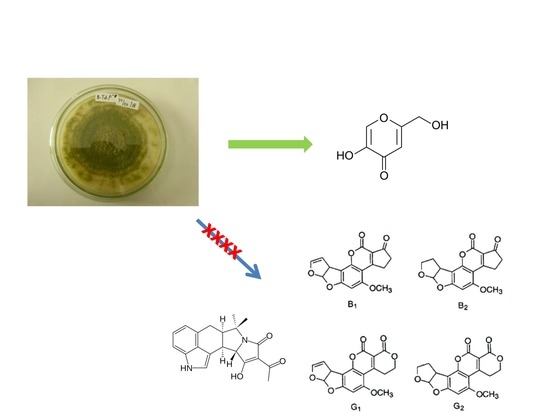
Single Production of Kojic Acid by Aspergillus flavus and the Revision of Flufuran
Abstract
:1. Introduction
2. Results and Discussion
3. Discussion
4. Materials and Methods
4.1. Isolation of Endophytic Fungi
4.2. Cultivation and Extraction of Secondary Metabolites
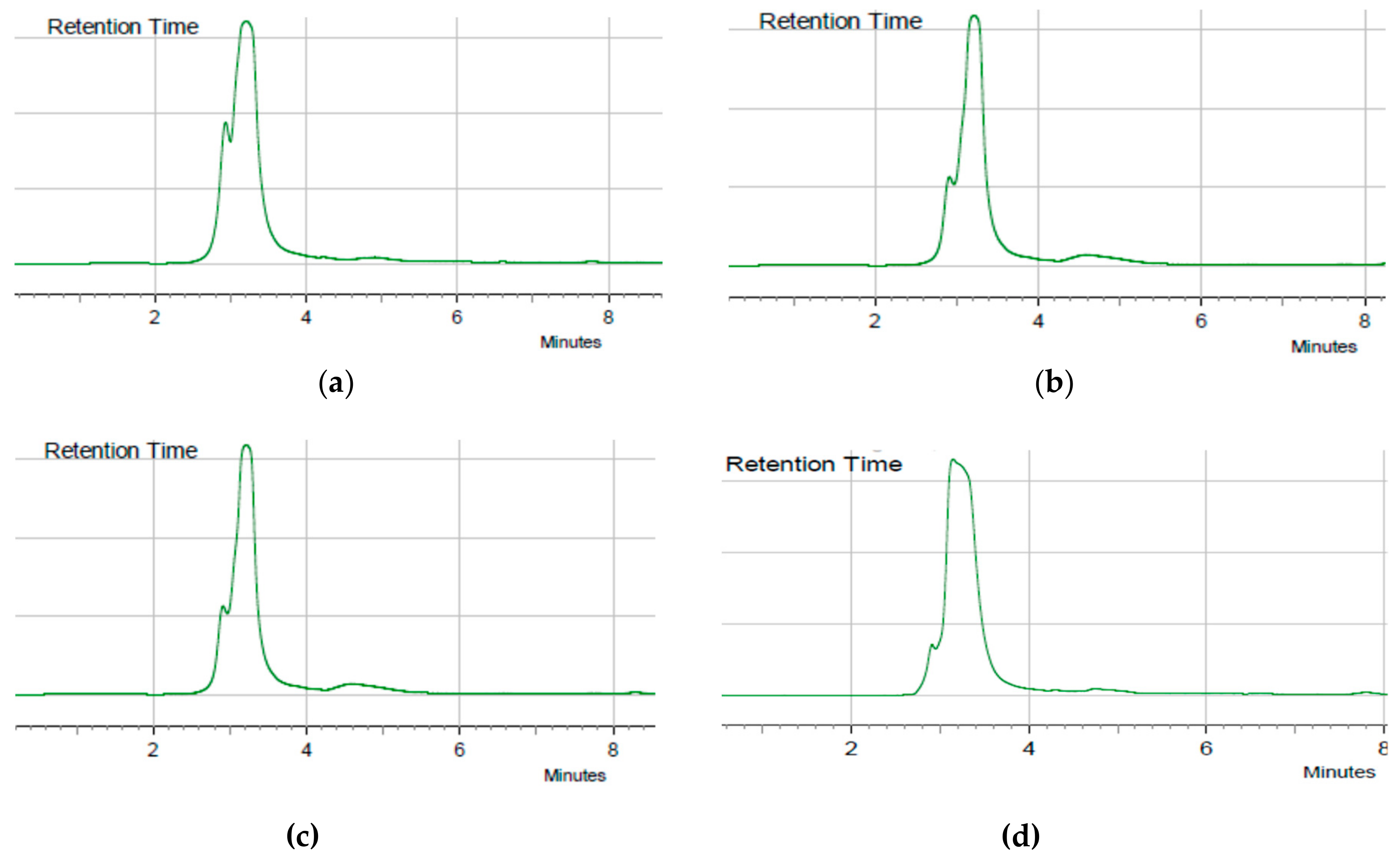
4.3. Analysis of Kojic Acid with HPLC
4.4. Fractionation and Identification of Kojic Acid
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-Derived Bioactive Compounds Produced by Endophytic Fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharm. 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. From miso, saké and shoyu to cosmetics: a century of science for kojic acid. Nat. Prod. Rep 2006, 23, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Parrish, F.W.; Wiley, B.J.; Simmons, E.G.; Long, L. Production of aflatoxins and kojic acid by species of Aspergillus and Penicillium. Appl. Microbiol. 1966, 14, 139. [Google Scholar] [PubMed]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008, 58, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Frisvad, J.C.; Samson, R.A. Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 2011, 69, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Basappa, S.C.; Sreenivasamurthy, V.; Parpia, H.A.B. Aflatoxin and Kojic Acid Production by Resting Cells of Aspergillus flavus Link. Microbiology 1970, 61, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Chang, P.-K.; Yu, J.; Cotty, P.J. Aflatoxin Biosynthesis Cluster Gene cypA Is Required for G Aflatoxin Formation. Appl. Environ. Microbiol. 2004, 70, 6518. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, G.M.; Julia Roberti, M.; Wright, J.E.; Seldes, A.M. Cryptoporic and isocryptoporic acids from the fungal cultures of Polyporus arcularius and P. ciliatus. Phytochemistry 2002, 61, 189–193. [Google Scholar] [CrossRef]
