-II [27] | 1 | 3.6 | 0.1 | 8.4 | 6.5 | 3.1 | 1.3 | 7.6 | 0.3 | 14.9 | 0 | 0.1 | 0.1 | 0.5 | |||||||||||||||
| Vell-III [27] | 0.4 | 1.7 | 0.1 | 3.9 | 3.9 | 2 | 1 | 5.1 | 0.1 | 8.3 | 0 | tr | tr | 0.3 | |||||||||||||||
| Aimp-1st [28] | 0.9 | 8.4 | tr | 27.5 | 9.2 | 0 | tr | 0.1 | 0.5 | 19.8 | 0 | tr | 0.3 | 0.2 | 0 | tr | |||||||||||||
| Aimp-2nd [28] | 0.5 | 7.4 | 0.2 | 24.2 | 14.8 | 0 | 0.2 | 0 | 0.3 | 22.5 | 0 | 0.2 | 0.2 | 0.5 | 0 | tr | |||||||||||||
| Naray-1st [28] | 2.7 | 5.9 | tr | 24.6 | 8.7 | 0 | 0.3 | 2.3 | 0.4 | 15.5 | 0 | 0.1 | 0.1 | 0.5 | 0 | tr | |||||||||||||
| Naray-2nd [28] | 0.3 | 6.4 | tr | 4.4 | 15.6 | 8.4 | 0.1 | 0 | 0.3 | 19.5 | 0 | 0.2 | 0.1 | 0.2 | 0 | tr | |||||||||||||
| Naray-3rd [28] | 1 | 2 | 0.1 | 13.9 | 4.8 | 0 | 0.2 | tr | 0.2 | 9.5 | 0 | tr | 0.2 | 0.1 | 0 | tr | |||||||||||||
| Neel-1st [28] | 1.6 | 6.5 | 0.2 | 27.3 | 11.3 | 0 | 1.3 | 7.9 | 0.5 | 18.6 | 0 | 0 | 0.1 | 0.6 | 0 | 0.2 | |||||||||||||
| Neel-2nd [28] | 1 | 5.6 | 0.2 | 23.9 | 7.8 | 0 | 0.4 | 0.5 | 0.3 | 15.9 | 0 | tr | 0.2 | 0.3 | 0 | 0.7 | |||||||||||||
| Neel-3rd [28] | 2.2 | 4.7 | tr | 23.2 | 9.8 | 0.3 | 0.4 | 0.1 | 0.1 | 12.9 | 0 | 0 | 0.3 | 0.3 | 0 | 0.1 | |||||||||||||
| Uthir-2nd [28] | 0.1 | 9.1 | 0.2 | 0.1 | 12.5 | 3.5 | 5 | 6.7 | 1.1 | 13.3 | 0 | 0.1 | 0 | 0.1 | 0 | 0.2 | |||||||||||||
| Kott-90 [31] | 2.5 | 7.4 | 0.1 | 18.8 | 15.4 | 0 | 0.3 | 0.2 | 0.2 | 23.8 | 0 | 0.2 | 0.3 | 0.5 | 0 | 0.8 | |||||||||||||
| Kott-91 [31] | 2.4 | 7.1 | 0.1 | 22.1 | 13.3 | 0 | 0.2 | 0.2 | 0.2 | 21.5 | 0 | 0.2 | 0.2 | 0.5 | 0 | 0.1 | |||||||||||||
| Kott-92 [31] | 1 | 3 | tr | 11.2 | 7.5 | 0 | 0.2 | tr | 0.1 | 12.7 | 0 | 0.3 | 0.2 | 0.1 | 0 | 0.2 | |||||||||||||
| Ott-90 [31] | 0.7 | 4.4 | 0.1 | 9.1 | 3.8 | 8.3 | 0.1 | tr | 0.1 | 15.5 | 0 | 0.2 | 0.1 | 0.1 | 0 | 0.1 | |||||||||||||
| Ott-91 [31] | 2 | 5.9 | 0.1 | 26.8 | 11 | 0 | 0.4 | 0.1 | 0.4 | 20.2 | 0 | 0.5 | 0.2 | 0.3 | 0 | 0.2 | |||||||||||||
| Ott-92 [31] | 0.6 | 1.8 | 0.1 | 0.1 | 11.7 | 18.6 | 0.2 | tr | 0.4 | 21.7 | 0 | 0.4 | 0.2 | 0.3 | 0 | 0.2 | |||||||||||||