- Evidente, A.; Cristinzio, G.; Punzo, B.; Andolfi, A.; Testa, A.; Melck, D. Flufuran, an Antifungal 3,5-Disubstituted Furan Produced by Aspergillus flavus Link. Chem. Biodiver. 2009, 6, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-M.; Ma, C.-C.; Li, T.; Wang, J. A new furan derivative from an endophytic Aspergillus flavus of Cephalotaxus fortunei. Nat. Prod. Res. 2016, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Pevzner, L.M.; Ignat’ev, V.M.; Ionin, B.I. Synthesis of isomeric phosphonomethylated N,N-diethylfurancarboxamides. Russ. J. Gen. Chem. 1999, 69, 551. [Google Scholar]
- DellaGreca, M.; De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; Becchimanzi, A.; Iuliano, M.; Andolfi, A. The Issue of Misidentification of Kojic Acid with Flufuran in Aspergillus flavus. Molecules 2019, 24, 1709. [Google Scholar] [CrossRef] [PubMed]
- Balde, E.S.; Andolfi, A.; Bruyère, C.; Cimmino, A.; Lamoral-Theys, D.; Vurro, M.; Damme, M.V.; Altomare, C.; Mathieu, V.; Kiss, R.; et al. Investigations of Fungal Secondary Metabolites with Potential Anticancer Activity. J. Nat. Prod. 2010, 73, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Cho, J.-Y.; Lee, Y.G.; Lee, H.J.; Lim, S.-I.; Lee, S.-Y.; Nam, Y.-D.; Moon, J.-H. Furan, phenolic, and heptelidic acid derivatives produced by Aspergillus oryzae. Food Sci. Biotechnol. 2016, 25, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Elsunni, M.A.; Yang, Z.-D. Secondary Metabolites of the Endophytic Fungi Penicillium polonicum and Their Monoamine Oxidase Inhibitory Activity. Chem. Nat. Compd. 2018, 54, 1018–1019. [Google Scholar] [CrossRef]
- Ola, A.R.B.; Tawo, B.D.; Belli, H.L.L.; Proksch, P.; Tommy, D.; Hakim, E.H. A New Antibacterial Polyketide from the Endophytic Fungi Aspergillus Fumigatiaffinis. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef]
- Ola, A.R.B.; Debbab, A.; Kurtán, T.; Brötz-Oesterhelt, H.; Aly, A.H.; Proksch, P. Dihydroanthracenone metabolites from the endophytic fungus Diaporthe melonis isolated from Annona squamosa. Tetrahedron Lett. 2014, 55, 3147–3150. [Google Scholar] [CrossRef]
Sample Availability: Samples of kojic is available from the authors. |
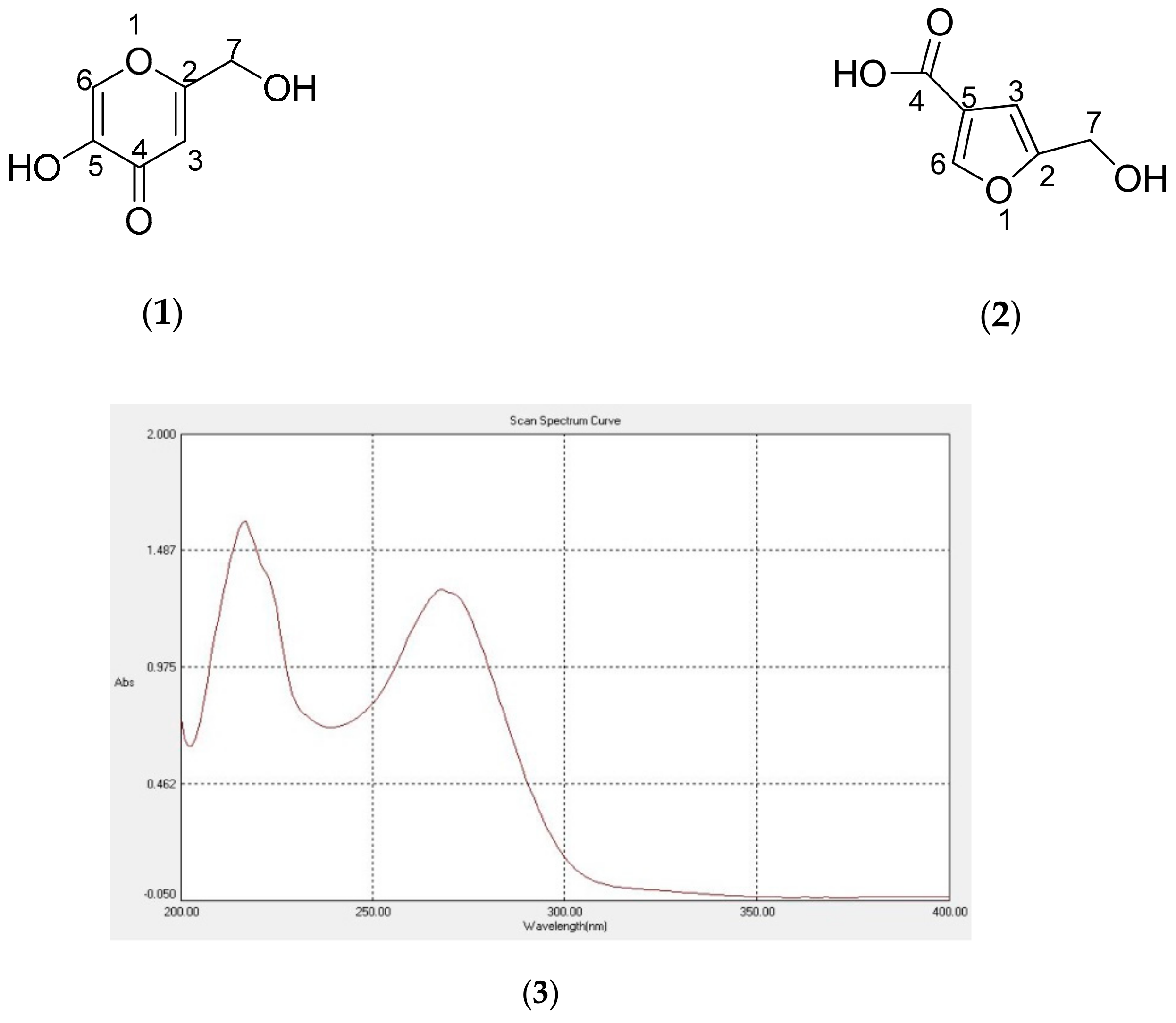
| No | Kojic Acid (1) | Flufuran Natural Products a,b | Flufuran Synthetic c | |||||
|---|---|---|---|---|---|---|---|---|
| 1H-NMR | 13C-NMR | 1H-NMRa | 13C-NMR a | 1H-NMR b | 13C-NMR b | 1H-NMR | 13C-NMR | |
| 1 | ||||||||
| 2 | - | 170.41 | 170.4 | 170.4 | 156.4 | |||
| 3 | 6.49 s, 1H | 110.74 | 6.53 | 110.7 | 6.49 | 110.8 | 6.62 | 106.7 |
| 4 | - | 176.86 | 176.9 | 176.9 | 165.1 | |||
| 5 | - | 147.37 | 147.5 | 147.4 | 120.1 | |||
| 6 | 7.95 s, 1H | 141.0 | 7.98 | 141.0 | 7.94 | 141.0 | 8.08 | 147.7 |
| 7 | 4.40 s, 2H | 61.18 | 4.43 | 61.2 | 4.4 | 61.2 | 4.53 | 55.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ola, A.R.B.; Metboki, G.; Lay, C.S.; Sugi, Y.; Rozari, P.D.; Darmakusuma, D.; Hakim, E.H. Single Production of Kojic Acid by Aspergillus flavus and the Revision of Flufuran. Molecules 2019, 24, 4200. https://doi.org/10.3390/molecules24224200
Ola ARB, Metboki G, Lay CS, Sugi Y, Rozari PD, Darmakusuma D, Hakim EH. Single Production of Kojic Acid by Aspergillus flavus and the Revision of Flufuran. Molecules. 2019; 24(22):4200. https://doi.org/10.3390/molecules24224200
Chicago/Turabian StyleOla, Antonius R. B., Gema Metboki, Caterina S. Lay, Yoseph Sugi, Philipi De Rozari, Dodi Darmakusuma, and Euis Holisotan Hakim. 2019. "Single Production of Kojic Acid by Aspergillus flavus and the Revision of Flufuran" Molecules 24, no. 22: 4200. https://doi.org/10.3390/molecules24224200
APA StyleOla, A. R. B., Metboki, G., Lay, C. S., Sugi, Y., Rozari, P. D., Darmakusuma, D., & Hakim, E. H. (2019). Single Production of Kojic Acid by Aspergillus flavus and the Revision of Flufuran. Molecules, 24(22), 4200. https://doi.org/10.3390/molecules24224200