| Kuth-90 [31] | 0.6 | 7.9 | 0.3 | 5.3 | 10.9 | 0.2 | 6.8 | 0 | 1 | 16.9 | 0 | 0 | 0.1 | 1.2 | 0 | 0.2 | |||||||||||||
| Kuth-92 [31] | 0.2 | 2.7 | 0.1 | 1.9 | 3.8 | 2 | 0.5 | 4.2 | 0.3 | 9 | 0 | 0.1 | tr | 0.7 | 0 | 0.3 | |||||||||||||
| Cher-90 [31] | 0.1 | 7.1 | 0.2 | 9.7 | 11.2 | 3 | 1.2 | 3.2 | 4 | 17.8 | 0 | 0.4 | 0.1 | 0.3 | 0 | 0.1 | 0.3 | 0.1 | |||||||||||
| Cher-91 [31] | 3.6 | 4 | 0.1 | 22.3 | 7.7 | 0 | 2.6 | 5.4 | 1.5 | 15.2 | 0 | 0.5 | 0.2 | 0.3 | 0 | 2.5 | 0.9 | 0.1 | |||||||||||
| Cher-92 [31] | 2 | 5 | 0.1 | 19.1 | 9.5 | 0.6 | 2.2 | 9.8 | 0.4 | 14.7 | 0 | 0.5 | 0.2 | 0.6 | 0 | 2.9 | 0.9 | 0.1 | |||||||||||
| Kall-90 [30] | tr | 10.1 | 0.4 | 8 | 11.7 | 0 | 9.8 | 0.9 | 0 | 18.1 | 0 | 0.1 | 0.1 | 1.3 | 0 | 0.8 | 0 | 0.1 | |||||||||||
| Kall-91 [30] | 0.4 | 5.8 | 0.2 | 7.1 | 8.1 | 0 | 6.1 | 3.7 | 0.9 | 19.1 | 0 | 0.2 | 0.1 | 1.3 | 0 | 0.8 | 0.1 | 0 | |||||||||||
| Kall-93 [30] | 0.2 | 3.5 | 0.1 | 1.8 | 4.4 | 2.6 | 0.4 | 4.5 | 0.3 | 10.7 | 0 | 0.2 | tr | 0.7 | 0 | 1.3 | 0.1 | 0.1 | |||||||||||
| Thom-91 [30] | 0.1 | 2.4 | 0.1 | 3.9 | 2 | 4 | 0.5 | 2.5 | 0.7 | 11.7 | 0 | 0 | 0.1 | 0.5 | 0 | 3.8 | 0.9 | 0 | |||||||||||
| Thom-93 [30] | 0.7 | 11.8 | 0.3 | 4.1 | 7.3 | 0.4 | 0.9 | 5.4 | 0.4 | 9.4 | 0 | 0.2 | 0.1 | 0.4 | 0 | 3 | 0.7 | 0 | |||||||||||
| Arak-90 [30] | 2.2 | 6.9 | 0.1 | 20.9 | 11.1 | 0 | 0.3 | 0.2 | 0 | 20.4 | 0 | 0.4 | 0.2 | 0.3 | 0 | 0.1 | 0.7 | tr | |||||||||||
| Arak-91 [30] | 0.4 | 4.4 | 0.6 | 5.5 | 12.5 | 2.4 | 0.2 | 0.1 | 0.2 | 21.9 | 0 | 0.4 | 0.1 | 0.1 | 0 | 0.1 | 0.6 | 1 | |||||||||||
| Arak-93 [30] | 0.6 | 2.4 | tr | 6.3 | 6.1 | 0.2 | 0.1 | 0.4 | 0.1 | 10.3 | 0 | 0.2 | 0.1 | 0.2 | 0 | 0.6 | 1.1 | tr |
| Compound | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 |
|---|---|---|---|---|
| α-Thujene | 0.610 | 1.510 | 0.323 | 1.250 |
| α-Pinene | 4.538 | 6.089 | 10.608 | 8.337 |
| Camphene | 0.173 | 0.125 | 0.288 | 0.036 |
| Sabinene | 5.892 | 19.141 | 2.179 | 0.175 |
| β-Pinene | 6.982 | 9.621 | 10.004 | 28.796 |
| Myrcene | 3.512 | 0.386 | 4.164 | 6.040 |
| α-Phellandrene | 2.169 | 0.688 | 2.686 | 0.485 |
| δ-3-Carene | 3.146 | 2.812 | 14.998 | 4.031 |
| p-Cymene | 0.758 | 0.546 | 1.324 | 0.074 |
| Limonene | 14.850 | 17.499 | 19.404 | 28.103 |
| β-Phellandrene | 0.000 | 0.173 | 0.111 | 0.027 |
| (E)-β-Ocimene | 0.588 | 0.190 | 0.111 | 0.053 |
| Terpinolene | 0.097 | 0.184 | 0.272 | 0.302 |
| Linalool | 0.473 | 0.398 | 0.412 | 1.083 |
| δ-Elemene | 0.019 | 0.223 | 0.675 | 0.049 |
| α-Cubebene | 1.661 | 0.777 | 0.477 | 0.844 |
| α-Copaene | 0.947 | 0.777 | 0.814 | 0.132 |
| β-Elemene | 0.148 | 0.402 | 0.515 | 0.205 |
| β-Caryophyllene | 33.815 | 20.759 | 17.184 | 8.951 |
| α-Guaiene | 0.183 | 0.202 | 0.088 | 0.000 |
| α-Humulene | 0.272 | 0.523 | 0.635 | 0.299 |
| Germacrene D | 0.012 | 0.015 | 0.465 | 0.014 |
| β-Selinene | 0.206 | 0.295 | 0.593 | 0.223 |
| α-Selinene | 0.483 | 0.500 | 0.674 | 0.091 |
| α-Farnesene | 0.000 | 0.000 | 1.088 | 2.495 |
| β-Bisabolene | 1.485 | 1.147 | 0.005 | 0.135 |
| δ-Cadinene | 0.134 | 0.381 | 0.165 | 0.120 |
| Elemol | 1.609 | 1.595 | 0.321 | 1.080 |
| Caryophyllene oxide | 1.923 | 1.182 | 0.863 | 1.135 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosoky, N.S.; Satyal, P.; Barata, L.M.; da Silva, J.K.R.; Setzer, W.N. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules 2019, 24, 4244. https://doi.org/10.3390/molecules24234244
Dosoky NS, Satyal P, Barata LM, da Silva JKR, Setzer WN. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules. 2019; 24(23):4244. https://doi.org/10.3390/molecules24234244
Chicago/Turabian StyleDosoky, Noura S., Prabodh Satyal, Luccas M. Barata, Joyce Kelly R. da Silva, and William N. Setzer. 2019. "Volatiles of Black Pepper Fruits (Piper nigrum L.)" Molecules 24, no. 23: 4244. https://doi.org/10.3390/molecules24234244
APA StyleDosoky, N. S., Satyal, P., Barata, L. M., da Silva, J. K. R., & Setzer, W. N. (2019). Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules, 24(23), 4244. https://doi.org/10.3390/molecules24234244